Abstract
Reactive oxygen species produced by activated neutrophils and monocytes are thought to be involved in mediating the loss of collagen and other matrix proteins at sites of inflammation. To evaluate their potential to oxidize the pyridinoline (Pyd) cross-links found in collagen types I and II, we reacted hydrogen peroxide (H2O2), hypochlorous acid/hypochlorite (HOCl/OCl−), and singlet oxygen (O2(1Δg)) with the Pyd substitutes, pyridoxamine dihydrochloride and vitamin B6, which share the same chemical structure and spectral properties of Pyd cross-links. Neither H2O2 (125–500 µm) nor O2(1Δg) (10–25 µm) significantly changed the spectral properties of pyridoxamine or vitamin B6. Reaction of HOCl/OCl− (12.5–50 µm) with pyridoxamine at pH 7.2 resulted in a concentration-dependent appearance of two new absorbance peaks and a decrease in fluorescence at 400 nm (excitation 325 nm). The new absorbance peaks correlated with the formation of an N-chloramine and the product of its subsequent reaction with pyridoxamine. In contrast, the extent to which HOCl reacted with vitamin B6, which lacks a primary amine group, was variable at this pH. At lysosomal pH 5.5, Cl2/HOCl/OCl− reacted with both pyridoxamine and vitamin B6. Four of the chlorinated products of this reaction were identified by gas chromatography-mass spectrometry and included 3-chloropyridinium, an aldehyde, and several chlorinated products with disrupted rings. To evaluate the effects of Cl2/HOCl/OCl− on Pyd cross-links in collagen, we exposed bone collagen type I and articular cartilage type II to HOCl. Treatment of either collagen type with HOCl at pH 5.0 or 7.2 resulted in the oxidation of amine groups and, for collagen type II, the specific decrease in Pyd cross-link fluorescence, suggesting that during inflammation both oxidations may be used by neutrophils and monocytes to promote the loss of matrix integrity.
Inflammation associated with articular cartilage, bone, and dentin surfaces is characterized by accumulation, adhesion, and activation of neutrophils and monocytes, which results in the destruction of cartilage and the loss of bone at these sites (1–4). These effects are thought to be mediated in part by the production of reactive oxygen species (ROS),1 a group of reactants that includes superoxide , hydrogen peroxide (H2O2), and the highly reactive species hypochlorous acid (HOCl) (5–8). ROS are produced by neutrophils and monocytes after they are recruited from the circulation to extravascular spaces. Once outside of the circulation, they adhere to extracellular matrix proteins (ECM) and undergo activation, which results in the production of ROS and release of proteolytic enzymes directly onto the matrix surface (4, 9–13). In inflammation associated with arthritic joints, the accumulation and activation of neutrophils and monocytes and increased synovial cell formation result in the loss of synovial membrane integrity and eventually to irreversible damage and destruction of articular cartilage in the afflicted joints (1, 4). Similarly, in periodontitis or inflammation associated with the tooth-supporting periodontal ligament, the recruitment of neutrophils and monocytes from the circulation and the subsequent activation and release of ROS and proteolytic enzymes can eventually result in a significant and irreversible loss of underlying bone matrix at these sites (2, 3). Despite the known participation of ROS in the inflammatory-mediated loss of underlying ECM, the mechanism(s) by which this occurs is not completely understood. This is particularly true for collagen, which is the major ECM protein in cartilage and bone.
Neutrophils and monocytes contain two enzymes that are responsible for producing ROS. The first is NADPH oxidase, which catalyzes the formation of by the transfer of electrons from NADPH to oxygen via cytochrome b558 (14–16). Superoxide is rapidly dismutated to H2O2 either spontaneously or via the enzyme superoxide dismutase (17). Neither nor H2O2 exhibit significant reactivity with biologic compounds (18). The second enzyme is myeloperoxidase (MPO), which catalyzes the formation of HOCl from H2O2 and Cl− (19). MPO has been localized to the primary granules of resting neutrophils and the extracellular space and phagolysosomes of phagocytically stimulated neutrophils (20, 21). MPO is also released from cytoplasmic granules of monocytes and some macrophages (22). The cationic nature of MPO allows it to adhere to cell and matrix surfaces and localize to sites of inflammation (23). It is at these sites that MPO produces HOCl, a highly reactive oxidant that readily reacts with primary amines to generate long lived N-chloramines (24–26). Although N-chloramines exhibit a lower oxidizing potential than HOCl, their much longer effective lifetime (~18 h) would enable them to cause damage at more distant sites than HOCl (26). Under acidic conditions, similar to the environment found in phagolysosomes, MPO generates Cl2 (27). MPO is also one of the pathways by which neutrophils generate O2(1Δg) (28).
Determining the ability of HOCl to contribute to the pathogenesis of inflammatory processes associated with rheumatoid arthritis and periodontitis is highly dependent on determining the relevant target(s) at these sites. The most likely protein target for neutrophil oxidants, including N-chloramines, is the ECM. ECM components found in or associated with articular cartilage or bone include hyaluronate, proteoglycans, fibronectin, several tissue-specific and nonspecific protein components, and collagen, the major component of these tissues. It has been reported that neutrophil-generated ROS mediate the degradation of hyaluronate (29), modify proteoglycan structure and/or synthesis, and alter the structure of fibronectin (29–31). Vissers and Winterbourn (32) reported an increase in proteolytic degradation of glomerular basement membrane collagen by elastase in response to myeloperoxidase/H2O2/Cl−. Davies et al. (33) reported that >1.0 mm HOCl was required to cause extensive fragmentation of collagen type II isolated from bovine articular cartilage or collagen type I isolated from bovine tendon. Davies et al. (33) also reported that N-chloramines did not cause direct fragmentation but greatly increased the degradation of collagen by collagenase and elastase. In general, there are very few studies available as to the susceptibility of collagen to oxidation by HOCl.
An important determinant for stability of the ECM is the degree of cross-linking. One important type of cross-link is pyridinoline (Pyd), which was first described by Fujimoto et al. (34) and later confirmed to be a non-reducible intermolecular cross-link of mature fibrillar collagen type I of bone (35). These cross-links were also found to be especially abundant in mature fibrillar collagen type II of articular cartilage (36), where they covalently link collagen type II to other type II helical regions, collagen type IX to the surface of type II, and bind collagen type IX to other molecules of collagen type IX. Their function is to stabilize the collagen fibrillar superstructure, or arrays, and make them more resistant to collagenolysis or proteolytic degradation (37).
The present study focuses on the susceptibility of Pyd crosslinks of collagen to reaction with HOCl. Pyd cross-links were chosen as potential oxidation targets because of their importance in maintaining the collagen superstructure and because their chemical structure suggested they would be targets of oxidative modification by OCl−/Cl2. Our findings indicate that these cross-links react with OCl−, Cl2, and N-chloramines and suggest that Pyd would be a site for ROS modification of collagen type I and II in bone and cartilage, respectively.
EXPERIMENTAL PROCEDURES
Pepsin-solubilized collagen types I and II isolated from human bone and articular cartilage, respectively, were kindly provided by Dr. David Eyre (University of Washington, Seattle). Pyridoxamine dihydrochloride, pyridoxine hydrochloride, 30% hydrogen peroxide, chloramine-T, 1,4-dimethylnaphthalene, methylene blue, and all other salts and buffer components were purchased from Sigma. Glacial acetic acid and dichloromethane were purchased from Fisher. Potassium iodide was purchased from Acros Organics (Fairlawn, NJ). Disodium anthracene-9,10-dipropionic acid was purchased from Molecular Probes (Eugene, OR). Sodium hypochlorite (NaOCl) (4–5% available chlorine) was purchased from Sigma, Acros Organics, Fisher, Alfa (Ward Hill, MA), and Cole-Palmer (Vernon Hills, IL); see comments about NaOCl below.
Preparation of Pyridoxamine and Vitamin B6
10 mm stock solutions of pyridoxamine dihydrochloride (pyridoxamine) and pyridoxine hydrochloride (vitamin B6) were made fresh weekly and stored at 4 °C with protection from light. Stock solutions were made in one of the following buffers: 0.5 m glacial acetic acid buffer, pH 3, or 0.5 m sodium phosphate buffer, pH 5.5 ± 0.2, pH 7.2 ± 0.2, or pH 8.0 ± 0.2. Where appropriate, 0.1 m NaCl was added to the buffers as a source of chloride ions.
Control and Reactive Oxygen Species Reaction with Pyridoxamine and Vitamin B6
On the day of use, stock solutions of pyridoxamine and vitamin B6 were diluted in appropriate buffer to 20 µm. 7.5 ml of the 20 µm solutions of pyridoxamine or vitamin B6 were then added to control and treated sample tubes so the final concentration of pyridoxamine or vitamin B6 in all samples was 15 µm in a total volume of 10 ml. Samples were brought to a total volume of 10 ml by the addition of 2.5 ml of appropriate buffer to control samples or 1.25 ml to hydrogen peroxide (H2O2) and sodium hypochlorite (NaOCl) samples. No buffer was added to samples treated with the combination of H2O2 and NaOCl (H2O2/NaOCl). H2O2 and NaOCl treatment solutions were prepared by diluting the stock solution of H2O2 to 1, 2, or 4 mm and diluting the stock solution of NaOCl to 0.1, 0.2, or 0.4 mm in appropriate buffer immediately before use. 1.25 ml of a diluted solution of H2O2 or NaOCl or 1.25 ml each of diluted solutions of H2O2 and NaOCl were added to appropriate tubes. The final concentrations of H2O2 were 125, 250, or 500 µm, and the final concentrations of NaOCl were 12.5, 25, or 50 µm. H2O2 was always added first to either the H2O2 or H2O2/NaOCl samples, and NaOCl was added immediately after, where appropriate. Samples were mixed after each addition. At pH 12, the NaOCl stock solution exists predominantly as the conjugate base, hypochlorite (OCl−).
H2O2 (30% or 12.92 m) and NaOCl (0.65 m) stock solutions as supplied by the manufacturer were stored tightly sealed at 4 °C with protection from light. Despite these precautions, both stock solutions decomposed over a period of 3–4 months after being opened. In general, decomposition of NaOCl could be identified by a yellowing of the solution. Yellowing was always accompanied by a distinctively greater reactivity of the NaOCl solutions, which we attributed to the breakdown of NaOCl to Cl− and eventually reactive chlorate (ClO3−) (38–40). Therefore, caution should be exercised when using NaOCl, since we found that even new solutions made by different companies showed signs of decomposition.
UV Absorbance and Fluorescence
After the addition of buffer, H2O2, NaOCl, or H2O2/NaOCl, control and treated samples were incubated for 15 min at RT (approximately 25 °C) in a Precision Scientific Low Temperature Incubator 815 (Chicago, IL). At the end of each incubation, the UV absorbance of a 50-µl aliquot of each sample was scanned from 400 to 200 nm in a Beckman DU-640 Spectrophotometer (Fullerton, CA), and the fluorescence intensity of a 3-ml aliquot of each sample was read in a PerkinElmer Life Sciences fluorescence spectrophotometer. Optimal fluorescence excitation and wavelengths were determined by referring to UV absorbance peaks and by prescanning samples for maximal excitation and peaks. The known excitation and peak wavelengths for both pyridoxamine and vitamin B6 are 324 nm excitation and 400 nm emission. A 200-µl aliquot of each control and treated sample was also stored at 4 °C after incubation for later N-chloramine analysis.
