Abstract
Purpose
We attempted to examine the correlation between metabolic syndrome and lower urinary tract symptoms (LUTS) in the aspect of gender-specific medicine.
Materials and Methods
A total of 922 patients participating in a health examination completed the International Prostate Symptom Score (IPSS) questionnaire and the Overactive Bladder Questionnaire Short Form (OABq-SF) symptom bother scale from March 2008 to July 2009. Metabolic syndrome was defined by using the National Cholesterol Education Program Adult Treatment Panel III criteria announced in 2001. We analyzed differences in lower urinary tract symptoms according to the presence of metabolic syndrome and the component elements of metabolic syndrome.
Results
The subjects were 538 males and 384 females with a mean age of 48.8±6.8 years. Among all patients, the number of patients with metabolic syndrome was 143 (15.5%); there were 110 males (20.4%) and 33 females (8.6%), showing a significant difference. There were no differences in scores on the IPSS or OABq-SF with respect to the presence or absence of metabolic syndrome in males. In females, however, there were significant differences in the IPSS and OABq-SF depending on the presence or absence of metabolic syndrome. In males and females, the IPSS total score was significantly correlated with age. Also, high-density lipoprotein (HDL) cholesterol in males and triglyceride in females was significantly correlated with the IPSS total score.
Conclusions
There are sex differences in the morbidity rate of metabolic syndrome and its effect on lower urinary tract symptoms. Therefore, it is necessary to consider gender-specific medicine in the diagnosis and treatment of LUTS.
Keywords: Gender identity, Metabolic syndrome X, Urologic diseases
INTRODUCTION
Gender-specific medicine is a medical methodology in which the gender differences between males and females are recognized and actively utilized in medical research, diagnosis, and treatment as well as in education. Gender-specific medicine assumes that gender is an important factor in the pathogenesis, disease progression, risk, and prognosis of many known diseases. According to gender-specific medicine, the management of certain diseases should be specified on the basis of a patient's gender, because males and females have different drug responses, risk factors, and prognoses.
Increasing evidence from clinical and epidemiological studies has shown associations between lower urinary tract symptoms (LUTS) and major chronic illnesses, such as heart disease and diabetes as well as related lifestyle factors, and these associations have motivated interest in the contribution of factors outside the urinary tract to urological symptoms-the so-called "beyond the bladder" hypothesis [1-4].
Data from the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) show a relationship between markers of metabolic syndrome and LUTS defined as having 3 of 4 urinary symptoms (nocturia, incomplete bladder emptying, weak stream, and hesitancy) [4]. Some symptoms, such as incontinence in women and nocturia in both men and women, have been associated with higher levels of bother or treatment seeking or both [5-7].
This study examined the correlations between metabolic syndrome and LUTS according to male and female gender in the aspect of gender-specific medicine.
MATERIALS AND METHODS
A total of 922 subjects who had participated in general health examinations at our hospital from March 2008 to July 2009 completed a general health examination questionnaire, the International Prostate Symptom Score (IPSS) questionnaire [8], and the Overactive Bladder Questionnaire Short Form (OABq-SF) symptom bother scale [9].
This study excluded cases that had been diagnosed with or administered a drug for a urologic disease. The diagnostic criteria for metabolic syndrome had to satisfy three or more of the NCEP-ATP III criteria, which are as follows: 1) hypertension (systolic blood pressure of 130 mmHg or higher or diastolic blood pressure of 85 mmHg or higher), 2) hyperglycemia (fasting blood sugar level of 110 mg/dl or higher), 3) obesity (waist circumference of 80 cm or greater or body mass index of 25 kg/m2 or heavier), 4) hypo-HDL-cholesterolemia of less than 50 mg/dl, and 5) hypertriglyceridemia of 150 mg/dl or higher.
The chi-square test, t-test, or multiple linear regression test was used to analyze 1) differences in the prevalence of metabolic syndrome of males and females, 2) differences in each component element of the IPSS and OABq-SF depending on the presence or absence of metabolic syndrome in males and females, and 3) correlations between A) age and IPSS total score, and B) component elements of metabolic syndrome and IPSS total score. All analyses were performed by using the SPSS program version 12.0 for Windows. In all comparisons of values, p-values less than 0.05 were considered to be statistically significant.
