Abstract
Purpose
To evaluate the clinical factors that impact ureteral stent-related lower urinary tract symptoms (LUTS) after ureteroscopic ureterolithotomy, including the stent position and medication.
Materials and Methods
Fifty-three patients who underwent ureteroscopic ureterolithotomy with indwelling a stent were distributed into three groups. On demand analgesics were given to the group 1 (n=18). Daily tamsulosin 0.2 mg was added for group 2 (n=15) and daily tamsulosin 0.2 mg and tolterodine 4 mg was added for group 3 (n=20). The patients were also subclassified into appropriate or inappropriate group according to stent position. All the patients completed a visual analogue scale (VAS) and International Prostate Symptom Score (IPSS) on the 1st and 7th postoperative days. The VAS and IPSS were analyzed according to the medication groups and the stent position.
Results
In the appropriate stent potion group, only the storage symptom scores of groups 2 and 3 on the 1st postoperative day were significantly lower than those of the group 1 (p=0.001). This medication effect on LUTS was not observed in the inappropriate stent position group. In this group, total IPSS (p=0.015) and storage symptom scores (p=0.002) were higher than in the appropriate stent position group on the 7th postoperative day.
Conclusions
Correct placement of the stent was more important than medication for lessening stent-related storage symptoms.
Keywords: Adrenergic alpha-antagonists, Cholinergic antagonists, Ureteroscopy, Urinary catheterization, Urological manifestations
INTRODUCTION
Placement of ureteral stents has been a common urological intervention since the first description of a cystoscopically placed endoluminal stent by Zimskind et al [1]. However, complications and morbidities associated with ureteral stents have been documented. In particular, general health and work performance are often affected by bothersome urinary symptoms (78%) and pain (80%) [2].
In an attempt to minimize the morbidity of stents, some authors postulated that the length and position have to be adequate [3,4]. Some authors suggested that the use of α1-blockers and anticholinergics would be effective. Tamsulosin acts as a selective inhibitor of α-1a and 1-d-mediated contraction of the distal ureter, bladder trigone, and proximal urethral smooth muscle [5]. It is thought that relaxing these smooth muscles decreases bladder outlet resistance and voiding pressure, thereby decreasing renal reflux and voiding symptoms. Tolterodine has been shown to have functional selectivity for muscarinic receptor subtypes for the bladder and is strongly indicated for treating patients with overactive bladder (OAB) [6,7].
The use of α1-blockers and anticholinergics to prevent stent-related symptoms is based on the similarity of these symptoms to benign prostatic hyperplasia-related lower urinary tract symptoms (LUTS), including urgency, frequency, and suprapubic pain, which are caused by involuntary contraction of the bladder mediated by muscarinic receptors. Tamsulosin and tolterodine are effective for the treatment of men with LUTS among patients with BPH and OAB [5]. Therefore, we evaluated the clinical factors that impact ureteral stent-related LUTS after ureteroscopic ureterolithotomy, including stent position and medication with α-blockers and/or anticholinergics.
MATERIALS AND METHODS
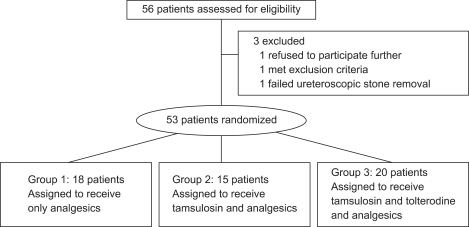
With the approval of our Institutional Review Board, all patients signed an informed consent form before participating. From June 2008 to July 2009, patients who underwent ureteroscopic ureterolithotomy for symptomatic ureteral calculi were enrolled prospectively. A total of 56 patients were enrolled and 3 patients were excluded. One patient refused to participate further, one met the exclusion criteria of urinary tract infection, and the ureteral stone failed to be removed in the last patients so he underwent open ureterolithotomy later. The 53 patients were randomly assigned into three groups. In group 1, 18 patients were prescribed analgesics only as a control. Fifteen patients in group 2 were given 0.2 mg of tamsulosin and analgesics daily, and 20 patients in group 3 took 0.2 mg of tamsulosin and 4 mg of tolterodine daily in addition to analgesics. All patients completed a visual analogue scale (VAS) for evaluation of pain and International Prostate Symptom Score (IPSS) for evaluation of LUTS. The patients were requested to complete the questionnaires on the 1st and 7th postoperative days to assess stent-related LUTS and pain.
