Abstract
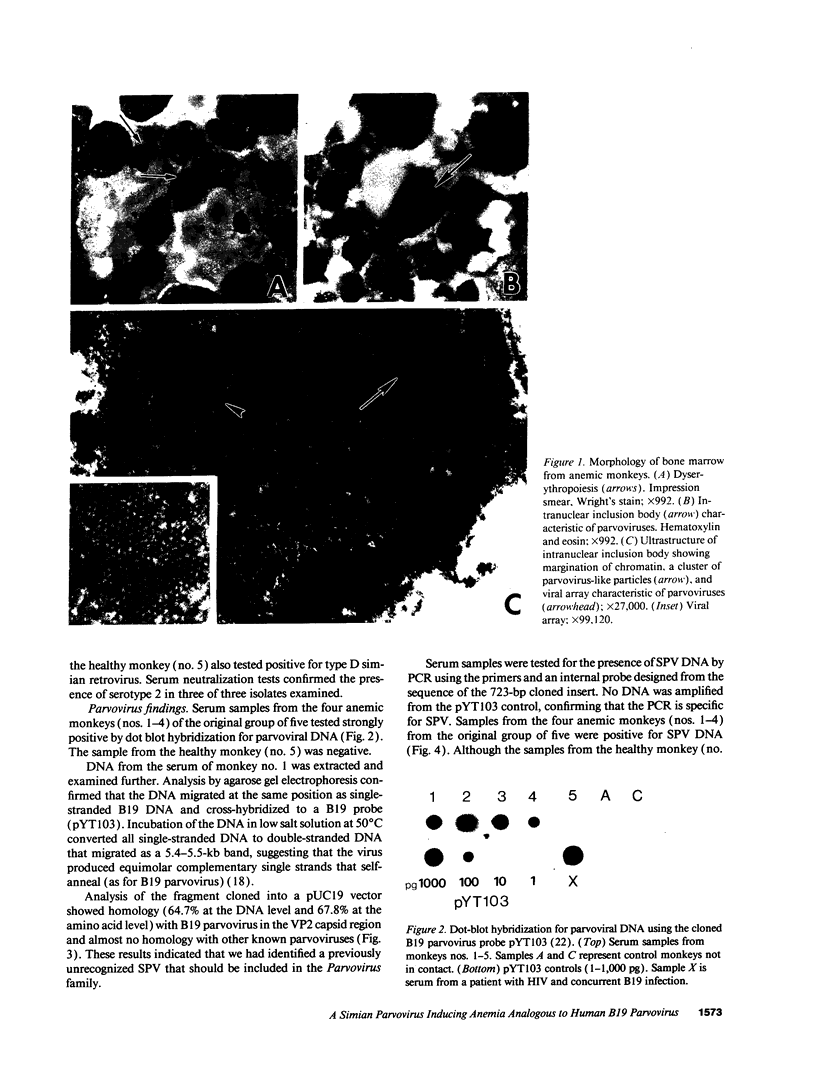
Although human B19 parvovirus infection has been clearly associated with a number of distinct syndromes (including severe anemia, abortion, and arthritis), detailed knowledge of its pathogenesis has been hindered by the lack of a suitable animal model. We have identified a novel simian parvovirus in cynomolgus monkeys with severe anemia. Sequencing of a 723-bp fragment of cloned viral DNA extracted from serum revealed that the simian parvovirus has 65% homology at the DNA level with the human B19 parvovirus but little homology with other known parvoviruses. Light microscopic examination of bone marrow from infected animals showed intranuclear inclusion bodies, and ultrastructural studies showed viral arrays characteristic of parvoviruses. Another striking feature was the presence of marked dyserythropoiesis in cells of the erythroid lineage, raising the possibility that B19 parvovirus infection may underlie related dyserythropoietic syndromes in human beings. Affected animals had concurrent infection with the immunosuppressive type D simian retrovirus, analogous to HIV patients who develop severe anemia because of infection with B19 parvovirus. The remarkable similarities between the simian and B19 parvoviruses suggest that experimentally infected cynomolgus monkeys may serve as a useful animal model of human B19 infection.