RESULTS
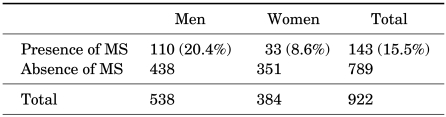
Analysis was conducted on a total of 922 subjects, of whom 538 were males and 384 were females with a mean age of 48.8±6.8 years (males, 48.8±6.2 years old; females, 48.9±7.6 years old). A total of 143 (15.5%) subjects satisfied the diagnostic criteria for metabolic syndrome, of whom 110 were males (20.4%) and 33 were females (8.6%), showing a significant difference (p<0.005) (Table 1).
TABLE 1.
Comparison of the prevalence rate of metabolic syndrome in males and females
MS: metabolic syndrome
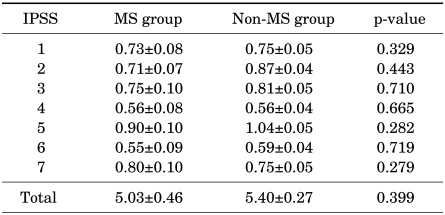
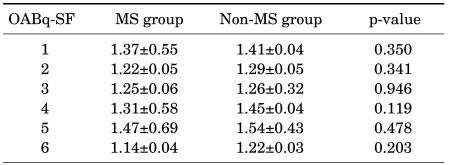
Male subjects were divided into two groups: a group without metabolic syndrome (428 subjects) and a group with metabolic syndrome (110 subjects). The differences in IPSS and OABq-SF depending on the presence or absence of metabolic syndrome were examined. There were no significances in either the IPSS (Table 2) or the OABq-SF (Table 3).
TABLE 2.
IPSS comparison according to the presence or absence of metabolic syndrome in males
IPSS: International Prostate Symptom Score, MS: metabolic syndrome
TABLE 3.
Comparison of OABq-SF depending on the presence or absence of metabolic syndrome in males
OABq-SF: Overactive Bladder Questionnaire Short Form, MS: metabolic syndrome
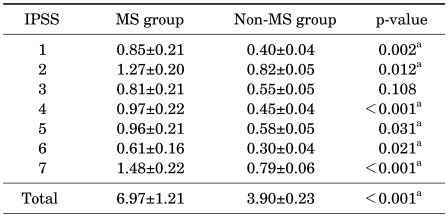
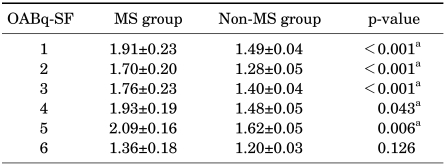
On the contrary, when the female patients were divided into a group without metabolic syndrome (351 subjects) and a group with metabolic syndrome (33 subjects), the differences in the IPSS and OABq-SF between these groups were found to be statistically significant. Of seven items and the total score for the IPSS, depending on the presence or absence of metabolic syndrome, significant differences were found in six items and the total score, which were as follows: incomplete emptying (p=0.002), frequency (p=0.012), urgency (p<0.001), weak stream (p=0.031), straining (p=0.021), nocturia (p<0.001), and total score (p<0.001) (Table 4). Also, of six items on the OABq-SF, depending on the presence or absence of metabolic syndrome, significant differences were found in five items, which were as follows: 1) uncomfortable urge to urinate (p<0.001), 2) sudden urge to urinate with little or no warning (p<0.001), 3) accidental loss of small amounts of urine (p<0.001), 4) nighttime urination (p=0.043), and 5) waking up at night because you had to urinate (p=0.006) (Table 5).
TABLE 4.
IPSS comparison according to the presence or absence of metabolic syndrome in females
IPSS: International Prostate Symptom Score, MS: metabolic syndrome, a: statistically significant
TABLE 5.
