Abstract
Purpose
The aim of this study was to evaluate the efficacy of a daily dose of tadalafil 5 mg as well as its safety for the cardiovascular system in men with erectile dysfunction.
Materials and Methods
This study included a total of 162 men who were administered a daily dose of tadalafil 5 mg between April and December of 2009. A total of 127 men completed the 8-week clinical trial. The International Index of Erectile Function (IIEF)-5, blood pressure, and heart rate were measured before treatment with tadalafil (V1) and 4 (V2) and 8 weeks (V3) after treatment with tadalafil. Adverse effects were assessed at V1, V2, and V3. In cases in which the International Prostate Symptom Score (IPSS) was ≥8 at V1, maximal flow rate (Qmax) and postvoid residual volume (PVR) were measured.
Results
The IIEF-5 values were 11.25±3.18, 14.56±3.79, and 16.91±3.56 at V1, V2, and V3, respectively, with significant improvement (V1 vs. V2, p<0.001; V1 vs. V3, p<0.001). The IPSS values were 10.59±5.56, 9.07±6.06, and 8.15±6.10 at V1, V2, and V3, respectively, and the differences were statistically significant (V1 vs. V2, p<0.001; V1 vs. V3, p<0.001). There were no significant differences in blood pressure or heart rate. Adverse effects were observed in 7 men (5.51%) at V2 and in 5 men (3.94%) at V3.
Conclusions
Tadalafil 5 mg administered once-a-day may be effective in improving erectile function. Adverse effects on the cardiovascular system may be minimal. In addition, it is believed that this may also be effective in improving voiding symptoms.
Keywords: Erectile dysfunction, Safety, Tadalafil, Treatment outcome
INTRODUCTION
Erectile dysfunction (ED) is defined as insufficient erection or inability to maintain erection for satisfactory sexual intercourse. ED is caused by a combination of factors such as physiologic, neurologic, hormonal, arterial, and cavernosal impairments [1]. ED affects about 150 million men worldwide, and the number of subjects who suffer from ED is expected to double by 2025 as the result of improved life expectancy and the age-related nature of ED [2]. The phosphodiesterase type-5 inhibitors (PDE5i) used worldwide are sildenafil, vardenafil HCl, and tadalafil, which have been used to treat about 40 million patients with ED [3].
Tadalafil is an oral selective PDE5 inhibitor that improves erectile function by provoking sexual stimulation through the nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) pathway [4]. It has a longer half-life than other drugs, and its duration of action is 36 hours. Many studies have demonstrated that once-a-day treatment with low-dose tadalafil is efficacious and safe. Daily treatment with low-dose PDE5i has clinical implications because it enables healthy men to have sexual intercourse at any time without taking the drug immediately before each intercourse attempt. After administration of tadalafil in a dose of 5 mg daily, tadalafil reaches a stable serum concentration within 5 days, and because this drug has the longest half-life among the PDE5i drugs, it is the most suitable for once-a-day treatment [5]. Recently, there was evidence that PDE5i improve lower urinary tract symptoms (LUTS). However, there have been few studies of once-a-day treatment with low-dose tadalafil in Korea. Therefore, this study was conducted to evaluate the efficacy, safety, and improvement in ED and LUTS after once-a-day treatment with tadalafil 5 mg.
MATERIALS AND METHODS
1. Subjects and study design
A total of 162 men with ED were enrolled between April and December of 2009. Of the 162 patients, 127 completed the 8-week clinical trial. This study was an open, prospective, noncomparative study conducted in three centers of urology in Korea and approved by the Institutional Review Board. All subjects were assessed by using the 5-item version of the International Index of Erectile Function (IIEF)-5, and those who had an IIEF-5 score of <18 were selected for the study. Subjects with ED were classified into 4 groups according to the cutoff value reported by Ahn et al [6]: those with no ED (IIEF-5 score≥18), mild ED (14≤IIEF-5 score<18), moderate ED (10≤IIEF-5 score<14), and severe ED (IIEF-5 score<10). The following subjects were included in the study: those who (1) were between 40 and 70 years old, (2) had an IIEF-5 score <18 on screening, and (3) had a willingness and the ability to participate in this clinical study. The following subjects were excluded from the study: those who (1) had shown hypersensitivity reactions to PDE5i, (2) were on medication with drugs affecting erectile function such as 5-alpha-reductase inhibitor within 1 month, (3) had been diagnosed with ED and had undergone surgery, and (4) were on medication with nitrate preparations and NO providers. Before the start of the study (V1), information on the duration of illness, smoking and drinking status, and past medical history was collected. In addition, a physical examination including the measurement of blood pressure (BP) and heart rate (HR), 12-lead electrocardiography, routine laboratory tests, and urinalysis was performed. All subjects were given tadalafil (Cialis®) orally in a dose of 5 mg once-a-day immediately after breakfast. They were asked to not take the drug more than once daily. Its efficacy and safety were assessed at 4 (V2) and 8 weeks (V3) after the initial administration of the drug. In 91 men (71.65%) with an International Prostate Symptom Score (IPSS) of ≥8 at V1, the IPSS, maximal flow rate (Qmax), and postvoid residual volume (PVR) as well as the IIEF-5 were measured at V1, V2, and V3.