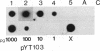
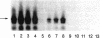
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman M. S., Rossmann M. G. Structure, sequence, and function correlations among parvoviruses. Virology. 1993 Jun;194(2):491–508. doi: 10.1006/viro.1993.1288. [DOI] [PubMed] [Google Scholar]
- Clewley J. P. Detection of human parvovirus using a molecularly cloned probe. J Med Virol. 1985 Feb;15(2):173–181. doi: 10.1002/jmv.1890150210. [DOI] [PubMed] [Google Scholar]
- Cohen B. J., Buckley M. M. The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol. 1988 Feb;25(2):151–153. doi: 10.1099/00222615-25-2-151. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Characterization and molecular cloning of a human parvovirus genome. Science. 1984 Dec 7;226(4679):1161–1165. doi: 10.1126/science.6095448. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., King N. W., Letvin N. L., Hunt R. D., Sehgal P. K., Desrosiers R. C. A new type D retrovirus isolated from macaques with an immunodeficiency syndrome. Science. 1984 Feb 10;223(4636):602–605. doi: 10.1126/science.6695172. [DOI] [PubMed] [Google Scholar]
- Field A. M., Cohen B. J., Brown K. E., Mori J., Clewley J. P., Nascimento J. P., Hallam N. F. Detection of B19 parvovirus in human fetal tissues by electron microscopy. J Med Virol. 1991 Oct;35(2):85–95. doi: 10.1002/jmv.1890350204. [DOI] [PubMed] [Google Scholar]
- Frickhofen N., Abkowitz J. L., Safford M., Berry J. M., Antunez-de-Mayolo J., Astrow A., Cohen R., Halperin I., King L., Mintzer D. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia in AIDS. Ann Intern Med. 1990 Dec 15;113(12):926–933. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- Frickhofen N., Young N. S. Polymerase chain reaction for detection of parvovirus B19 in immunodeficient patients with anemia. Behring Inst Mitt. 1990 Aug;(85):46–54. [PubMed] [Google Scholar]
- Henrickson R. V., Maul D. H., Lerche N. W., Osborn K. G., Lowenstine L. J., Prahalada S., Sever J. L., Madden D. L., Gardner M. B. Clinical features of simian acquired immunodeficiency syndrome (SAIDS) in rhesus monkeys. Lab Anim Sci. 1984 Apr;34(2):140–145. [PubMed] [Google Scholar]
- Knisely A. S., O'Shea P. A., McMillan P., Singer D. B., Magid M. S. Electron microscopic identification of parvovirus virions in erythroid-line cells in fatal hydrops fetalis. Pediatr Pathol. 1988;8(2):163–170. doi: 10.3109/15513818809022293. [DOI] [PubMed] [Google Scholar]
- Kurtzman G. J., Cohen B., Meyers P., Amunullah A., Young N. S. Persistent B19 parvovirus infection as a cause of severe chronic anaemia in children with acute lymphocytic leukaemia. Lancet. 1988 Nov 19;2(8621):1159–1162. doi: 10.1016/s0140-6736(88)90233-4. [DOI] [PubMed] [Google Scholar]
- Lerche N. W., Marx P. A., Osborn K. G., Maul D. H., Lowenstine L. J., Bleviss M. L., Moody P., Henrickson R. V., Gardner M. B. Natural history of endemic type D retrovirus infection and acquired immune deficiency syndrome in group-housed rhesus monkeys. J Natl Cancer Inst. 1987 Oct;79(4):847–854. [PubMed] [Google Scholar]
- Marx P. A., Maul D. H., Osborn K. G., Lerche N. W., Moody P., Lowenstine L. J., Henrickson R. V., Arthur L. O., Gilden R. V., Gravell M. Simian AIDS: isolation of a type D retrovirus and transmission of the disease. Science. 1984 Mar 9;223(4640):1083–1086. doi: 10.1126/science.6695196. [DOI] [PubMed] [Google Scholar]
- Osborn K. G., Prahalada S., Lowenstine L. J., Gardner M. B., Maul D. H., Henrickson R. V. The pathology of an epizootic of acquired immunodeficiency in rhesus macaques. Am J Pathol. 1984 Jan;114(1):94–103. [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. F., Nerlich A., Hottenträger B., Jäger G., Wiest I., Kantimm S., Roggendorf H., Schultz M., Gloning K. P., Schramm T. Parvovirus B19 infection of the fetus. Histology and in situ hybridization. Am J Clin Pathol. 1991 Jul;96(1):121–126. doi: 10.1093/ajcp/96.1.121. [DOI] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Young N., Harrison M., Moore J., Mortimer P., Humphries R. K. Direct demonstration of the human parvovirus in erythroid progenitor cells infected in vitro. J Clin Invest. 1984 Dec;74(6):2024–2032. doi: 10.1172/JCI111625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. Hematologic and hematopoietic consequences of B19 parvovirus infection. Semin Hematol. 1988 Apr;25(2):159–172. [PubMed] [Google Scholar]