Comparison of OABq-SF depending on the presence or absence of metabolic syndrome in females
OABq-SF: Overactive Bladder Questionnaire Short Form, MS: metabolic syndrome, a: statistically significant
As such, there were no differences in LUTS with respect to the presence or absence of metabolic syndrome in male subjects, whereas significant differences were found in LUTS depending on the presence or absence of metabolic syndrome in females.
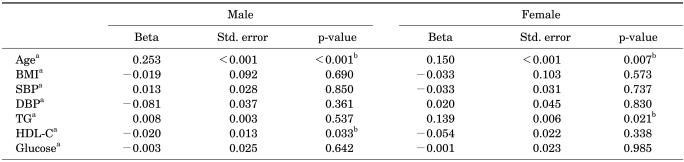
In order to find which component of metabolic syndrome affects voiding symptoms, we investigated correlations between 1) age and component elements of the diagnostic criteria of metabolic syndrome and 2) IPSS total score for men and women. Multiple regression analysis was performed for age and components of metabolic syndrome [systolic and diastolic blood pressure (BP), blood glucose, triglyceride, high-density lipoprotein (HDL) cholesterol, and body mass index (BMI)] and the total score on the IPSS in males and females. There were significant correlations between age and the IPSS total score in both males (p<0.001) and females (p=0.007). In addition, significant correlations between HDL cholesterol and the IPSS total score in males were found (p=0.033). In females, however, significant correlations were found between triglyceride and the IPSS total score (p=0.021) (Table 6).
TABLE 6.
Correlation between IPSS total scorea and age or components of metabolic syndrome in males and females
IPSS: International Prostate Symptom Score, Std. error: standard error, BMI: body mass index, SBP: systolic blood pressure, DBP: diastolic blood pressure, TG: triglyceride, HDL-C: High-density lipoprotein cholesterol, a: continuous variable, b: statistically significant
DISCUSSION
Metabolic syndrome is a cluster of disorders associated with the development and mortality of cardiovascular disease with a common feature of insulin resistance. Patients with metabolic syndrome are twice as likely to have cardiovascular disease and four times as likely to have type II diabetes mellitus as are patients without metabolic syndrome. The mortality rate due to cardiovascular or coronary arterial diseases is also increased 2.9 to 4.0 times in patients with metabolic syndrome [10-12].
The prevalence rate of metabolic syndrome in the United States has a diverse range, showing figures from 2.4% to 43.5%. In this country, a high morbidity rate of 21.5% was shown in a population of patients with an age of 20 years old or older [13]. This study also showed a similar result with a prevalence rate of 15.5%.
One study reported that Asian women have a higher prevalence of the metabolic syndrome than do Asian men [14]. However, this study showed a prevalence rate of 20.4% for males and of 8.6% for females, showing a higher prevalence for males.
Geer and Shen discussed the gender differences observed in body composition with regard to adiposity and insulin resistance [15]. In men, greater visceral fat and elevated fat content in the liver are most likely due to the absence of the protective effect of estrogen, which is found in premenopausal women. This absence leads to increased insulin resistance in individuals with increased visceral adiposity. Compared with men, premenopausal women are protected from cardiovascular disease. However, once postmenopausal, women rapidly catch up [15]. The high prevalence rate of males in this study was due to the relatively high proportion of premenopausal women.
The basic pathophysiology of metabolic syndrome is known to be hyperinsulinism developed secondarily from insulin resistance due to insulin-mediated glucose absorption disorder [16-18]. Insulin resistance or hyperinsulinism affects the ventromedial nucleus, which regulates the activation of the sympathetic nervous system of the hypothalamus. It increases blood and tissue catecholamines, stimulates the peripheral sympathetic nervous system, and increases the activity of the sympathetic nervous system. Accordingly, the prostate or bladder neck, which has a wide distribution of the sympathetic system, also becomes stimulated and LUTS may be manifested [19].
In this study, there was no difference in LUTS in males depending on the presence or absence of metabolic syndrome. However, there were statistically significant differences for most items in females.