2. Assessment of efficacy and safety
The efficacy of tadalafil 5 mg once-a-day was assessed by using the IIEF-5 and Global Assessment Question (GAQ). The IIEF-5 is a self-administered questionnaire that consists of 5 domains assessing erectile function and intercourse satisfaction. Higher scores in each domain reflect better sexual function. The assessment of the efficacy of tadalafil was supplemented by the dichotomous question (GAQ), "Has the treatment you have taken over the past 8 weeks improved your erections?" For evaluation of the effects of tadalafil for LUTS, we measured the IPSS in all subjects and LUTS were assessed only in subjects who had an IPSS of ≥8 (n=91). In these subjects, Qmax and PVR together with the IIEF-5 score were assessed at V1, V2, and V3. The subjects were further divided into 2 subgroups [those with Qmax≥12 ml/sec (n=61, 67.03%) and those with Qmax<12 ml/sec (n=30, 32.97%)], and the efficacy and safety of tadalafil were assessed for each. Safety evaluation included history taking, physical examination, and recording of adverse effects. The association of adverse effects with tadalafil was determined by the principal investigator.
3. Statistical analysis
The intercourse satisfaction of the subjects was divided into 4 categories: very satisfied, somewhat satisfied, somewhat dissatisfied, and very dissatisfied. "Being very satisfied" and "being somewhat satisfied" were regarded as intercourse satisfaction. Statistical comparisons of IPSS, Qmax, and PVR before and after the use of tadalafil were made with the paired t-test. Statistical analyses were performed by using Open Office.org Calc (Open Office.org® version 3.2.0, Oracle Corp, Redwood Shores, CA, USA) and MedCalc (MedCalc® version 11.2.1.0, MedCalc Software, Mariakerke, Belgium). A p-value of <0.05 was considered statistically significant.
RESULTS
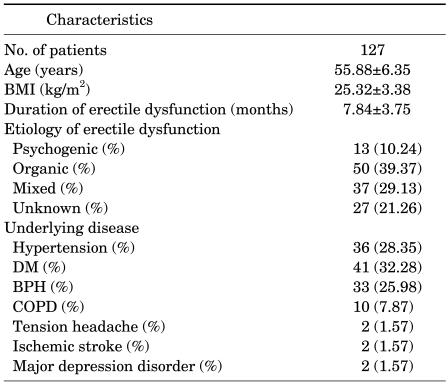
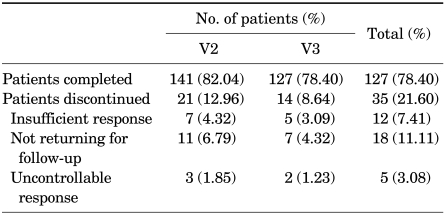
The mean age of the subjects was 55.88±6.35 years, and the duration of ED was 7.84±3.75 months. The subjects' mean body mass index (BMI) was 25.32±3.38 kg/m2, and their mean abdominal circumference was 87.50±5.31 cm. The causes of ED were as follows: psychogenic etiologies in 13 men (10.24%), organic etiologies in 50 (39.37%), mixed etiologies in 37 (29.13%), and unknown etiologies in 27 (21.26%). The subjects with organic etiologies were classified into small groups according to guidelines [7]. The organic etiologies were as follows: vascular ED in 22 (17.32%), neurogenic in 9 (7.09%), anatomical or structural in 5 (3.94%), hormonal in 4 (3.15%), and drug-induced in 10 (7.87%). The concurrent diseases were as follows: hypertension in 36 men (28.35%), diabetes mellitus (DM) in 41 (32.28%), benign prostatic hyperplasia (BPH) in 33 (25.98%), chronic obstructive pulmonary disease (COPD) in 10 (7.87%), tension headache in 2 (1.57%), ischemic stroke in 2 (1.57%), and major depression disorder in 2 (1.57%) (Table 1). In severity, 35 men (27.56%) had mild disease, 52 (40.94%) had moderate, and 40 (31.5%) had severe. At V2, 21 men (12.96%) and at V3, 14 men (9.93%) dropped out of the study. Table 2 shows the reasons for dropout.