N-Chloramine Assay
The presence of N-chloramines was determined by the method of Witko et al. (41). This method is based on the colorimetric measurement of triiodide ions formed by the oxidation of potassium iodide (KI) in solution. Chloramine-T (N-chloro-p-toluene-sulfonamide sodium salt), a commercially available source of N-chloramine, was used to calibrate the assay. A 100 mm stock solution of chloramine-T was made fresh weekly in distilled H2O and stored at 4 °C with protection from light. The 100 mm chloramine-T solution was then diluted in appropriate buffer to final concentrations of 25, 50, 75, or 100 µm immediately before use. We extended the RT incubation from 2 (41) to 5 min and found no significant difference in results. The direct oxidation of KI by H2O2, NaOCl, or H2O2/NaOCl was also determined, and these values were subtracted as background from the correspondingly treated samples. The resulting difference represented the amount of N-chloramine present in each sample.
Chloramine-T Reactivity
On the day of use, 10 mm stock solutions of pyridoxamine or vitamin B6 were diluted to a concentration of 1 mm, and a 100 mm stock solution of chloramine-T was diluted to final concentrations of 25, 50, 75, or 100 µm in appropriate buffer. Control and chloramine-T-treated samples contained 15 µl of the diluted 1 mm pyridoxamine or vitamin B6 solution plus 1 ml of appropriate buffer (control) or 1 ml of 25, 50, 75, or 100 µm chloramine-T solution. The final concentration of pyridoxamine or vitamin B6 for all control and chloramine-T-treated samples was 15 µm. Following preparation, samples were incubated for 15 min at RT. At the end of each incubation, the UV absorbance of a each sample was scanned from 400 to 200 nm to look for any changes in the absorbance of pyridoxamine or vitamin B6 due to a reaction with chloramine-T and to identify the absorbance peaks for N-chloramines and pyridoxamine-chloramine or vitamin B6-chloramine reaction products. In addition, the N-chloramine assay of Witko et al. (41) was performed to determine if a reaction of chloramine-T with pyridoxamine or vitamin B6 had occurred as indicated by a decrease in the amount of chloramine-T available to oxidize KI.
1,4-Dimethyl-1,4-naphthalene Endoperoxide (DNE) Synthesis and Release of O2(1Δg)
DNE, a pure chemical source of O2(1Δg) that thermally releases O2(1Δg) at 37 °C, was synthesized by the method of Wasserman and Larsen (42, 43). A duplicate set of coverslips was coated with either dichloromethane or a solution of DNE in dichloromethane (3.6 mg/100 µl) by surface evaporation at 4 °C. 200 µl of either a 15 µm pyridoxamine or vitamin B6 solution in appropriate buffer was added to a duplicate set of dichloromethane- (control) and DNE-coated cover-slips. One set of control and DNE-coated coverslips was incubated overnight at 4 °C and another at 37 °C. The release of O2(1Δg) from DNE was confirmed after overnight incubation at 37 °C by following the decrease in absorbance at 400 nm of anthracene-9,10-diproprionic acid (AAP) (1 × 10−4 m) in 0.5 m sodium phosphate buffer, pH 7.2, according to the method of Deby-Dupont et al. (44).
Gas Chromatography-Mass Spectrometry
Gas chromatography-mass spectrometries (GC-MS) using electron ionization-mass spectrometry were performed by M-Scan, Inc., West Chester, PA. In brief, reacted samples containing vitamin B6 alone or vitamin B6 in a 1:1 ratio with NaOCl were lyophilized, dissolved in 40 µl in dimethyl formamide, and sialylated derivatives prepared by the addition of 100 µl of N, O-bis(trimethylsilyltrifluoro-acetamide with trimethylchlorosilane (Supelco, PA 16823) followed by heating to 35 °C for 5 min. Derivatized products were concentrated to ~50 µl under anhydrous N2 and analyzed on capillary column (PerkinElmer Life Sciences PE-5MS, 30 m × 0.25 mm × 25 µm) by GC-MS (PerkinElmer Life Sciences Auto System XL gas chromatograph with Turbomass Quadrupole mass spectrometer) in the positive electron ionization mode. Electron ionization-mass spectrometry was used to identify the structure of individual compounds in each GC peak. The source and interface temperatures were both 200 °C. The injector temperature was maintained at 280 °C, and the initial GC oven temperature was 70 °C for 2 min followed by an increase to 140 °C/min to 300 °C.
Preparation of Pepsin-solubilized Collagen
The lyophilized collagen samples were dissolved in 0.5 m acetic acid at a concentration of 1.2 mg/ml overnight at 4 °C with gentle stirring and protection from light. Samples were then dialyzed against 0.02 m dibasic sodium phosphate buffer, pH 9.0, for 48 h at 4 °C using a dialysis cassette made by Pierce Slide-A-lyzer (Pierce) according to manufacturer’s instructions. The samples were split into 2 equal volumes and dialyzed for an additional 48 h at 4 °C with protection from light against 0.5 m sodium phosphate buffer to bring the pH to approximately 5.0 ± 0.3 or 7.2 ± 0.3. After dialysis, samples were removed from cassettes and stored at 4 °C until use. The collagen suspensions were turbid and contained fibrils and/or a variety of polymorphic forms of collagen in equilibrium with monomers (45, 46). These preparations represent a mixture of fibrils, cross-linked trimers, dimers, and α-subunits of collagen (γ-, β-, and α- bands, respectively) as visualized by PAGE. At pH 7.2, collagen type II preparations also contained a small amount of aggregated/particulate material, also in equilibrium with monomers. The collagen preparations did not require sonication for suspension (47) and could be quantitatively and reproducibly loaded onto nitrocellulose or into wells for SDS-PAGE fractionation.
Reactive Oxygen Species Treatment of Collagen
On the day of use, 50 µg of collagen type I or II were added to 1.5-ml Eppendorf tubes. Control samples received appropriate buffer only, and ROS-treated samples received appropriate buffer containing H2O2, HOCl, or the combination of H2O2 and HOCl (H2O2/HOCl). Final concentrations of H2O2 were 125, 250, or 500 µm; final concentrations of HOCl were 12.5, 25, or 50 µm in a total volume of 200 µl. Samples were mixed after each addition and then incubated for 1 h at 37 °C.
Oxyblot for the Detection of Carbonyl (Aldehyde and Ketones) Formation
Carbonyl groups are formed as a consequence of protein oxidation and in the reaction of HOCl with pyridinium compounds. The 2,4-dinitrophenylhydrazine assay for carbonyls (48) was performed according to kit instructions in the Oxyblot-oxidized protein detection kit (Oncor, Gaithersburg, MD) without SDS-PAGE separation. In brief, aliquots of each collagen sample were reacted with 2,4-dinitrophenylhydrazine to derivatize carbonyl groups to the product 2,4-dinitrophenylhydrazone. After derivatization, aliquots of each collagen sample were diluted in SDS-PAGE sample buffer and spotted onto dry nitrocellulose, and the derivatized product was detected by chemiluminescence using a horseradish peroxidase-conjugated antibody that specifically recognized 2,4-dinitrophenylhydrazone. The spot intensities were quantified by scanning densitometry (Arcus II flatbed scanner) using NIH Image version 1.57 software (Wayne Rasband, National Institutes of Health, Bethesda).
Acetone Precipitation
Cold precipitation of collagen samples was performed according to the Pierce BCA Applications Note 13 (Pierce). 200 µl of −20 °C acetone were added to 50 µl of each sample, vortexed, and placed at −20 °C for 30 min. The samples were then centrifuged at 12,000 × g for 10 min in a microcentrifuge at 4 °C; supernatants were removed, and the remaining acetone was evaporated by leaving samples uncovered for 30 min at RT.
Fluorescamine and o-Phthalaldehyde Primary Amine and Imino Acid Measurements
Fluorescamine reacts directly with primary amines or imino acids to yield highly fluorescent derivatives that emit fluorescence at 475 nm when excited at 390 nm (49) and was used according to the method of Bohlen et al. (50). o-Phthalaldehyde also reacts with primary amines and imino acids and was used according to manufacturer’s instructions (Pierce). Both assays were performed on aliquots of each collagen sample, acetone precipitates of each sample, or sample supernatants of acetone precipitates after ROS and/or protease treatments. Triplicates of each sample (200 µl) were placed in a 96-well cytoplate (CFCPN9610, Millipore Corp.; Bedford, MA), and the fluorescence was read at an excitation wavelength of 340 ± 20 nm and an emission wavelength of 400 ± 20 nm (Cytofluor 2350, Perspective Biosystems, Inc.; Cambridge, MA).
SDS-PAGE
4-µg aliquots of acetone-precipitated collagen or non-precipitated collagen samples were resuspended in SDS-PAGE sample buffer and subjected to electrophoresis using a 5% stacking gel and a 10% separating gel prepared according to a modified Laemmli procedure previously described in detail (51) or using precast linear gradient gels (4–15% acrylamide) purchased from Bio-Rad. The gels were stained with silver using the Bio-Rad silver stain kit (Bio-Rad). The collagen gels were quantified by scanning densitometry (Arcus II flatbed scanner). Band intensities were analyzed using NIH Image version 1.57 software (Wayne Rasband, National Institutes of Health, Bethesda). To determine the reactivity of silver with protein after reaction with HOCl, [14C]bovine serum albumin was reacted with increasing concentrations of HOCl under the conditions described above for collagen. After reaction, the [14C]bovine serum albumin was subjected to gel electrophoresis, stained with silver, dried, and exposed to x-ray film. Silver staining intensity of each band was then compared with autoradiogram band intensity of the same gel.
Data Analysis
Data are expressed as the mean ± S.E. To evaluate the treatment effects, the data were grouped by experiment and time point for statistical analysis. Statistical significance of differences between the vehicle only (control) and treatment values for an individual experiment and time point was determined by a pairwise comparison of correlated groups using Student’s t test from the GB-STAT statistics software version 5.4.1.