Several studies have suggested a relationship between voiding symptoms and the presence of the metabolic syndrome [2,4]. Rohrmann et al, in a large population-based survey, found that the components of the metabolic syndrome were likely to be associated with LUTS in older men and, recently, in a population-based sample of African-American men aged 40-79 years [4].
However, Temml et al reported that the metabolic syndrome was not associated with IPSS, the IPSS obstructive or irritative subscore, or LUTS. The proportion of LUTS and the mean IPSS did not differ significantly regarding the presence or absence of the metabolic syndrome in either sex [20]. Park et al reported that no significant differences were found in the mean IPSS or quality-of-life score between men with or without the metabolic syndrome [21].
The pathogenesis of LUTS is currently considered to be a sex-independent, multifactorial process with the involvement of structural changes in the urinary bladder, infection, comorbidity, medication, neurologic factors, and hormones [20]. Kupelian et al reported statistically significant associations between urological symptoms and type 2 diabetes or increased blood sugar [22].
One study suggested that vascular risk factors play a role in the development of LUTS in both sexes [23]. In that study, the IPSS was identical in men with 0 (6.2±4.1) and 1 (6.2±4.4) vascular risk factor, yet increased to 7.7±5.5 in those with ≥2 risk factors (p=0.01). In women, the IPSS increased from 4.8±4.6 in those with no vascular risk factors to 5.7±5.3 (+18.7%) in those with 1 and to 7.0±5.7 (+45.8%) in those with ≥2 factors (p=0.05).
In our study, we found significant correlations between HDL cholesterol and the total score on the IPSS in males (p=0.033). In females, significant correlations were found between triglyceride and the IPSS total score (p=0.021) (Table 6). Park et al also reported that hypertriglyceridemia is associated with moderate to severe LUTS in men [21].
In 1997, Legato, a cardiologist at Columbia Medical School, asserted that the recognition of gender differences was very important in medical research and medical treatment and established a new medical concept called "genderspecific medicine" in which differences between males and females are researched [24]. Programs of gender difference and gender-specific education at medical centers and medical schools that apply the concept of gender difference in medical treatment are rapidly increasing.
Men and women differ in bone, cardiovascular system, brain, pain, immune system, lung, and especially the urogenital system. Gender-specific medicine still means women's health to all but the indoctrinated few, and many continue to view women's health as a feminist, commercial, or boutique issue more suited to marketing teams for hospital centers than to serious practitioners of medicine [24]. In the future, studies in gender-specific medicine may become vigorous in areas such as pharmacologic action of drugs; chromosomal body and genetic substance; hormones and the endocrine system, for which male and female differences are typically distinctive; adjustment of the biological function of the nervous system; various infections and degenerative diseases; metabolic diseases; tumors; psychiatric medicine; behavior; studies on emotion; environmental medicine; public health; and medical ethics.
The necessity of gender-specific medicine will be inevitable in the study of metabolic syndrome and in the diagnosis and treatment of LUTS, as dealt with in this study. Furthermore, metabolic syndrome has a different morbidity rate for males and females and its correlation with LUTS may also differ in males and females. Thus, gender differences must be considered in the prevention or treatment of LUTS in patients with metabolic syndrome. The importance of gender-specific medicine in such areas will be significant, and further study on this issue is needed.
Finally, there is a limitation to our study. Because this study consisted of a single institution in a metropolitan area, there may be a potential selection bias. Thus, our study represents a specific class of patients.
CONCLUSIONS
The proportion of metabolic syndrome was higher in males. In males, there were no significant differences in LUTS depending on the presence or absence of metabolic syndrome, whereas differences in LUTS secondary to the presence or absence of metabolic syndrome may be seen in females. However, a study with participants selected from a larger area may be necessary.
In view of gender-specific medicine, which has recently come into focus, the morbidity rate of metabolic syndrome and its effect on LUTS may differ depending on gender. Therefore, such aspects must be considered in the establishment of a treatment policy.
Footnotes
The authors have nothing to disclose.