TABLE 1.
Patient characteristics at baseline
BMI: body mass index, DM: diabetes mellitus, BPH: benign prostatic hyperplasia, COPD: chronic obstructive pulmonary disease
TABLE 2.
Reasons for drug discontinuation
V2: 4 weeks, V3: 8 weeks
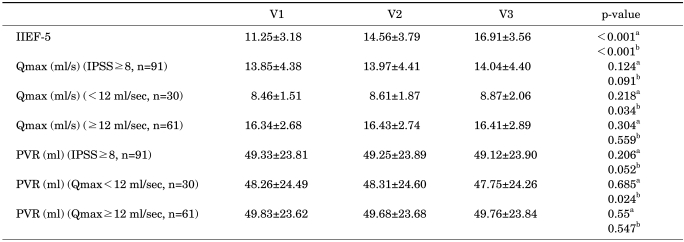
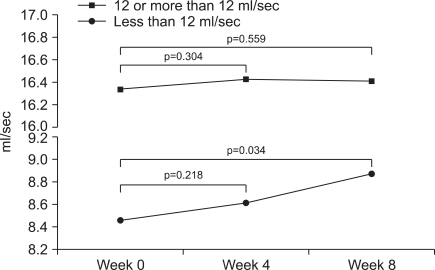
The mean IIEF-5 score showed significant improvements (Table 3). Regarding questions, the scores for all questions were significantly higher (Fig. 1). At V1 with IPSS≥8, Qmax and PVR were not significantly different. Only the IPSS showed a significant difference between the groups. But, in subjects with Qmax<12 ml/sec, Qmax was improved significantly at V3. PVR was also improved significantly at V3. Otherwise, in subjects with Qmax≥12 ml/sec, Qmax and PVR were not improved significantly (Table 3). There were significant differences in Qmax (Fig. 2) and PVR between the 2 subgroups.
TABLE 3.
Result of IIEF-5, IPSS, Qmax, and PVR
IIEF-5: International Index of Erectile Function-5, IPSS: International Prostate Symptom Score, Qmax: maximal flow rate, PVR: post-void residual volume, V2: 4 weeks, V3: 8 weeks, a: V1 vs. V2, b: V1 vs. V3
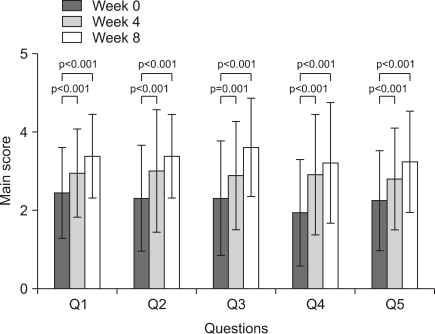
FIG. 1.
There was a significant domain score increase in all questions (Q1 to Q5) of the International Index of Erectile Function (IIEF)-5.
FIG. 2.
In the group with maximal flow rate (Qmax) less than 12 ml/sec (n=30, 32.97%), there was a significant increase in Qmax compared with that in the group with Qmax of 12 or more than 12 ml/sec (n=61, 67.03%).
On the GAQ, 84 men (66.14%) reported "yes" at V2, and 95 men (74.80%) reported "yes" at V3. In intercourse satisfaction after the endpoint of tadalafil administration, 59 men (46.46%) were "very satisfied," 33 (25.98%) were "somewhat satisfied," 26 (20.47%) were "somewhat dissatisfied," and 9 (7.09%) were "very dissatisfied." Intercourse satisfaction was observed in 92 men (72.44%), whereas 35 men expressed dissatisfaction by reason of ineffectiveness (n=24, 68.57%), financial problems (n=5, 14.29%), and over effect (n=6, 17.14%). A total of 74 men (58.27%) thought that they could maintain normal erection during sexual intercourse.