RESULTS
UV Absorbance and Fluorescence Emission of Pyridoxamine and Vitamin B6
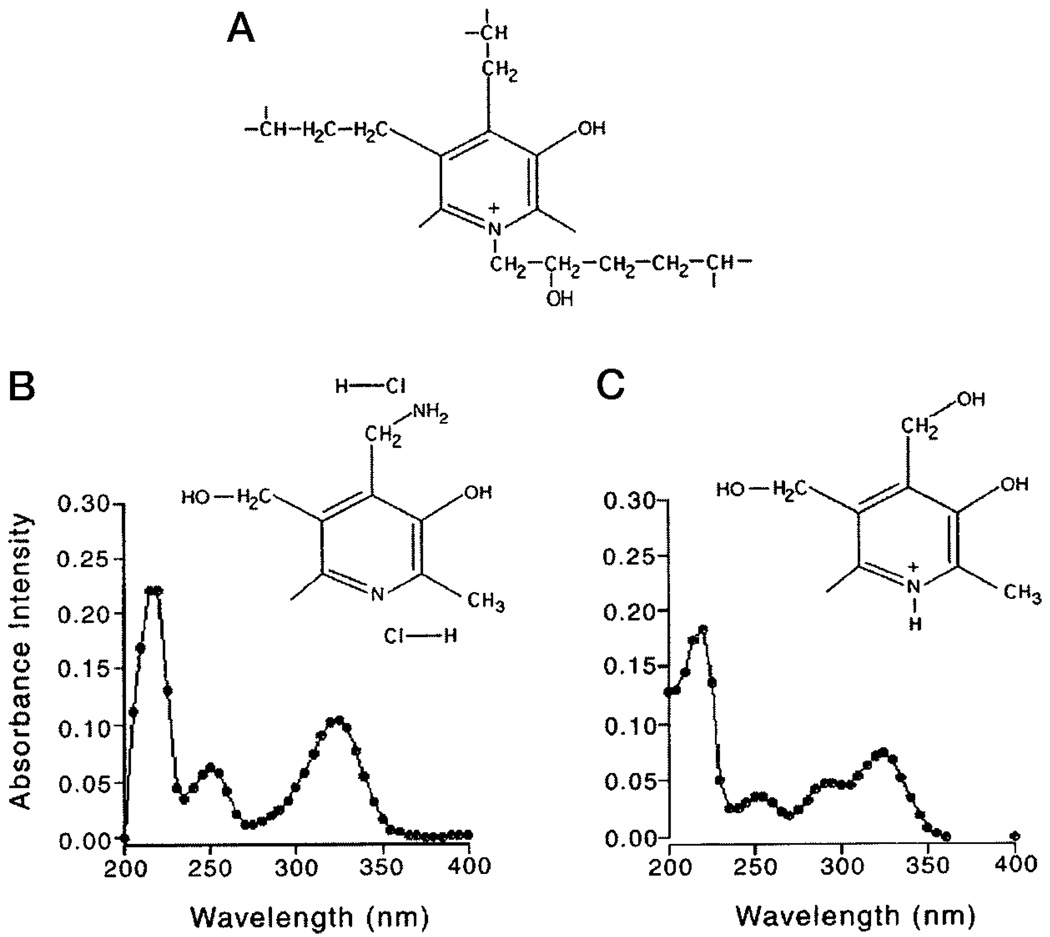
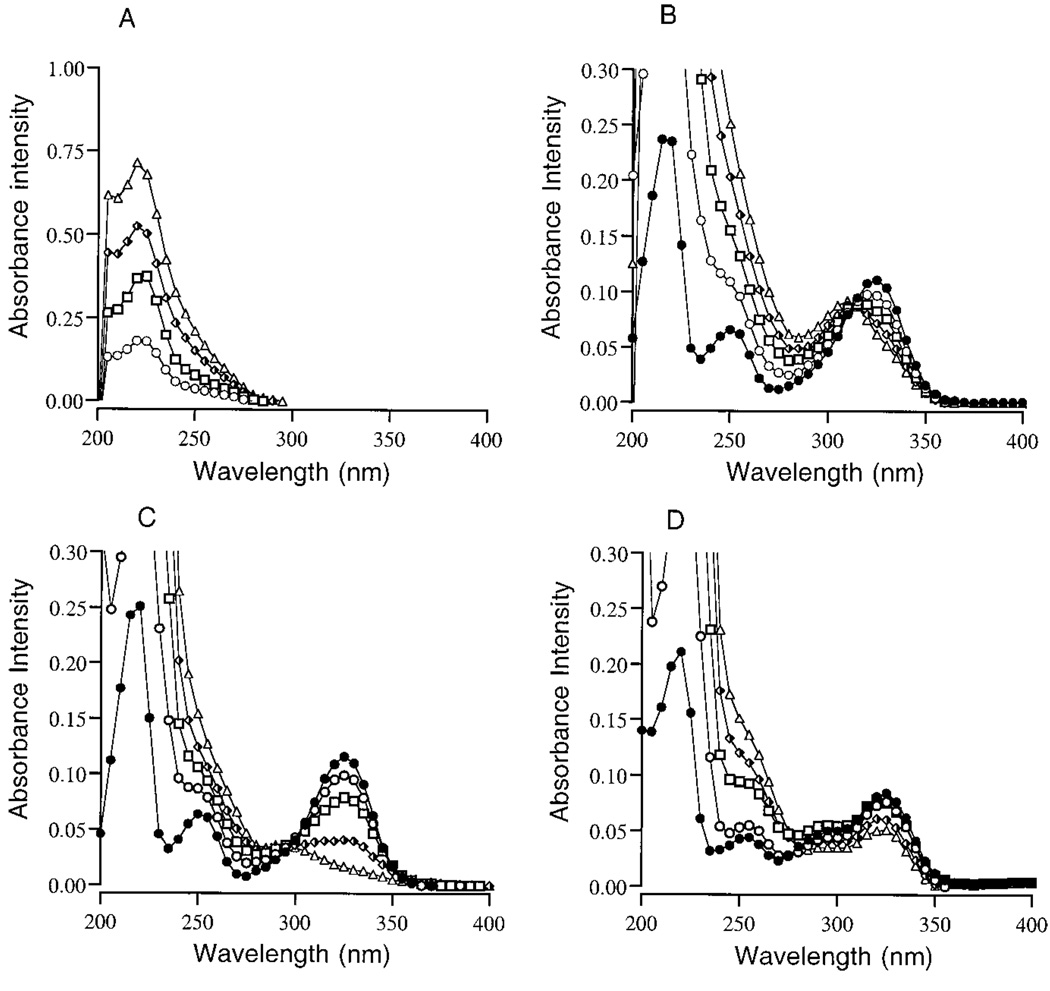
Pyridoxamine dihydrochloride (Fig. 1B) and vitamin B6 (Fig. 1C) share the pyridinium ring structure and spectral properties of the pyridinoline (Pyd) trifunctional crosslinks (Fig. 1A) of collagens, including collagen types I, II, III, IX, and XI. The spectral properties of pyridoxamine, vitamin B6, and Pyd include their characteristic UV absorbance and excitation maximum at 325 nm, pH 7.2 (Fig. 1B). The absorbance characteristics of pyridoxamine and vitamin B6 include three peaks at 217–219, 251–252, and 321–325 nm. At pH 5.5, a hydrogen ion binds to the nitrogen group (52) of the pyridinium ring of vitamin B6, resulting in an additional peak at 292 nm (Fig. 1C).
FIG. 1.
Chemical structures of pyridinoline (A), a cross-link involved in maintaining the interaction and structural integrity of collagen types I, II, III, IX, and XI, pyridoxamine dihydrochloride (B), and vitamin B6 (C), two chemical substitutes for pyridinoline. B, UV absorbance peaks of 15 µm pyridoxamine or vitamin B6 in 0.5 m sodium phosphate buffer, pH 7.2, are 217–219, 251–252, and 321–325 nm. C, at pH 5.5, vitamin B6 has an additional absorbance peak at 292 nm.
Oxidative Modification of Pyridoxamine and Vitamin B6 after Exposure to the ROS, H2O2, HOCl/OC−, or O2(1Δg)
To evaluate the oxidation of pyridoxamine or vitamin B6 by H2O2, HOCl/OCl− (which will be referred to as HOCl), or O2(1Δg), a 15 µm solution of pyridoxamine or vitamin B6 dissolved in 0.5 m sodium phosphate buffer, pH 7.2 ± 0.2, was exposed for 15 min at RT to 125, 250, or 500 µm of H2O2, 12.5, 25, or 50 µm of HOCl, or a combination of H2O2/HOCl in a ratio of 10:1. The above concentrations of HOCl and H2O2 are within the predicted range generated by activated neutrophils or monocytes at sites of inflammation (6). Concentrations of H2O2 and HOCl in this range are possible within specialized microenvironments, such as phagocytic vacuoles or the neutrophil or macrophage attachment sites, because the aqueous volumes in these microenvironments are thought to be nanoliters or less, resulting in µm to mm concentrations of ROS (53). A ratio of 10:1 was used in the present study because the amounts of HOCl generated by activated neutrophils are 5–20 times less than relative amounts of H2O2 generated by the same cells stimulated under the same conditions (6).
O2(1Δg) is generated when both HOCl and H2O2 are added together (Reaction 1) (54) or when HOCl is added in buffer containing Cl− pH 7.2 (Reaction 2) (55).
| Reaction 1 |
| Reaction 2 |
The amount of O2(1Δg) generated in the reaction of H2O2 with HOCl is pH-dependent (56), with the greatest amount of O2(1Δg) being produced at alkaline pH, intermediate amounts at neutral pH, and essentially non-measurable amounts at acidic pH due to assay limitations and interference by chlorine (Cl2). No O2(1Δg) is produced by HOCl in the absence of Cl− or at pH < 4.2 or = 8.0 (55).
At pH 7.2, HOCl would exist in almost equal concentrations with OCl− (pKa = 7.4) (56), and acidification of HOCl (pH below 6.0, peaking at pH 5.25) in the presence of Cl− results in the evolution of chlorine (Cl2), Reaction 3 (38, 54, 56).