References
- 1.Fitzgerald MP, Link CL, Litman HJ, Travison TG, McKinlay JB. Beyond the lower urinary tract: the association of urologic and sexual symptoms with common illnesses. Eur Urol. 2007;52:407–415. doi: 10.1016/j.eururo.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joseph MA, Harlow SD, Wei JT, Sarma AV, Dunn RL, Taylor JM, et al. Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol. 2003;157:906–914. doi: 10.1093/aje/kwg051. [DOI] [PubMed] [Google Scholar]
- 3.Michel MC, Mehlburger L, Schumacher H, Bressel HU, Goepel M. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol. 2000;163:1725–1729. [PubMed] [Google Scholar]
- 4.Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III) Int J Obes (Lond) 2005;29:310–316. doi: 10.1038/sj.ijo.0802881. [DOI] [PubMed] [Google Scholar]
- 5.Roe B, Doll H, Wilson K. Help seeking behaviour and health and social services utilisation by people suffering from urinary incontinence. Int J Nurs Stud. 1999;36:245–253. doi: 10.1016/s0020-7489(99)00020-6. [DOI] [PubMed] [Google Scholar]
- 6.Teunissen D, van Weel C, Lagro-Janssen T. Urinary incontinence in older people living in the community: examining help-seeking behaviour. Br J Gen Pract. 2005;55:776–782. [PMC free article] [PubMed] [Google Scholar]
- 7.Yu HJ, Chen FY, Huang PC, Chen TH, Chie WC, Liu CY. Impact of nocturia on symptom-specific quality of life among community-dwelling adults aged 40 years and older. Urology. 2006;67:713–718. doi: 10.1016/j.urology.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 8.Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 9.Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11:563–574. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 10.Hedblad B, Nilsson P, Engström G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med. 2002;19:470–475. doi: 10.1046/j.1464-5491.2002.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Meigs JB. Epidemiology of the metabolic syndrome, 2002. Am J Manag Care. 2002;8(11 Suppl):S283–S292. [PubMed] [Google Scholar]
- 12.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 13.Nugent AP. The metabolic syndrome. Nutr Bull. 2004;29:36–43. [Google Scholar]
- 14.DECODA Study Group. Prevalence of the metabolic syndrome in populations of Asian origin. Comparison of the IDF definition with the NCEP definition. Diabetes Res Clin Pract. 2007;76:57–67. doi: 10.1016/j.diabres.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(1 Suppl):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 17.Rett K, Wicklmayr M, Mehnert H. New aspects of insulin resistance in hypertension. Eur Heart J. 1994;15(Suppl C):78–81. doi: 10.1093/eurheartj/15.suppl_c.78. [DOI] [PubMed] [Google Scholar]
- 18.Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61:1081–1090. [PubMed] [Google Scholar]
- 19.Berne C, Pollare T, Fagius J. The sympathetic outflow in vasoconstrictor nerve fascicles to muscle is increased during euglycacemic hyperinsulinemia. Diabetologia. 1989;32(Suppl):465A. [Google Scholar]
- 20.Temml C, Obermayr R, Marszalek M, Rauchenwald M, Madersbacher S, Ponholzer A. Are lower urinary tract symptoms influenced by metabolic syndrome? Urology. 2009;73:544–548. doi: 10.1016/j.urology.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Park HK, Lee HW, Lee KS, Byun SS, Jeong SJ, Hong SK, et al. Relationship between lower urinary tract symptoms and metabolic syndrome in a community-based elderly population. Urology. 2008;72:556–560. doi: 10.1016/j.urology.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Kupelian V, McVary KT, Kaplan SA, Hall SA, Link CL, Aiyer LP, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area Community Health Survey. J Urol. 2009;182:616–624. doi: 10.1016/j.juro.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenfeld OZ, Meir KS, Yutkin V, Gofrit ON, Landau EH, Pode D. Do atherosclerosis and chronic bladder ischemia really play a role in detrusor dysfunction of old age? Urology. 2005;65:181–184. doi: 10.1016/j.urology.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 24.Legato MJ. Beyond women's health the new discipline of gender-specific medicine. Med Clin North Am. 2003;87:917–937. doi: 10.1016/s0025-7125(03)00063-4. [DOI] [PubMed] [Google Scholar]