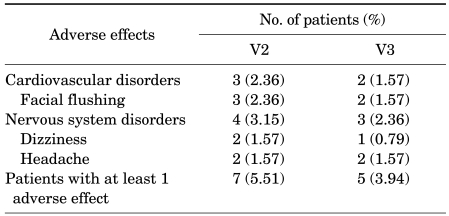
Adverse effects were observed in 7 men (5.51%) at V2 and in 5 men (93.94%) at V3. Facial flushing was the most common adverse effect (n=3, 2.36% at V2; n=2, 1.57% at V3), followed by headache (n=2, 1.57% each at V2 and V3), and dizziness (n=2, 1.57% at V2; n=1, 0.79% at V3) (Table 4). No subjects dropped out of the study as the result of adverse effects.
TABLE 4.
Adverse effects 4 and 8 weeks after tadalafil treatment
V2: 4 weeks, V3: 8 weeks
The mean standing systolic BP was 126.95±7.18 mmHg at V1, 127.55±7.87 mmHg at V2, and 127.28±7.83 mmHg at V3 (V1 vs. V2, p=0.118; V1 vs. V3, p=0.396). The mean standing diastolic BP was 82.12±4.00 mmHg at V1, 82.28±4.89 mmHg at V2, and 81.65±4.54 mmHg at V3 (V1 vs. V2, p=0.535; V1 vs. V3, p=0.087). The mean sitting systolic BP was 121.33±8.49 mmHg at V1, 121.82±7.76 mmHg at V2, and 120.17±6.38 mmHg at V3 (V1 vs. V2, p=0.111; V1 vs. V3, p=0.118). The mean sitting diastolic BP was 77.22±5.52 mmHg at V1, 77.28±4.86 mmHg at V2, and 77.13±5.19 mmHg at V3 (V1 vs. V2, p =0.890; V1 vs. V3, p=0.855). The mean standing HR was 76.28±5.38 at V1, 76.48±7.12 at V2, and 75.94±5.22 at V3 (V1 vs. V2, p=0.676; V1 vs. V3, p=0.073). The mean sitting HR was 76.25±5.51 at V1, 76.21±5.44 at V2, and 76.19±5.43 at V3 (V1 vs. V2, p=0.740; V1 vs. V3, p=0.697). None of the differences in cardiovascular system variables were statistically significant.
DISCUSSION
ED was reported to affect 18 million men in the United States and 189 million men globally in 2004, 193 million men in 2005, and 198 million men in 2006 [8,9]. ED is related to chronic conditions such as atherosclerosis, diabetes mellitus, and obesity that reduce arterial blood flow. Endothelial dysfunction caused by such conditions is considered to be an important factor for ED [10,11].
PDE5i suppress the degradation of cGMP in the smooth muscle cells of the corpus spongiosum, which maintains the relaxation of the smooth muscle cells induced by NO or nitrate [12]. After the introduction of sildenafil citrate (Viagra®, Pfizer Inc, New York, NY, USA) in 1998, more than 30 million men with ED have taken these drugs [13]. Thereafter, vardenafil HCI (Levitra®, Ganyer-GSK, Ratitan, NJ, USA), tadalafil (Cialis®, Lilly-ICOS, Indianapolis, IN, USA), udenafil (Zydena®, Dang-A Pharmaceutial Company, Seoul, Korea), and mirodenafil (Mvix®, SK Chemical, Seoul, Korea) have been developed, with a high efficacy, a low incidence of adverse effects, rapid onset of action, a longer duration of action, and a high specificity for PDE5 [14-17]. PDE5i are currently first-line drugs for ED because of their high efficacy, safety, and ease of use. Wespes et al have recommended that men with ED should select the most effective drug for themselves after the use of all available PDE5i [18].
Previous studies have demonstrated that tadalafil improves sexual functioning, intercourse satisfaction, and quality of life for men and their female partners [19-21]. Tadalafil administered once-a-day eliminates the conception that the use of this drug is limited to the sexual act itself and instead improves sexual quality of life, unlike on-demand products. Eardley et al reported that most men have sexual intercourse within 30 minutes of their attempt, regardless of the presence or absence of ED [22]. Fisher et al reported in a FEMALES study that 30% of men and 34% of women do not perform a sexual act at a fixed time [23]. These results suggest that sexual acts are not performed at an expected time or during a fixed time period and that there is a limitation in the use of PDE5i. The theoretical evidence for tadalafil administered once-a-day is that desire for sexual intercourse is unexpected and sexual intercourse is usually performed within a short period after the decision.