| Reaction 3 |
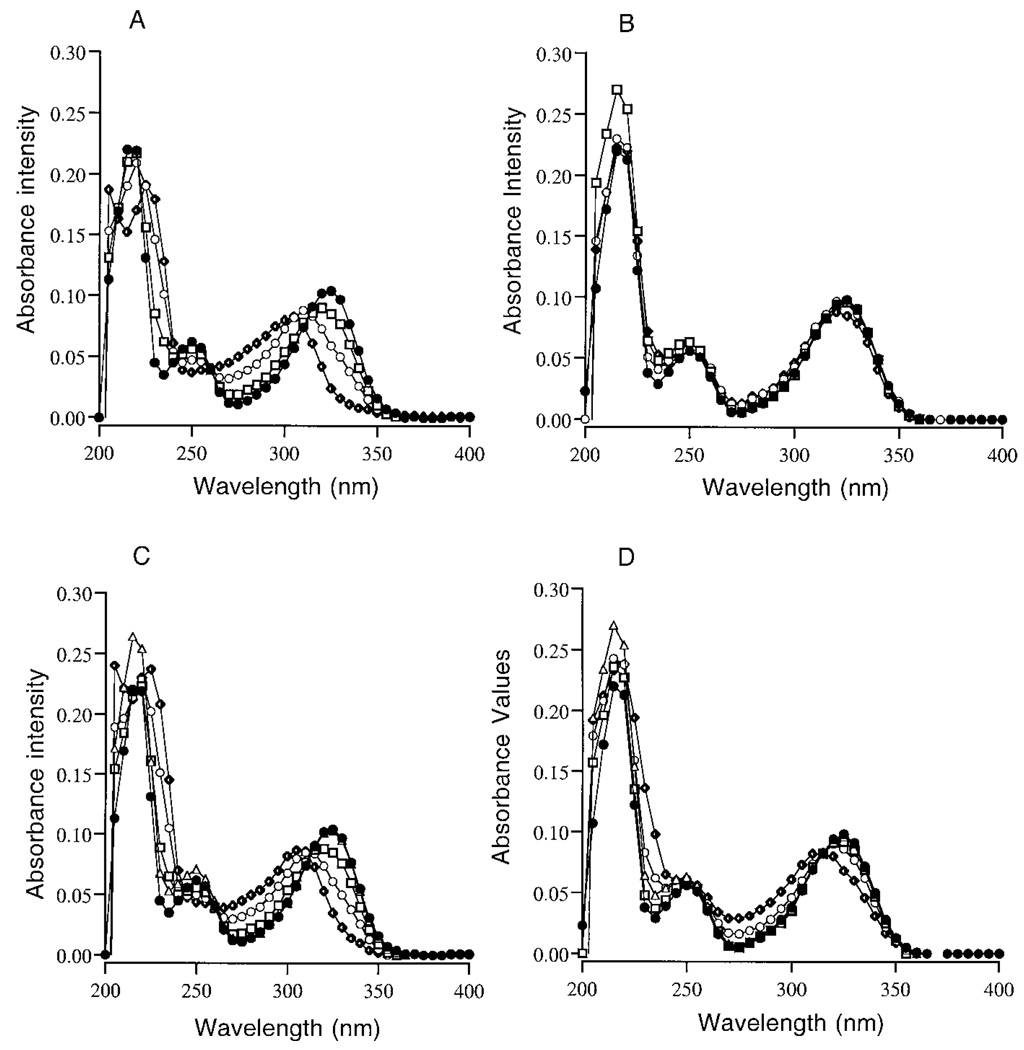
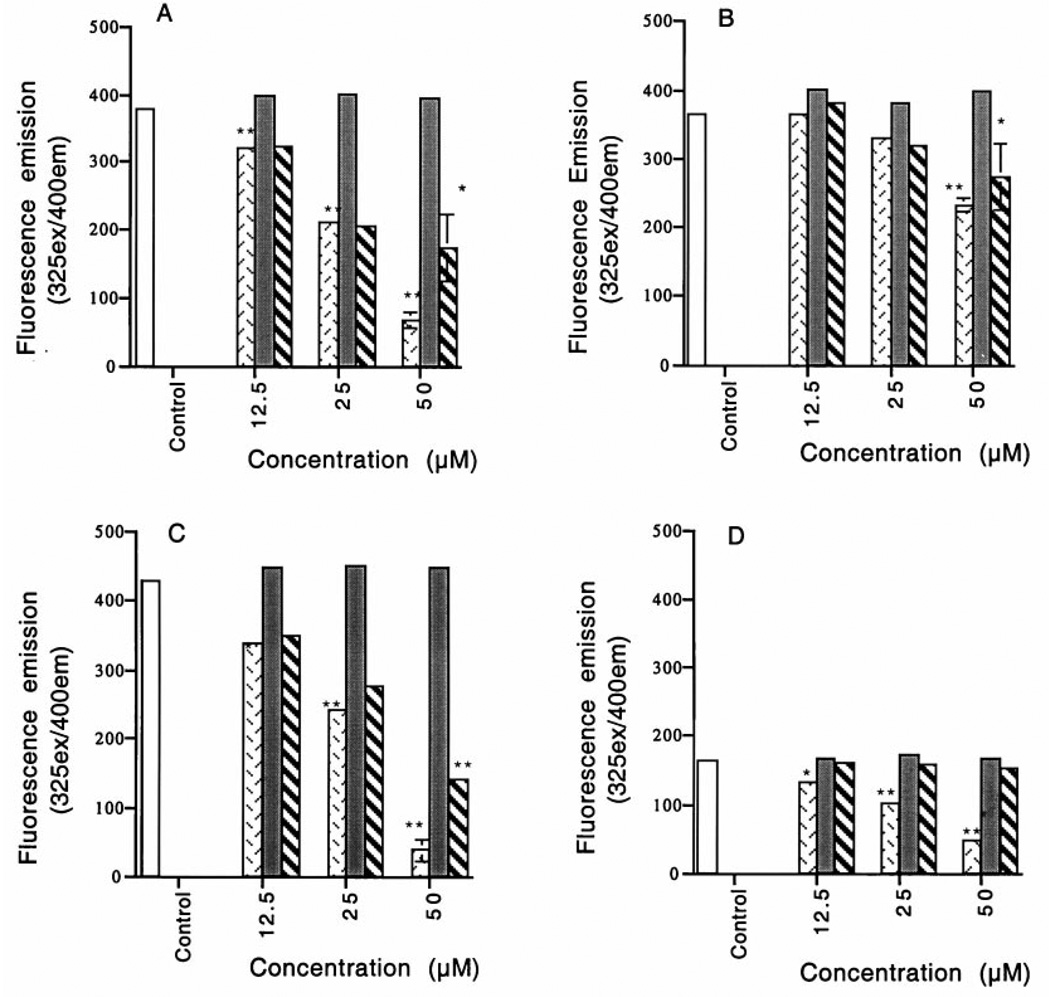
The UV absorbance data for a typical experiment using 0.5 m sodium phosphate buffer containing 0.1 m NaCl, pH 7.2, is presented in Fig. 2. The UV absorbance scans of pyridoxamine treated with HOCl showed an immediate concentration-dependent shift in maximum absorbance at 217 and 325 nm (Fig. 2A). Accompanying the shift in UV absorbance in response to increasing concentrations of HOCl was the appearance of two new absorbance peaks at 220–225 and 307–320 nm. In parallel, pyridoxamine excitation at 325 and fluorescence at 400 nm was dramatically decreased in response to increasing concentrations of HOCl. The fluorescence data for HOCl-treated samples are presented in Fig. 3A as the mean ± S.E. (n = 3, **p < 0.01 and *p < 0.05). No other changes in fluorescence excitation or emission were observed. These findings follow the predicted increase in reactivity rate of HOCl in the presence of Cl− (38–40, 57).
FIG. 2.
The UV absorbance spectra of 15 µm pyridoxamine in 0.5 m sodium phosphate buffer ± 0.1 m NaCl, pH 7.2, reacted for 15 min at RT with 0 µm (●), 12.5 µm (□), 25 µm (●), or 50 µm HOCl (♦) in the presence of 0.1 m NaCl (A) and without NaCl present (B). The UV absorbance of pyridoxamine reacted for 15 min at RT with H2O2 or a 10:1 mix of H2O2 and HOCl; Δ, 500 µm H2O2; ●, 0 µm H2O2/HOCl; □, 125 and 12.5 µm H2O2/HOCl; ○, 250 and 25 µm H2O2/HOCl, or ♦, 500 and 50 µm H2O2/ HOCl in the presence of 0.1 m NaCl (C) or without NaCl present (D). Graphs are a representative set of data from experiments that were repeated three times.
FIG. 3.
Fluorescence of 15 µm pyridoxamine in 0.5 m sodium phosphate buffer ± 0.1 m NaCl, reacted for 15 min at RT with (□) CT, ( ) HOCl, (■) H2O2 (1 × 101) or (
) HOCl, (■) H2O2 (1 × 101) or ( ) H2O2 (1 × 101)/HOCl, at pH 7.2 in the presence of 0.1 m NaCl (A) or without NaCl present (B). Pyridoxamine reacted at pH 5.5 in the presence of 0.1 m NaCl (C) or vitamin B6 in the presence of 0.1 m NaCl at pH 5.5 (D). After reaction, 3 ml of each sample were excited at 325 nm, and the emission intensity at 400 nm was determined. The fluorescence data are presented as the mean ± S.E. of a set of experiments repeated three times. Statistical significance of differences between control and treatment values was determined by a pairwise comparison of correlated groups using Student’s t test, and statistical significance is defined as **p < 0.01 or *p < 0.05.
) H2O2 (1 × 101)/HOCl, at pH 7.2 in the presence of 0.1 m NaCl (A) or without NaCl present (B). Pyridoxamine reacted at pH 5.5 in the presence of 0.1 m NaCl (C) or vitamin B6 in the presence of 0.1 m NaCl at pH 5.5 (D). After reaction, 3 ml of each sample were excited at 325 nm, and the emission intensity at 400 nm was determined. The fluorescence data are presented as the mean ± S.E. of a set of experiments repeated three times. Statistical significance of differences between control and treatment values was determined by a pairwise comparison of correlated groups using Student’s t test, and statistical significance is defined as **p < 0.01 or *p < 0.05.
The combination treatment of H2O2/HOCl also shifted the absorbance (Fig. 2C) and decreased the fluorescence (Fig. 3A) of pyridoxamine. The absorbance and fluorescence changes in response to H2O2/HOCl were similar to the changes observed when pyridoxamine was treated with the corresponding concentrations of HOCl alone. When 0.1 m NaCl was omitted from the buffer system, only minor effects on the absorbance and fluorescence were observed in response to either HOCl (Fig. 2B and Fig. 3B) or H2O2/HOCl (Fig. 2D and Fig. 3B). H2O2 alone had no effect on the UV absorbance or fluorescence of pyridoxamine (Fig. 2C and Fig. 3A) in either buffer system, although the absorbance of H2O2 around 219 nm contributed to the slight increase in absorbance at this wavelength in samples treated with this ROS.
As shown in Fig. 1, vitamin B6 has essentially the same chemical structure as pyridoxamine, except for a −CH2OH group in the para position of the pyridinium ring instead of the −CH2NH2 group. This difference is important because a reaction of HOCl with the primary amine, −CH2NH2, of pyridoxamine is favored at pH 7.2 (58) over a reaction with the nitrogen of the pyridinium ring. The reaction of HOCl with the primary amine of pyridoxamine would produce an N-chloramine/–CH2NHCl group/N-chloropyridoxamine. In contrast to HOCl, the reactivity of O2(1Δg) with the nitrogen of the pyridinium ring has been reported (59). Despite the potential of O2(1Δg), and possibly HOCl, to react with the ring nitrogen, there was essentially no reaction of H2O2 or H2O2/HOCl and little reaction of HOCl with vitamin B6 in the presence or absence of NaCl, although the degree to which HOCl reacted with vitamin B6 varied (data not shown).
Oxidation of Pyridoxamine and Vitamin B6 at pH 5.5 ± 0.2
The pH of the phagolysosomes of neutrophils during the first 15 min following the ingestion of opsonized particles is 7.4–7.8 (60, 61), but after 15 min, the pH within the phagolysosomes, and presumably the underlying extracellular attachment site, decreases to pH 5.5–6.0. pH 5.5 is also the optimal pH for the generation of Cl2 and O2(1Δg) by HOCl in solutions containing 0.1 m NaCl (53–54). To evaluate the reactivity of HOCl, H2O2, or H2O2/HOCl with pyridoxamine and vitamin B6 at pH 5.5 ± 0.2, ROS were added to 15 µm pyridoxamine in 0.5 m sodium phosphate buffer ± 0.1 m NaCl. Pyridoxamine or vitamin B6 control and treated samples were then incubated at RT for 15 min. The UV absorbance data for a typical pyridoxamine experiment is presented in Fig. 4. At pH 5.5 the absorbance of pyridoxamine at 217 and 325 nm, and to a lesser extent at 252 nm, decreased after treatment with HOCl (Fig. 4, A and B), and a new absorbance peak at 228–229 nm, which is within the range of known N-chloramine absorbance peaks, was formed (27).
FIG. 4.
The UV absorbance spectra of 15 µm pyridoxamine in 0.5m sodium phosphate buffer ± 0.1 m NaCl, pH 5.5, reacted for 15 min at RT with 0 µm (●), 12.5 µm (□), 25 µm (○), or 50 µm (♦) HOCl in the presence or absence of 0.1 m NaCl (A) or 15 mm pyridoxamine reacted with 500 µm H2O2 (Δ), 0 µm H2O2/HOCl (●), 125 µm, 12.5 µm H2O2/HOCl (□), 250 µm, 25 µm H2O2/ HOCl (○), or 500 µm, 50 µm H2O2/HOCl (♦) in the presence or absence of 0.1 m NaCl (B). The UV absorbance spectra of 15 µm vitamin B6 in 0.5 m sodium phosphate buffer ± 0.1 m NaCl, pH 5.5, reacted for 15 min at RT with 0 µm (●), 12.5 µm (□), 25 µm (○), or 50 µm HOCl (♦) in the presence of 0.1 m NaCl (C) or without NaCl present (D). Graphs present a representative set of data from experiments that were repeated three times.