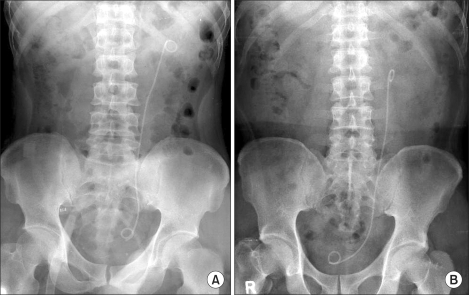
The patients with a history of previous pelvic surgery and prostate surgery, evidence of urinary tract infection, chronic medication with α-blockers or anticholinergics, and severe complications during the procedure were excluded. Ureteroscopic ureterolithotomy was performed with a Wolf 7.5/9 Fr dual operating channel semirigid ureteroscope, and intracorporeal lithotripsy was performed with a pneumatic lithotripter (50-60 Hz). A 6 Fr ureteral stent (Percuflex® material with Hydroplus™, Boston Scientific, MA, USA) was inserted in all patients after stone removal, and the length of the ureteral stent was adjusted according to the patient's height. The location of the distal curling of the ureteral stents was evaluated postoperatively by plain KUB (Fig. 1A). When the distal curling exceed the midline (symphysis pubis), the stent was considered to be in an inappropriate position (Fig. 1B). The stents were removed at the outpatient department by use of a rigid 17.5 Fr cystoscope 2 or 3 weeks after ureteroscopic surgery according to the difficulty of the surgery. On-demand use of ketorolac tromethamine 10 mg was prescribed for pain control when the patients were discharged. All consenting patients were fully informed regarding the potential side effects of the medication.
FIG. 1.
The position of the ureteral stent. (A) The distal curling of the ureteral stent is positioned in the ipsilateral side. (B) The distal curling of the ureteral stent crossed the midline of the bladder.
Statistical analysis was performed by using chi-square and ANOVA tests with commercially available software (Stata 11, StataCorp LP, TX, USA), and p<0.05 was considered statistically significant.
RESULTS
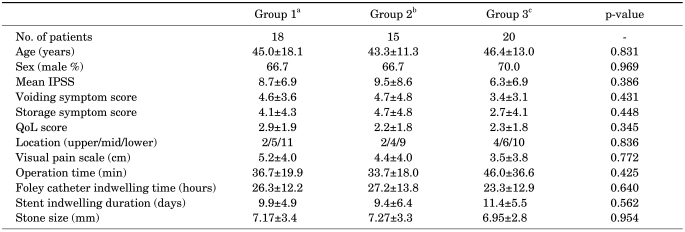
Fifty-three patients completed the study protocol (Fig. 2). No significant statistical differences were observed in the clinicopathological characteristics among the patients in the three groups (Table 1). The mean age of the patients was 45 years (range, 20-80 years), and 36 patients (68%) were male. Baseline LUTS did not differ significantly among the groups.
FIG. 2.
Study flow diagram.
TABLE 1.
Baseline patient characteristics
IPSS: International Prostate Symptom Score, QoL: quality of life, a: control group, patients were prescribed analgesics only, b: patients were given 0.2 mg of tamsulosin and analgesics daily, c: patients took 0.2 mg of tamsulosin and 4 mg of tolterodine daily in addition to analgesics
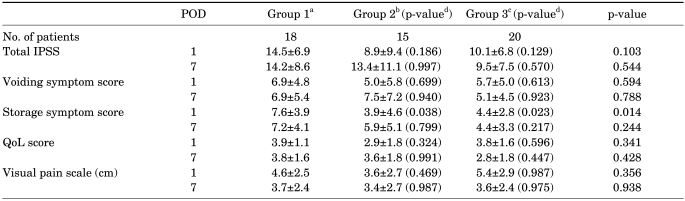
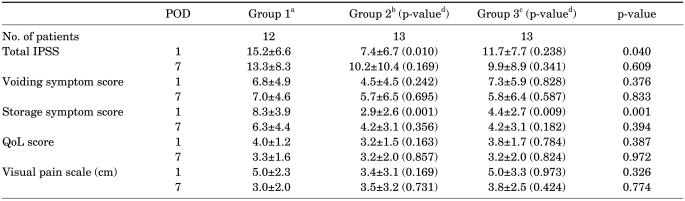
The mean total IPSS showed no significant statistical difference in the three groups on the 1st postoperative day (14.5, 8.9, and 10.1, respectively; p>0.05) or on the 7th postoperative day (14.2, 13.4, and 9.5, respectively; p> 0.05). Voiding symptom scores also demonstrated no difference in the three groups on the 1st postoperative day (6.9, 7.5, and 5.1, respectively; p>0.05) or on the 7th postoperative day (6.9, 5.0, and 5.7, respectively; p>0.05). However, storage symptom scores were significantly lower in groups 2 and 3 than in group 1 on the 1st postoperative day (7.6, 3.9, and 4.4, respectively; p=0.014). The mean quality of life (QoL) scores and the mean VAS of the three groups on the 1st postoperative day were 3.9, 2.9, and 3.8 (p>0.05) and 4.6, 3.6, and 5.4, respectively (p>0.05). On the 7th postoperative day, the mean QoL scores and the mean VAS were 3.8, 3.6, and 2.8 (p>0.05) and 3.7, 3.4, and 3.6, respectively (p>0.05) (Table 2). The use of analgesics did not differ significantly among the groups.