Althof et al indicated that once-a-day treatment with tadalafil 5 mg improves erection, vaginal penetration, and overall sexual satisfaction compared with that in a placebo group and that in both men with ED and their female partners, erection achievement (99.0% and 96.6%, respectively), vaginal penetration (98.6% and 97.4%, respectively), and overall intercourse satisfaction (84.3% and 82.8%, respectively) were excellent compared with the placebo group [24]. McVary et al stated that the IIEF-EF domain score and IPSS were significantly higher 6 and 12 weeks after once-a-day treatment with tadalafil 5 mg in the treatment group than in the placebo group [25]. In our study, IIEF-5 scores for all items were significantly increased after once-a-day treatment with tadalafil 5 mg. As for intercourse satisfaction, most men reported had intercourse satisfaction after the use of tadalafil. Roehrborn et al reported that LUTS secondary to BPH were improved 4, 8, and 12 weeks after the once-a-day administration of tadalafil 5 mg in the treatment group as compared with the placebo group and that there were no significant differences in Qmax between the treatment and placebo groups [26]. Similarly, the IPSS was significantly improved in this study, but Qmax and PVR were not. However, in men with Qmax<12 ml/sec, Qmax and PVR were improved. Kaplan and Hatzichristou said, "It is reasonable to assume that the reason why Qmax did not improve is because flow rate was normal at baseline [27]. There were no upper limits or exclusion criteria based on flow rate and consequently, how can one expect to improve a 'normal' flowrate?" So we think this was a very meaningful study because there are no reports based on low Qmax patients.
Because PDE5 exists in the vessels, bronchus, esophagus, anal sphincter, urethra, and prostate, tadalafil causes adverse effects in these organs. Seftel et al reported that the most common treatment-emergent adverse events were headache (15.7% vs. 6.3% with placebo), back pain (8.8% vs. 0%), and dyspepsia (7.5% vs. 0%) after on-demand 20 mg tadalafil administration during 12 weeks [28]. Roehrborn et al documented that most men with ED tolerate a wide range of doses, so they can take the drug continually, and that there were no significant differences in tadalafil-associated adverse effects between the treatment and placebo groups [26].
Our study is subject to some limitations. First, there were no placebo and control groups comparable to each other. Second, this study had a limitation stemming from a relatively short follow-up period (8 weeks). Third, we did not restrict the use of alpha-blocker medication; therefore, the results concerning LUTS cannot be regarded as a pure effect of tadalafil. However, to the best of our knowledge, this is the first such study in Korea. Further studies with a larger sample size and a longer follow-up period are needed to confirm our results.
CONCLUSIONS
There were a few mild adverse effects in men with ED taken tadalafil 5 mg once-a-day. This treatment improved sexual functioning, overall intercourse satisfaction, and LUTS. The results of this study suggest that tadalafil 5 mg administered once-a-day may be effective in the treatment of ED with acceptable tolerability.
Footnotes
The authors have nothing to disclose.
References
- 1.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 2.McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000;12(Suppl 4):S6–11. doi: 10.1038/sj.ijir.3900567. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Moon DG. Past, present and future of PDE5 inhibitor. Korean J Androl. 2008;26:49–60. [Google Scholar]
- 4.Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–1336. doi: 10.1016/S0022-5347(05)64442-4. [DOI] [PubMed] [Google Scholar]
- 5.Masson P, Lambert SM, Brown M, Shabsigh R. PDE-5 inhibitors: current status and future trends. Urol Clin North Am. 2005;32:511–525. doi: 10.1016/j.ucl.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Ahn TY, Lee DS, Kang W, Hong JH, Kim YS. Validation of an abridged Korean version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Korean J Urol. 2001;42:535–540. [Google Scholar]