At pH 5.5, HOCl also reacted with vitamin B6, which resulted in a decrease in the absorbance at 292 and 325 nm (Fig. 4C). In the absence of NaCl, vitamin B6 samples treated with HOCl showed only a slight decrease in absorbance in response to 50 µm HOCl (Fig. 4D), suggesting that either Cl2 or O2(1Δg) are involved in this reaction.
The fluorescence data for all samples are presented in Fig. 3, C and D, as the mean ± S.E. (n = 3, **p < 0.01, *p < 0.05). The fluorescence of pyridoxamine (Fig. 3C) and vitamin B6 (Fig. 3D) at 400 nm was decreased in response to either HOCl or H2O2/HOCl treatments. The decrease in absorbance (Fig. 4) and fluorescence (Fig. 3) of both pyridoxamine and vitamin B6 suggests that the ring structure is disrupted by this reaction.
N-Chloramine Formation in HOCl-treated Samples
To determine whether the reaction of HOCl with pyridoxamine or vitamin B6 leads to the formation of N-chloramines, 15 µm pyridoxamine or vitamin B6 in 0.5 m sodium phosphate ± 0.1 m NaCl, pH 7.2, was reacted with increasing concentrations of HOCl. The oxidation of potassium iodide (KI) to triiodide by HOCl or by N-chloramines formed in the reaction of HOCl with pyridoxamine was then determined after incubation for 15 min at RT.
In the absence of NaCl, HOCl in solution at pH 7.2 was slow to decompose, and the direct oxidation of KI by HOCl remained high even after 24 h incubation at 37 °C (Table I). Due to the high background, the formation of N-chloramines by HOCl in the absence of NaCl could not be determined, although an immediate reaction of HOCl with pyridoxamine could be observed as a decrease in KI oxidation in these samples (data not shown).
TABLE I. N-Chloramine formation determined by the oxidation of potassium iodide after the reaction of pyridoxamine with HOCl (0.5 m sodium phosphate, pH 7.2).
The data are presented as the mean ± S.E. of a set of experiments repeated three times. Statistical significance of differences between control and treatment values was determined by a pairwise comparison of correlated groups using Student’s t test.
| Additions | HOCl | |||
|---|---|---|---|---|
| 0 µm | 12.5 µm | 25 µm | 50 µm | |
| No NaCl | 0 | 13.4 ± 1.2 | 24.3 ± 1.0 | 48.0 ± 3.2 |
| 0.1 m NaCl | 0 | 9.4 ± 0.5 | 18.2 ± 2.0 | 3.1 ± 0.4a |
| 15 µm pyridoxamine + 0.1 m NaCl | 0.6 ± 1.1 | 0.5 ± 0.5 | 0.4 ± 1.1 | 15.1 ± 2.5b |
Statistical significance is defined as p < 0.01.
Statistical significance is defined as p < 0.05.
In the presence of NaCl, there was an immediate decrease in background oxidation of KI by unreacted HOCl and the production of N-chloramines could be detected, after 15 min, in pyridoxamine samples reacted with 50 µm HOCl (Table I). As would be expected from the limited effect of HOCl on the absorbance and fluorescence of vitamin B6, there was no formation of N-chloramines in the reaction of HOCl with vitamin B6 at pH 7.2.
Despite the more rapid decomposition of HOCl or the rapid reaction of HOCl with NaCl at pH 5.5, the formation of N-chloramines could not be determined at this pH. The increased oxidation of KI under these conditions may be due to the production of O2(1Δg).
Reaction of Chloramine-T with Pyridoxamine and Vitamin B6
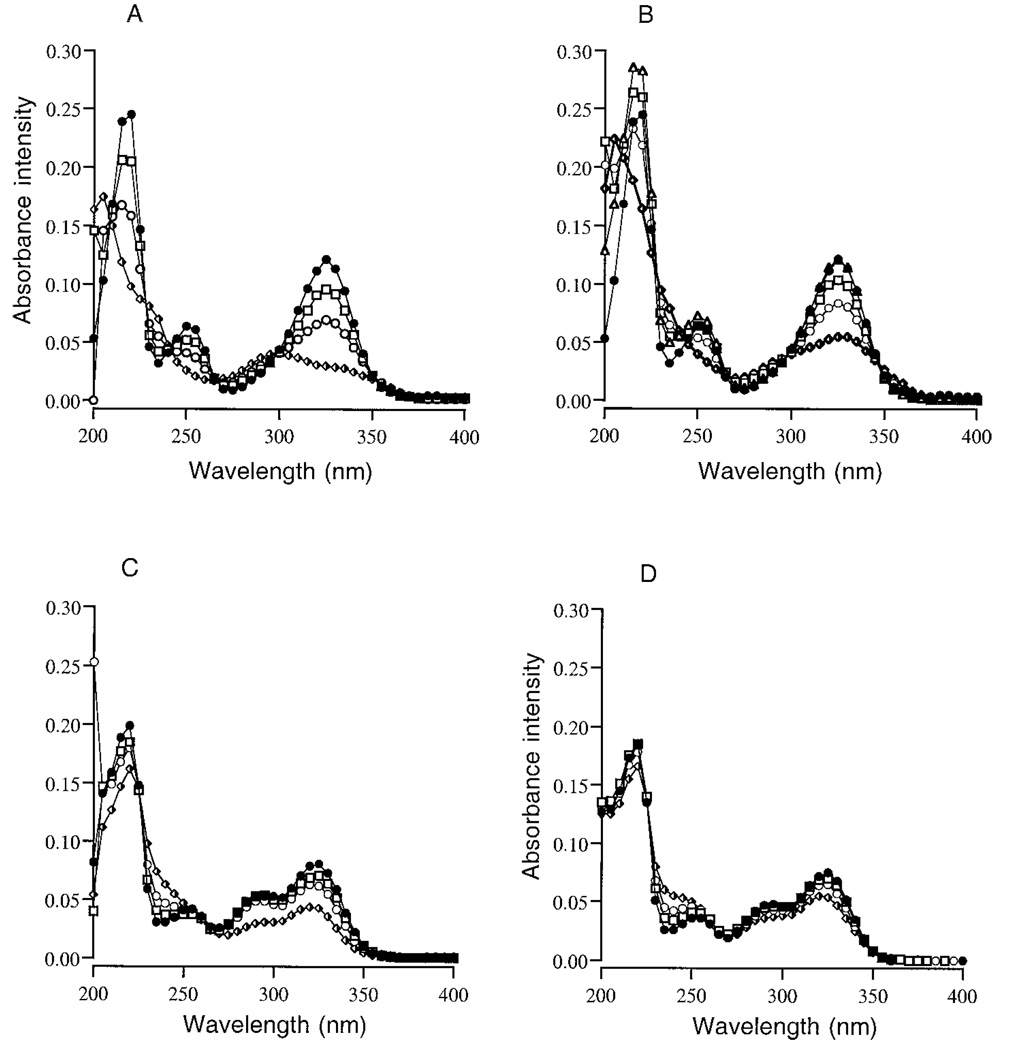
To evaluate further the formation of N-chloropyridoxamine in the reaction of HOCl with pyridoxamine, the absorbance of chloramine-T in 0.5 m sodium phosphate buffer, pH 7.2 and 5.5, was determined (Fig. 5A). The absorbance peaks for chloramine-T were 219–224 nm and corresponded with the initial absorbance peaks of HOCl-treated pyridoxamine samples (Fig. 2), indicating the reaction of HOCl with pyridoxamine generates N-chloropyridoxamine. The absorbance of unreacted chloramine-T remained constant at both pH values. To evaluate the reaction of N-chloramines with pyridoxamine or vitamin B6, increasing concentrations of chloramine-T were added to 15 µm pyridoxamine or vitamin B6 in 0.5 m sodium phosphate buffer, pH 7.2 or 5.5, and the samples were incubated for 15 min RT, 2 h at 37 °C, or overnight at 37 °C. Chloramine-T reacted with pyridoxamine at pH 7.2 as indicated by the concentration-dependent shifts in the major absorbance peak of pyridoxamine from 325 to 312–318 nm (Fig. 5, B and C). There was essentially no reaction of chloramine-T with vitamin B6.
FIG. 5.
The UV absorbance peaks for 0 µm (●), 25 µm (○), 50 µm (□), 75 µm (♦), and 100 µm (Δ) chloramine-T in 0.5 m sodium phosphate buffer ± 0.1 m NaCl at pH 7.2 (A) or pH 5.5 (C). One major peak at 219–224 nm characterized the absorbance for chloramine-T. The UV absorbance for 15 µm pyridoxamine reacted with increasing concentrations of chloramine-T ± 0.1 m NaCl for 15 min RT at pH 7.2 (B) or pH 5.5 (C). The UV absorbance scans for 15 µm vitamin B6 reacted with increasing concentrations of chloramine-T in 0.5 m sodium phosphate buffer pH 5.5 ± 0.1 m NaCl for 15 min at RT (D), and then incubated overnight (16–18 h) at 37 °C.
At pH 7.2, the reaction of chloramine-T with pyridoxamine was verified using the N-chloramine assay as described above. There was a decrease over time in the amount of chloramine-T available to oxidize KI after reaction with pyridoxamine, but not vitamin B6 at pH 7.2 (data not shown). The reaction of chloramine-T with pyridoxamine was immediate and continued to increase over time.
At pH 5.5, chloramine-T reacted with both pyridoxamine (Fig. 5C) and vitamin B6 (Fig. 5D), although the vitamin B6 reaction was not immediate. The reactivity of chloramine-T with vitamin B6 suggests that the nitrogen in the pyridinium ring is reactive at this pH, although to a lesser degree compared with the primary amine group of pyridoxamine.
Thermal Release of O2(1Δg) from DNE
DNE, a pure chemical source of O2(1Δg), can provide a reaction system that does not rely on the presence of other ROS that may interfere or react with the target of interest to evaluate more definitively a reaction of O2(1Δg) with pyridoxamine or vitamin B6. We incubated 200 µl of 15 µm pyridoxamine or vitamin B6 in 0.5 m sodium phosphate containing 0.1 m NaCl, pH 7.2 or 5.5, on a DNE-coated coverslip. Duplicate samples were incubated for 24 h at 4 or 37 °C, and the absorbance readings were compared. Control coverslips were coated with the dichloromethane solvent. There were no changes in the absorbance of either pyridoxamine or vitamin B6 in response to O2(1Δg) (data not shown). The release of O2(1Δg) was verified using AAP (44). After 24 h at 4 °C, the absorbance of AAP at 400 nm was 0.715 ± 0.003, and AAP incubated on a DNE-coated coverslip had an absorbance of 0.702 ± 0.005. For duplicate samples incubated at 37 °C, the absorbance of AAP after 24 h on control coverslips was 0.718 ± 0.003, and the absorbance of AAP incubated on DNE-coated coverslips was 0.588 ± 0.008. The decrease in absorbance of AAP on DNE-coated coverslips after incubation at 37 °C confirmed that O2(1Δg) was released and reacted with AAP in the aqueous buffer systems used in our study. Taken together these findings eliminate O2(1Δg) in the reaction of HOCl with either pyridoxamine or vitamin B6 at pH 5.5, leaving Cl2 and HOCl as the remaining reactants.