TABLE 2.
Symptom changes among the groups
POD: postoperative day, IPSS: International Prostate Symptom Score, QoL: quality of life, a: control group, patients were prescribed analgesics only, b: patients were given 0.2 mg of tamsulosin and analgesics daily, c: patients took 0.2 mg of tamsulosin and 4 mg of tolterodine daily in addition to analgesics, d: p-value compared with group 1 by Student's t-test
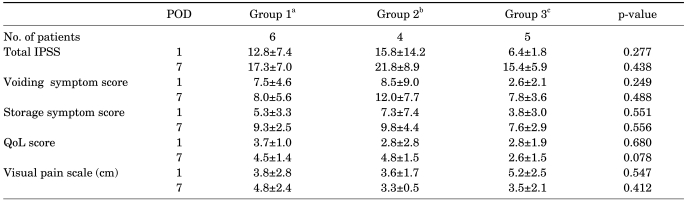
In the appropriate stent location group, the results were similar to the results of the total enrolled patients (Table 3). On postoperative day 1 only, the patients taking tamsulosin showed improved total IPSS (p=0.01) and storage symptoms (p=0.001) and the patients taking tamsulosin and tolterodine showed improved storage symptoms (p=0.007) compared with the control group. However, an effect of medication was not observed in the inappropriate stent location group (Table 4).
TABLE 3.
Symptom changes in the appropriate stent group
POD: postoperative day, IPSS: International Prostate Symptom Score, QoL: quality of life, a: control group, patients were prescribed analgesics only, b: patients were given 0.2 mg of tamsulosin and analgesics daily, c: patients took 0.2 mg of tamsulosin and 4 mg of tolterodine daily in addition to analgesics, d: p-value compared with group 1 by Student's t-test
TABLE 4.
Symptom changes in the inappropriate stent group
POD: postoperative day, IPSS: International Prostate Symptom Score, QoL: quality of life, a: control group, patients were prescribed analgesics only, b: patients were given 0.2 mg of tamsulosin and analgesics daily, c: patients took 0.2 mg of tamsulosin and 4 mg of tolterodine daily in addition to analgesics
None of the patient who took tamsulosin 0.2 mg experienced dizziness caused by orthostatic hypotension. Minor adverse effects including fatigue and dyspepsia occurred in two patients (13%), who were treated conservatively. In group 3, one patient experienced dry mouth and was also managed conservatively.
DISCUSSION
Ureteral stents have been widely used to manage various urinary tract diseases. Ureteral stents prevent urinary tract obstruction from ureteral swelling and divert urine. They also make the ureteral mucosa heal faster after iatrogenic injury, dilate the ureter, and assist in stone passage [8]. However, many patients experience significant stent-related morbidities, and additional procedures are needed to remove the stent.
Although the pathophysiology of stent-related symptoms has not been clearly proved, Thomas suggested that the high pressure transmitted to the renal pelvis during micturition and trigonal irritation caused by the intravesical portion of the stent may be contributing factors [9]. Deliveliotis et al reported that stent-related pain and urinary frequency may be related to lower ureteral spasm or local trigone sensitivity [10].
In the results of the current study, only storage symptom scores were significantly lower during the immediate postoperative period in the treatment groups (group 2 and group 3) than in the control group. Although it was not significant in the statistical analysis, a tendency for improved storage symptom scores in these groups was demonstrated on the 7th postoperative day. However, other parameters such as total IPSS, storage symptoms, voiding symptoms, QoL score, and VAS were not significantly different between the treatment groups and the control group irrespective of the duration after ureteroscopic ureterolithotomy. These findings are not consistent with those of recently reported prospective trials that showed the effect of α-blockers and anticholinergics in preventing ureteral stent-related urinary tract symptoms, pain, and QoL during variant periods from 3 days to 6 weeks [11-14]. It is hard to definitely conclude the efficacy of these agents on stent-related LUTS and pain. Therefore, future studies pooling these data will be needed to determine the overall treatment effect and the optimal management.
Park et al reported that alfuzosin and tolterodine ER improved stent-related urinary symptoms and body pain according to the validated Ureteral Stent Symptom Questionnaire (USSQ) [14]. There was no significant difference in urinary symptoms or pain between the alfuzosin and tolterodine ER groups. In our studies, there was no additional effect in the tamsulosin and tolterodine group compared with the tamsulosin only group. There are not many reports about the role of anticholinergics; accordingly, further study is needed to define the role of anticholinergics in stent-related morbidity.