- 7.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–814. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–157. doi: 10.1016/j.amjmed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Rubio-Aurioles E, Casabe A, Torres LO, Quinzanos L, Glina S, Filimon I, et al. Efficacy and safety of tadalafil in the treatment of Latin American men with erectile dysfunction: results of integrated analyses. J Sex Med. 2008;5:1965–1976. doi: 10.1111/j.1743-6109.2008.00860.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW, Park WJ, Choi YS, Cho SY. The correlation between erectile dysfunction and the severity of coronary artery involvement in patients with coronary artery disease. Korean J Urol. 2007;48:94–102. [Google Scholar]
- 11.Kam SC, Choi SM, Jeh SU, Lee SH, Hwa JS, Jung KH, et al. Efficacy and safety of a herbal formula that mainly consists of cornus officinalis for erectile dysfunction: a double-blind, placebo-controlled study. Korean J Urol. 2007;48:741–747. [Google Scholar]
- 12.Lee U, Lee M, Kim SY, Ji YH, Hong JH, Ahn TY. Clinical efficacy and safety of sildenafil in the men with erectile dysfunction in Korea. Korean J Urol. 2001;42:435–440. [Google Scholar]
- 13.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA Sildenafil Study Group. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 14.Yoo C, Park J, Kim W, Hong B, Hong J, Ahn TY. Comparison of the efficacy, safety and patient preference of the phosphodiesterase type 5 inhibitors for the patients with erectile dysfunction. Korean J Urol. 2007;48:219–225. [Google Scholar]
- 15.Ormrod D, Easthope SE, Figgitt DP. Vardenafil. Drugs Aging. 2002;19:217–227. doi: 10.2165/00002512-200219030-00005. [DOI] [PubMed] [Google Scholar]
- 16.Eardley I, Cartledge J. Tadalafil (Cialis) for men with erectile dysfunction. Int J Clin Pract. 2002;56:300–304. [PubMed] [Google Scholar]
- 17.Kuan J, Brock G. Selective phosphodiesterase type 5 inhibition using tadalafil for the treatment of erectile dysfunction. Expert Opin Investig Drugs. 2002;11:1605–1613. doi: 10.1517/13543784.11.11.1605. [DOI] [PubMed] [Google Scholar]
- 18.Wespes E, Amar E, Hatzichristou D, Hatzimouratidis K, Montorsi F, Pryor J, et al. EAU Guidelines on erectile dysfunction: an update. Eur Urol. 2006;49:806–815. doi: 10.1016/j.eururo.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Althof SE, Eid JF, Talley DR, Brock GB, Dunn ME, Tomlin ME, et al. Through the eyes of women: the partners' perspective on tadalafil. Urology. 2006;68:631–635. doi: 10.1016/j.urology.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 20.Rubio-Aurioles E, Kim ED, Rosen RC, Porst H, Burns P, Zeigler H, et al. Impact on erectile function and sexual quality of life of couples: a double-blind, randomized, placebo-controlled trial of tadalafil taken once daily. J Sex Med. 2009;6:1314–1323. doi: 10.1111/j.1743-6109.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- 21.Carson CC, Rajfer J, Eardley I, Carrier S, Denne JS, Walker DJ, et al. The efficacy and safety of tadalafil: an update. BJU Int. 2004;93:1276–1281. doi: 10.1111/j.1464-410X.2004.04819.x. [DOI] [PubMed] [Google Scholar]
- 22.Eardley I, Dean J, Barnes T, Kirby M, Glasser D, Solanki J. The sexual habits of British men and women over 40 years old. BJU Int. 2004;93:563–567. doi: 10.1111/j.1464-410x.2003.04684.x. [DOI] [PubMed] [Google Scholar]
- 23.Fisher WA, Rosen RC, Eardley I, Sand M, Goldstein I. Sexual experience of female partners of men with erectile dysfunction: the female experience of men's attitudes to life events and sexuality (FEMALES) study. J Sex Med. 2005;2:675–684. doi: 10.1111/j.1743-6109.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 24.Althof SE, Rubio-Aurioles E, Kingsberg S, Zeigler H, Wong DG, Burns P. Impact of tadalafil once daily in men with erectile dysfunction-including a report of the partners' evaluation. Urology. 2010;75:1358–1363. doi: 10.1016/j.urology.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 25.McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177:1401–1407. doi: 10.1016/j.juro.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008;180:1228–1234. doi: 10.1016/j.juro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan SA, Hatzichristou D. Open to debate. The motion: PDE5 inhibitors will have a significant role in the treatment of BPH. Eur Urol. 2007;52:1523–1527. doi: 10.1016/j.eururo.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Seftel AD, Wilson SK, Knapp PM, Shin J, Wang WC, Ahuja S. The efficacy and safety of tadalafil in United States and Puerto Rican men with erectile dysfunction. J Urol. 2004;172:652–657. doi: 10.1097/01.ju.0000132857.39680.ce. [DOI] [PubMed] [Google Scholar]