Gas Chromatography and Mass Spectrometry
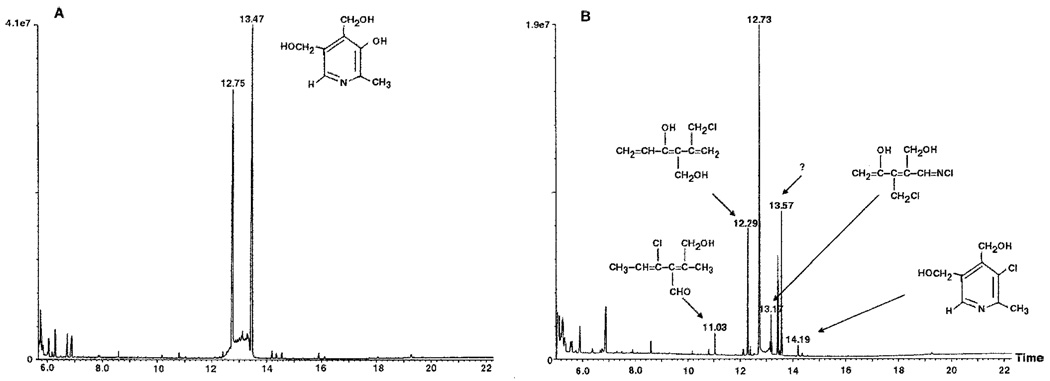
Samples of vitamin B6 and vitamin B6 reacted with 50 µm HOCl for 15 min were analyzed by GC-MS as described under “Experimental Procedures.” Fig. 6 presents the results of these analyses. Electron ionization-mass spectrometry of the vitamin B6-GC peak showed two peaks at 12.73 and 13.42 (Fig. 6A). After reaction with 50 µm HOCl at pH 5.5 (Fig. 6B), four chlorinated products were identified at 11.03, 12.29, 13.17, and 14.19 min as 4-chloro-2-hydroxymethyl-2,4-hexadiene-3-carboxaldehyde, 5-chloromethyl-3-hydroxy-4-hydroxymethyl-1,3,5-hexatriene, N-chloro-3-chloromethyl-4-hydroxy-2-hydroxymethyl-1-imino-2,4-pentadiene, and 3-chloro-4,5-dihydroxymethyl-2-methylpyridine (3-chloropyridinium), respectively. The product at 13.57 min was not chlorinated, and no further characterization was carried out.
FIG. 6.
Gas chromatography and electron ionization-mass spectrometry (GC-MS) of vitamin B6 (A) and vitamin B6 reacted with 50 µm HOCl for 15 min at pH 5.5 (B). Two peaks at 12.73 and 13.42 min characterized the GC-MS for vitamin B6 (A), and four chlorinated products were identified at 11.03, 12.29, 13.17, and 14.19 min for vitamin B6 (B) after reaction with HOCl and were identified as 4-chloro-2-hydroxymethyl-2,4-hexadiene-3-carboxaldehyde, 5-chloromethyl-3-hydroxy-4-hydroxymethyl-1,3,5-hexatriene, N-chloro-3-chloromethyl-4-hydroxy-2-hydroxy-methyl-1-imino-2,4-pentadiene, and 3-chloro-4,5-dihydroxymethyl-2-methylpyridine (3-chloropyridinium), respectively. The product at 13.57 min was not chlorinated, and no further characterization was carried out.
Carbonyl (Aldehyde and Ketone) Formation after Exposure of Collagen to H2O2, HOCl, or H2O2/HOCl
To evaluate the oxidation of human collagen types I (bone) and II (articular cartilage) by H2O2, HOCl, or H2O2/HOCl, 50 µg of collagen in 0.5 m sodium phosphate buffer containing 0.1 m NaCl, pH 7.2 or pH 5.0, were exposed for 1 h at 37 °C to 125, 250, or 500 µm H2O2, 12.5, 25, or 50 µM HOCl, or a combination of H2O2/HOCl in a ratio of 10:1. The above concentrations of HOCl and H2O2 are within the predicted range generated by activated neutrophils or monocytes at sites of inflammation (6). A ratio of 10:1 was used in the present study because the amounts of HOCl generated by activated neutrophils is 5–20 times less than relative amounts of H2O2 generated by the same cells stimulated under the same conditions (6). The pH values were chosen based on the neutral pH of the neutrophil phagolysosomes during the first 15 min following the ingestion of opsonized particles (60, 61) and the acidic pH after 15 min (62).
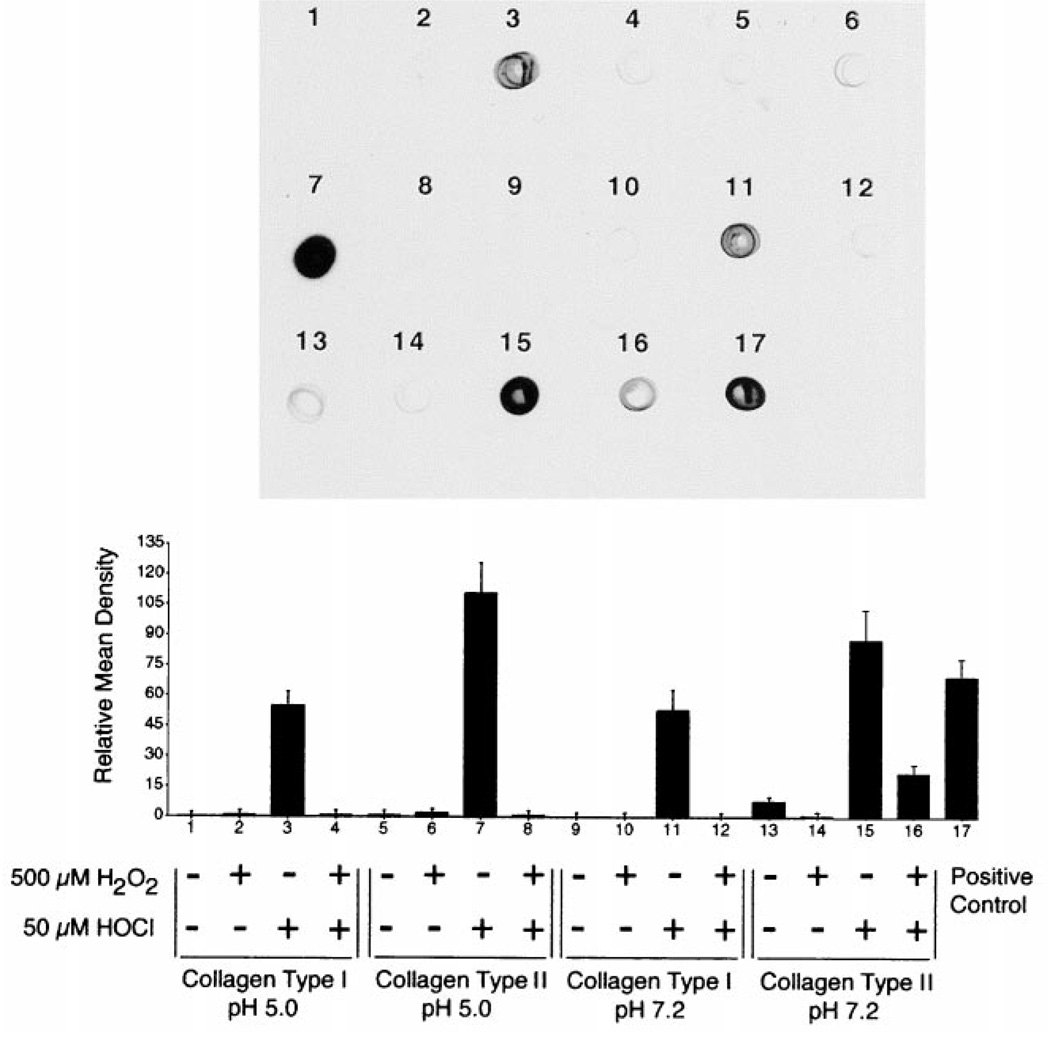
To determine the oxidation of primary amines or pyridinoline cross-links by H2O2 or HOCl, immuno-chemiluminescence analysis (Oxyblot kit) was used to assess the presence of carbonyl groups within the protein of interest. A concentration-dependent increase in the number of reactive carbonyl groups in both collagen types was found after exposure to HOCl, with the amount varying depending on collagen type. The results for collagen reacted with 500 µm H2O2, 50 µm HOCl, or both are presented in Fig. 7. Lesser amounts of carbonyls were generated in the reaction of 25 and 12.5 µm HOCl with both collagen types (data not shown). In general, collagen type I was less reactive at both pH 5.0 and pH 7.2 as compared with collagen type II, as indicated by relative mean density (Fig. 7).
FIG. 7.
50 µg of human collagen type I or type II were incubated at pH 5.0 or pH 7.2 for 1 h at 37 °C in 0.5 m sodium phosphate buffer (control) or buffer containing H2O2 and/or HOCl as indicated. After incubation, 0.1 µg of each sample was analyzed for carbonyl content. Only samples reacted with HOCl showed increased carbonyl formation. The immuno-chemiluminescence (Oxyblot kit) data is presented for a typical experiment performed three times. The bar graph shows the relative mean density ± S.E. for all three experiments.
No change in carbonyl content was observed relative to untreated collagen control samples after treatment of either collagen type with H2O2 or a combination of H2O2/HOCl (Fig. 7). Adding H2O2 and HOCl together decreased the effect of HOCl, suggesting that H2O2 is reacting with HOCl and decreasing the availability of HOCl to react with collagen.
Pyridinoline Cross-link and Aromatic Amino Acid Fluorescence of Collagen before and after Exposure to HOCl
To assess oxidation of the pyridinoline cross-links and aromatic amino acids of collagen types I and II by HOCl, the fluorescence emission of HOCl-treated collagen was measured and compared with untreated control samples subjected to the same conditions. The fluorescence of collagen type I samples was below measurable levels, and no further fluorescence studies were done on this collagen type. Exposure of 0.125 mg/ml collagen type II to HOCl at pH 5.0 and 7.2 for 15 min at 37 °C resulted in a concentration-dependent decrease in fluorescence emission at 400 nm (excitation 325 nm) (Table II). No other changes in fluorescence emission or excitation were observed.
TABLE II. Exposure of human articular collagen type II to HOCl resulted in a decrease in fluorescence emission at 400 nm (excitation 325 nm) after a 15-min incubation at 37 °C.
Data are presented as a percentage decrease in fluorescence emission relative to unreacted collagen incubated under the same conditions. Values represent the mean ± S.E. (n = 2).
| HOCl | |||
|---|---|---|---|
| 1 µm | 3 µm | 4 µm | |
| 0.125 mg/ml collagen, pH 5.5 | 8.5 ± 5.0% | 9.4 ± 4.5% | 10.0 ± 4.5% |
| 0.125 mg/ml collagen, pH 7.2 | 10.0 ± 5.7% | 18.5 ± 7.0% | 21.0 ± 6.0% |
| Pyd:HOCla | 1:1.2 | 1:3.5 | 1:4.6 |
Ratio of Pyd:HOCl, assuming the amount of 2 mol of Pyd/mol of collagen remains unchanged by pepsin-solubilization of the collagen type II.