In our studies, stent-related LUTS were associated more with the location of the stent than with medication use during the stent period. Therefore, the correct placement of a ureteral stent and verification of its location is important. Al-Kandari et al reported that flank pain was not affected by the length of the stent, whereas urgency, dysuria, and QoL were significantly affected [4].
The potential limitations of the current study are as follows. First, even though the IPSS is the most widely used questionnaire for the assessment of LUTS, it is not specific for stent-related symptoms. Joshi et al first developed and validated a questionnaire for the evaluation of stent-related symptoms, the USSQ, which has good evaluative and discriminate properties [15]. Second, the number of patients in each group was relatively small, so the power to detect an effect was limited.
CONCLUSIONS
Stent-related LUTS were affected more by the location of the ureteral stent than by medication. When the stent position was adequate, administration of tamsulosin or tolterodine combined with tamsulosin improved the storage symptoms only immediately after ureteroscopic ureterolithotomy.
Footnotes
The authors have nothing to disclose.
References
- 1.Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967;97:840–844. doi: 10.1016/S0022-5347(17)63130-6. [DOI] [PubMed] [Google Scholar]
- 2.Joshi HB, Stainthorpe A, MacDonagh RP, Keeley FX, Jr, Timoney AG, Barry MJ. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol. 2003;169:1065–1069. doi: 10.1097/01.ju.0000048980.33855.90. [DOI] [PubMed] [Google Scholar]
- 3.Pilcher JM, Patel U. Choosing the correct length of ureteric stent: a formula based on the patient's height compared with direct ureteric measurement. Clin Radiol. 2002;57:59–62. doi: 10.1053/crad.2001.0737. [DOI] [PubMed] [Google Scholar]
- 4.Al-Kandari AM, Al-Shaiji TF, Shaaban H, Ibrahim HM, Elshebiny YH, Shokeir AA. Effects of proximal and distal ends of double-J ureteral stent position on postprocedural symptoms and quality of life: a randomized clinical trial. J Endourol. 2007;21:698–702. doi: 10.1089/end.2007.9949. [DOI] [PubMed] [Google Scholar]
- 5.Shibasaki M, Sudoh K, Inagaki O, Uchida W, Honda K. Effect of the optical isomers of YM-12617 on increased intra-urethral pressure induced by phenylephrine in anaesthetized dogs. J Auton Pharmacol. 1992;12:263–268. doi: 10.1111/j.1474-8673.1992.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung DE, Te AE. Tolterodine extended-release for overactive bladder. Expert Opin Pharmacother. 2009;10:2181–2194. doi: 10.1517/14656560903167965. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319–2328. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 8.Chew BH, Knudsen BE, Denstedt JD. The use of stents in contemporary urology. Curr Opin Urol. 2004;14:111–115. doi: 10.1097/00042307-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Thomas R. Indwelling ureteral stents: impact of material and shape on patient comfort. J Endourol. 1993;7:137–140. doi: 10.1089/end.1993.7.137. [DOI] [PubMed] [Google Scholar]
- 10.Deliveliotis C, Chrisofos M, Gougousis E, Papatsoris A, Dellis A, Varkarakis IM. Is there a role for alpha1-blockers in treating double-J stent-related symptoms? Urology. 2006;67:35–39. doi: 10.1016/j.urology.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 11.Damiano R, Autorino R, De Sio M, Giacobbe A, Palumbo IM, D'Armiento M. Effect of tamsulosin in preventing ureteral stent-related morbidity: a prospective study. J Endourol. 2008;22:651–656. doi: 10.1089/end.2007.0257. [DOI] [PubMed] [Google Scholar]
- 12.Beddingfield R, Pedro RN, Hinck B, Kreidberg C, Feia K, Monga M. Alfuzosin to relieve ureteral stent discomfort: a prospective, randomized, placebo controlled study. J Urol. 2009;181:170–176. doi: 10.1016/j.juro.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Wang CJ, Huang SW, Chang CH. Effects of tamsulosin on lower urinary tract symptoms due to double-J stent: a prospective study. Urol Int. 2009;83:66–69. doi: 10.1159/000224871. [DOI] [PubMed] [Google Scholar]
- 14.Park SC, Jung SW, Lee JW, Rim JS. The effects of tolterodine extended release and alfuzosin for the treatment of double-J stent-r elated symptoms. J Endourol. 2009;23:1913–1917. doi: 10.1089/end.2009.0173. [DOI] [PubMed] [Google Scholar]
- 15.Joshi HB, Newns N, Stainthorpe A, MacDonagh RP, Keeley FX, Jr, Timoney AG. Ureteral stent symptom questionnaire: development and validation of multidimensional quality of life measure. J Urol. 2003;169:1060–1064. doi: 10.1097/01.ju.0000049198.53424.1d. [DOI] [PubMed] [Google Scholar]