SDS-PAGE Results of Collagen Types I or II Reacted with H2O2, HOCl, or a Combination of H2O2/HOCl
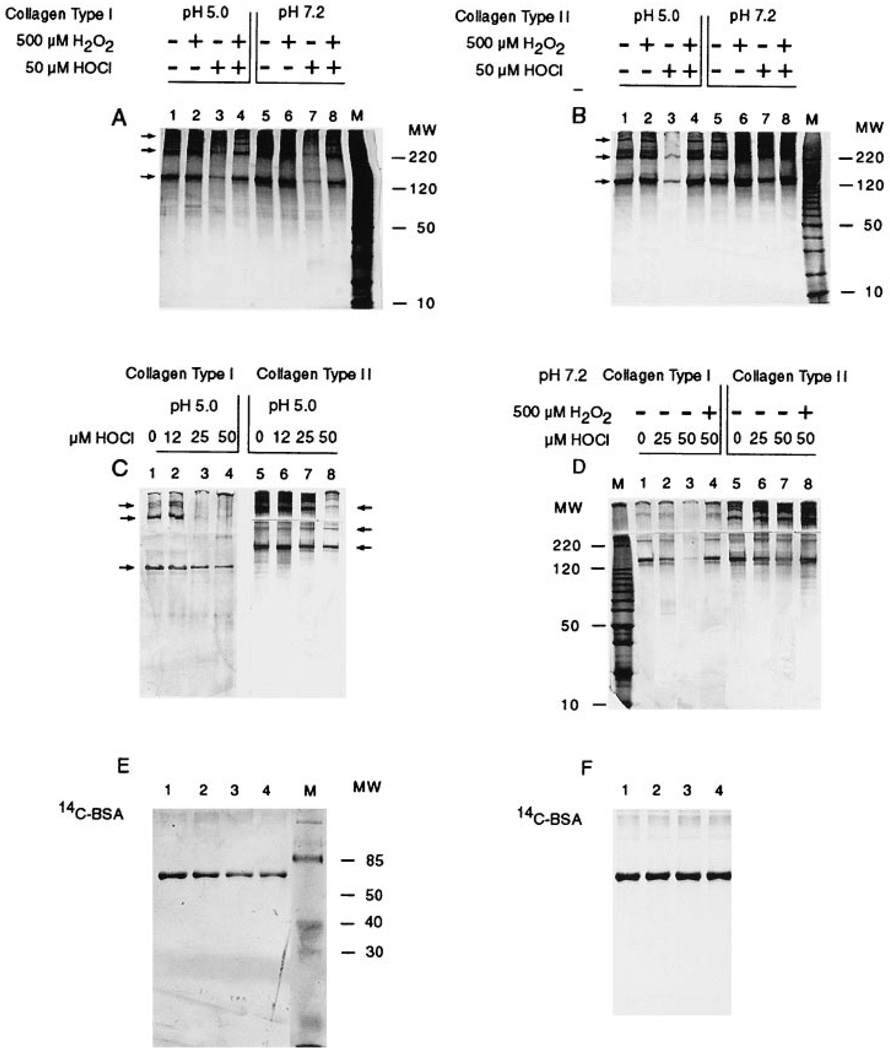
After exposure to ROS as described above, 4-µg aliquots of each collagen sample were subjected to SDS-PAGE analysis (4–15% gradient) and stained with silver. Results of a representative gel of an experiment performed three times are presented in Fig. 8. Exposing collagen types I (Fig. 8A, lanes 3 and 7) or II (Fig. 8B, lane 3) to 50 µm HOCl resulted in a 60–80% decrease in the intensity of collagen electrophoretic band staining by silver (arrows indicating form top to bottom, γ, β, and α). Except at pH 7.2, the decrease in silver staining for collagen type II reacted with HOCl was much less (Fig. 8B, lane 7). Concentration-dependent decreases in silver staining are shown in Fig. 8, C and D, for collagen reacted with HOCl at pH 5.0 and 7.2. No smearing (fragmentation) or low molecular weight bands were observed after any treatment. A decrease in reactivity of silver with the HOCl-reacted collagen monomers is consistent with the formation of N-chloroamines (33) in the reaction of HOCl with collagen and ultimately the spontaneous deamination and decarboxylation of N-chloramines (α-amino groups) to form aldehydes (26, 63), thus suggesting that the collagen monomers are still intact but have been oxidatively modified and no longer react with silver. The oxidative modification of amine groups by HOCl would no longer make them available for reaction with silver.
FIG. 8.
50-µg samples of collagen type I (A)and collagen type II (B) were reacted at pH 5.0 and pH 7.2 or collagen types I or II were reacted at pH 5.0 (C) or pH 7.2 (D) for 1 h at 37 °C in 0.5 m sodium phosphate buffer alone or buffer containing H2O2 and/or HOCl as indicated. After ROS exposure, 4 µg of human collagen type I, type II, or [14C]bovine serum albumin (14C-BSA) were subjected to SDS-PAGE analysis and stained with silver. E, 14C-BSA was reacted at pH 7.2 in buffer alone or buffer containing HOCl at 12.5 (lane 2), 25 (lane 3), or 50 µm (lane 4). Exposing collagens type I, type II, or [14C]BSA to 12.5–50 µm HOCl resulted in a concentration-dependent decrease in the intensity of the electrophoretic band staining by silver (arrows) without causing a loss of protein as indicated by the single and equally intense (F) autoradiograph band for [14C]BSA in lanes 1–4. Lanes marked M contain molecular mass markers (kDa). The presented data represent typical results of an experiment performed three times.
To determine if the decrease in silver reactivity with collagens after oxidation by HOCl is due to oxidative modification of primary amine groups and not protein fragmentation or both, we reacted 14C-labeled bovine serum albumin (BSA) with increasing concentrations of HOCl (12.5–50 µm). After exposure to HOCl as described above, 4-µg aliquots of [14C]BSA were subjected to SDS-PAGE analysis (10% acrylamide), stained with silver, then dried and exposed to autoradiographic film. Silver staining and autoradiogram results are presented in Fig. 8, C and D, respectively. A concentration-dependent decrease in silver staining intensity was observed after treatment of [14C]BSA with 12.5–50 µm HOCl, without affecting the auto-radiogram band intensity of these same samples. This provided us with some supportive evidence that the decrease in collagen staining after reaction with HOCl is due to oxidative modification rather than protein fragmentation or a combination of both processes.
Fluorescamine and o-Phthalaldehyde Primary Amine and Imino Acid Measurements
To determine if the decrease in detectable electrophoretic band staining was the result of complete collagen fragmentation by HOCl, aliquots of each collagen sample, acetone precipitates of each sample, and sample supernatants of acetone precipitates were analyzed by fluorescamine and o-phthalaldehyde assays. Both assays detect primary amines and imino acids, which would increase if collagen were fragmented. However, neither assay detected an increase in free amine or imino acid groups in any of the collagen samples.
DISCUSSION
ROS are produced and released into extracellular spaces and contribute to the development and progression of inflammatory diseases. However, little is known about direct ROS-induced molecular modifications of individual matrix proteins and the consequence of these modifications on the structure, physical properties, or function of the matrix. The present study tested the hypothesis that HOCl participates in the inflammatory-mediated loss of connective tissue collagen by oxidizing the Pyd cross-links found in abundance in adult articular cartilage. HOCl, produced by the enzyme MPO, is the major highly reactive oxidant produced by activated neutrophils and to a lesser extent by monocytes and some macrophages. Based on its reactivity, HOCl has the potential to cause the bulk of the tissue damage at sites of acute inflammation (64). Pyd cross-links are of particular importance because their function is to help maintain the structure of the collagen fibrils and make them more resistant to collagenolysis or proteolytic degradation (37, 65). Our current findings indicate that OCl−/Cl2, and to a lesser extent N-chloramines, chlorinate pyridinium compounds with structures similar to Pyd and that these HOCl species react with both collagen types I and II, resulting in the oxidation of amine groups and Pyd cross-links.
Our findings indicate that HOCl rapidly reacts with and chlorinates pyridinium compounds, but the chlorination reaction and the N-chlorination sites are pH-dependent. At pH 7.2 ± 0.2, HOCl/−OCl preferentially reacted with the para-CH2NH2 group of pyridoxamine to formN-chloropyridoxamine, which was detected using the KI oxidation assay for N-chlor-amine and by the appearance of the characteristic N-chloramine absorbance peak at 220–225 nm. In pyridoxamine samples treated with HOCl, a second absorbance peak at 307–320 nm was observed. This peak corresponds to a peak observed for the product that results from the reaction of pyridoxamine with chloramine-T, a commercial N-chloramine standard. Based on the presence of these two peaks, we suggest that as soon as N-chloropyridoxamines are formed in the reaction of pyridoxamine and HOCl, they in turn react with other pyridoxamine molecules and initiate the formation of N-chloropyridoxamine-pyridoxamine dimers. The reaction of HOCl/−OCl with the para-CH2NH2 of pyridoxamine is in agreement with the report of Davies et al. (33) stating that the preferred reaction of HOCl/OCl− with collagen at neutral pH is with the primary amine groups.
At pH 5.5 ± 0.2 and in the presence of Cl−, HOCl, and N-chloramines reacted with both pyridoxamine and vitamin B6. The reactivity of HOCl at this pH with the pyridinium compounds is OCl− with compounds that possess extensive p electrons (ring nitrogen) (66). The significant loss in absorbance and fluorescence of either compound after reaction with HOCl at pH 5.5 suggests that a percentage of the pyridinium ring structure was disrupted in these reactions. Disruption of the ring structure was confirmed by GC-MS analysis and is consistent with the formation of an aldehyde as a result of the spontaneous deamination and decarboxylation of an N-chloramine (in this case the ring nitrogen) to form an aldehyde (58, 63).
The reactivity of HOCl with vitamin B6 at pH 5.5 in the presence of Cl− is also consistent with the evolution of Cl2 (55). GC-MS analysis also identified 3-chloropyridinium as one of the products of this reaction, similar to a previous report of 3-chlorotyrosine formation in the reaction of Cl2 with the aromatic amino acid, l-tyrosine (27). The reactivity of Cl2 is also of interest because it has been demonstrated that neutrophils generate Cl2 via the MPO-H2O2-Cl− system (66).
In contrast to HOCl, H2O2 alone was without effect under any condition, which is in keeping with its low reactivity with biological molecules. The third of the nonradical species tested in this study, O2(1Δg), is a relatively long lived (2 µs) and highly reactive oxidant produced by HOCl at pH 5.5 and to a lesser extent at pH 7.2 in the presence of Cl− (55). Because O2(1Δg) would be produced by both of these ROS systems, it would seem likely that O2(1Δg) would react with the pyridinium ring nitrogen (59). However, O2(1Δg) did not significantly contribute to the derivatization of pyridoxamine, and at pH 7.2 the production of O2(1Δg) by the reaction of H2O2 with HOCl actually interfered with the reaction of HOCl and pyridoxamine. Similarly, at pH 5.5 HOCl should generate maximal amounts of O2(1Δg) in the presence of NaCl (55); however, even at this pH our findings indicate that HOCl and not O2(1Δg) is the major reactant with either pyridoxamine or vitamin B6. Finally, the thermal release of O2(1Δg) by DNE, a pure chemical source of O2(1Δg), did not result in an absorbance or fluorescence change in the pyridoxamine or vitamin B6 spectra. It has been reported that O2(1Δg) can react with pyridinium compounds, resulting in cleavage of the nitrogen-carbon bond between the nitrogen-containing pyridinium ring and the terminal carbon of a substituted group at this site (59). This reaction leaves the pyridinium ring structure intact and unchanged. Although we did not detect a reaction of O2(1Δg) with an unsubstituted nitrogen group in the pyridinium rings of pyridoxamine and vitamin B6, it is still possible that a reaction between O2(1Δg) and the Pyd cross-links could take place in vivo when the nitrogen is covalently linked to the triple helical region of a collagen molecule. This type of reaction would also result in the disruption of the intermolecular bond between two molecules of collagen.
In a previous study by Davies et al. (33) the oxidation of bovine collagen type I isolated from tendon and collagen type II isolated from articular cartilage was assessed as the amount of collagen fragmentation taking place in response to HOCl or N-chloramines. Only at superphysiological concentrations of 1–5 mm did HOCl cause extensive fragmentation (smearing) of collagen. In contrast, the addition of N-chloramines (5–50 µm) did not cause fragmentation but, instead, greatly increased the degradation of collagen by collagenase and elastase. The mechanism by which N-chloramines increased the proteolytic susceptibility of collagen was not specifically determined, although it was assumed that N-chloramines were reacting with amine groups and disrupting the secondary and tertiary structure of the collagen molecules. In many cases, oxidation of proteins appears to result in the partial unfolding of target proteins, exposing hydrophobic regions that are normally shielded and promoting the preferential degradation of these proteins by proteases (67–69). Disruption of the secondary and tertiary structure of proteins by oxidative damage has been reported to expose hydrophobic regions of proteins and promote their preferential degradation by proteases (67–71). We would also suggest that oxidation and disruption of the Pyd cross-links could result in the loss of functional interactions of collagens, destabilization of the structural integrity of collagen fibrils, and/or an increase in the susceptibility of collagen to proteolytic degradation. Any of these changes would result in potentially irreversible damage of the tissue.
Our present findings agree with those of Davies et al. (33) that no fragmentation of either collagen takes place in response to physiological concentrations of HOCl (12.5–50 µm). However, we show that at these concentrations HOCl directly oxidizes human collagen type I from bone and collagen type II from articular cartilage. We were able to demonstrate the direct oxidation of collagen types I and II by taking advantage of the fact that N-chloramines, formed by the reaction of HOCl with α-amino groups, decompose to aldehydes (26, 63) and that the formation of N-chloramines contributes to the decrease in silver staining intensity of HOCl-reacted protein. We were also able to demonstrate the direct oxidation of Pyd cross-links in collagen type II by taking advantage of the fact that aldehydes are also formed in the reaction of HOCl with pyridinium compounds and that the formation of aldehydes results in the disruption of the pyridinium ring and the loss of Pyd fluorescence (325ex/400em). We have focused on the oxidation of the Pyd cross-links because their chemical makeup suggests that they should be reactive with HOCl (27) and possibly O2(1Δg) (72). Using the decrease in fluorescence emission as a measure for the reaction of HOCl with the Pyd cross-links is supported by the fact that other fluorescent groups present or generated by oxidation of collagen do not fluoresce at 400 nm (325 nm excitation) or have increased fluorescence at this wavelength. These groups include tryptophan residues (275ex/334em) that are not present in these collagens, glycation end products (350ex/430em), and bityrosines (325ex/400em), which when formed have an increased fluorescence emission at 400 nm (27, 30).
Although both collagen types I and II were oxidized by HOCl, the reactions differed. Oxidized collagen type II showed a greater amount of carbonyl formation and a decrease in fluorescence (325ex/400em), as compared with oxidized collagen type I. The increase in carbonyl formation may have resulted from the reaction of HOCl with Pyd cross-links that are more abundant in this collagen type (36). This reaction would also account for, but may not completely explain, the more intense silver staining of collagen type II since a reaction of HOCl with Pyd cross-links would result in a greater amount of carbonyl formation without a corresponding decrease in amine groups. In support of a reaction of HOCl with the Pyd cross-links, we detected a decrease in fluorescence emission at 400 nm (excitation 325 nm) in the collagen type II samples reacted with HOCl. The formation of aldehydes in the reaction of HOCl with pyridinium compounds is rapid and can be detected after only 15 min of incubation at 37 °C as opposed to the slower decomposition of N-chloramines to aldehydes, which can take hours (25).
In summary, activated neutrophils and monocytes are present in early inflammatory lesions and in later focal regions of acute inflammation in arthritic joints (1) and periodontitis (3). The presence of activated neutrophils results in the deposition of MPO onto the ECM surface and the production of the reactive oxidant, HOCl. Unlike a reaction with HOCl, the Pyd cross-links need not be on the surface of the fibril to be accessible to attack by Cl2 or N-chloramines, which are small and less reactive, so they can diffuse from their sites of production. Thus, suggesting that the Pyd cross-links linking collagen types IX–II and II–II in articular cartilage would be assessable to reaction during inflammation associated with articular cartilage. However, the Pyd and deoxypyridinoline cross-links of bone collagen type I are probably less susceptible to oxidation, because this collagen is more densely packed. First, the mineralization of bone collagen type I reduces the diffusibility of Cl2 or N-chloramines in this matrix. Second, the collagen fibril itself is more tightly aligned (i.e. lateral packing of the molecules within the fibril and the spacing between collagen molecules is smaller in bone than cartilage (45, 46)) allowing less solvent and small molecule access to inner spaces. Finally, our findings suggest that if formed in vivo, 3-chloropyridinium might serve as a specific marker for the production of HOCl and the involvement of MPO in inflammation of tissues containing significant quantities of Pyd cross-links.
Acknowledgments
We thank Dr. James San Antonio (Department of Medicine, Thomas Jefferson University, Philadelphia), Dr. Noreen Hickok (Department of Orthopaedic Surgery, Thomas Jefferson University, Philadelphia), and Professor Morris J. Karnovsky (Department of Pathology, Harvard Medical School, Boston) for the help they provided in reading and commenting on the work presented in this manuscript. We also thank Dr. John Buzby, ChiRex Cauldron Process Chemistry, Malvern, PA, for providing the chemical names of the GC-MS products.
Footnotes
This work was supported by NIDCR Grants DE-08507 and DE-11082 from the National Institutes of Health.
The abbreviations used are: ROS, reactive oxygen species; , superoxide anion; H2O2, hydrogen peroxide; OCl−/HOCl, hypochlorite/hypochlorous acid; O2(1Δg), singlet oxygen; MPO, myeloperoxidase; ECM, extracellular matrix; Pyd, pyridinoline cross-links; ECM, extracellular matrix; AAP, anthracene-9,10-diproprionic acid; RT, room temperature; DNE, 1,4-dimethyl-1,4-naphthalene endoperoxide; PAGE, polyacrylamide gel electrophoresis; GC-MS, gas chromatography-mass spectrometry; chloramine-T, N-chloro-p-toluene-sulfonamide sodium salt; BSA, bovine serum albumin.
Contributor Information
Kathleen M. Daumer, Department of Orthopaedic Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania 19107
Ahsan U. Khan, Department of Chemistry, New York University, New York, New York 10003
Marla J. Steinbeck, Department of Orthopaedic Surgery, Thomas Jefferson University, Philadelphia, Pennsylvania 19107.
REFERENCES
- 1.Woolley DE, et al. Ann. Rheum. Dis. 1997;56:151–161. doi: 10.1136/ard.56.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fredriksson M, Gustafsson A, Asman B, Bergstrom K. J. Clin. Periodontol. 1998;25:394–398. doi: 10.1111/j.1600-051x.1998.tb02461.x. [DOI] [PubMed] [Google Scholar]
- 3.Fredriksson MI, Figueredo CM, Gustafsson A, Bergstrom KG, Asman BE. J. Periodontol. 1999;70:1355–1360. doi: 10.1902/jop.1999.70.11.1355. [DOI] [PubMed] [Google Scholar]
- 4.Harris EDJ. In: Inflammation: Basic Principles and Clinical Correlates. Gallin JI, Goldstein IM, Snyderman R, editors. New York: Raven Press, Ltd.; 1988. pp. 751–774. [Google Scholar]
- 5.Babior BM. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- 6.Klebanoff SJ. In: Inflammation: Basic Principles and Clinical Correlates. Gallin JI, Goldstein IM, Snyderman R, editors. New York: Raven Press, Ltd.; 1988. pp. 391–444. [Google Scholar]
- 7.Halliwell B. Ann. Rheum. Dis. 1995;54:505–510. doi: 10.1136/ard.54.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwald RA. Semin. Arthritis Rheum. 1991;20:219–240. doi: 10.1016/0049-0172(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 9.Ginis I, Tauber AI. Blood. 1990;76:1233–1239. [PubMed] [Google Scholar]
- 10.Weiss SJ, LoBuglio AF. Lab. Invest. 1982;47:5–18. [PubMed] [Google Scholar]
- 11.Harlan JM. Blood. 1985;65:513–525. [PubMed] [Google Scholar]
- 12.Vissers MC, Day WA, Winterbourn CC. Blood. 1985;66:161–166. [PubMed] [Google Scholar]
- 13.Henson PM, Johnston RB., Jr J. Clin. Invest. 1987;79:669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babior BM, Kipnes RS, Curnutte JT. J. Clin. Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segal AW, Jones OT, Webster D, Allison AC. Lancet. 1978;2:446–449. doi: 10.1016/s0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- 16.Rossi F. Biochim. Biophys. Acta. 1986;853:65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- 17.McCord JM, Fridovich I. J. Biol. Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 18.Gartner A, Weser U. Top. Curr. Chem. 1986;132:1–61. [Google Scholar]
- 19.Harrison JE, Schultz J. J. Biol. Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 20.Bainton DF, Farquhar MG. J. Cell Biol. 1968;39:299–317. doi: 10.1083/jcb.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briggs RT, Drath DB, Karnovsky ML, Karnovsky MJ. J. Cell Biol. 1975;67:566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurst JK, Barrette WC., Jr Crit. Rev. Biochem. Mol. Biol. 1989;24:271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj RJ, Zgliczynski JM, Paul BB, Sbarra AJ. J. Infect. Dis. 1978;137:481–485. doi: 10.1093/infdis/137.4.481. [DOI] [PubMed] [Google Scholar]
- 24.Weiss SJ, Klein R, Slivka A, Wei M. J. Clin. Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss SJ, Lampert MB, Test ST. Science. 1983;222:625–628. doi: 10.1126/science.6635660. [DOI] [PubMed] [Google Scholar]
- 26.Thomas EL, Grisham MB, Jefferson MM. Methods Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 27.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. J. Clin. Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinbeck MJ, Khan AU, Karnovsky MJ. J. Biol. Chem. 1992;267:13425–13433. [PubMed] [Google Scholar]
- 29.Greenwald RA, Moak SA. Inflammation. 1986;10:15–30. doi: 10.1007/BF00916037. [DOI] [PubMed] [Google Scholar]
- 30.Vissers MC, Winterbourn CC. Arch. Biochem. Biophys. 1991;285:53–59. doi: 10.1016/0003-9861(91)90327-f. [DOI] [PubMed] [Google Scholar]
- 31.Vissers MC, Winterbourn CC. Arch. Biochem. Biophys. 1991;285:357–364. doi: 10.1016/0003-9861(91)90372-p. [DOI] [PubMed] [Google Scholar]
- 32.Vissers MC, Winterbourn CC. Biochim. Biophys. Acta. 1986;889:277–286. doi: 10.1016/0167-4889(86)90190-4. [DOI] [PubMed] [Google Scholar]
- 33.Davies JM, Horwitz DA, Davies KJ. Free Radic. Biol. Med. 1993;15:637–643. doi: 10.1016/0891-5849(93)90167-s. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto D, Moriguchi T, Ishida T, Hayashi H. Biochem. Cell Biol. 1978;84:52–57. doi: 10.1016/0006-291x(78)90261-9. [DOI] [PubMed] [Google Scholar]
- 35.Eyre DR. In: The Chemistry and Biology of Mineralized Connective Tissues. Veis A, editor. Amsterdam: Elsevier/North-Holland Biomedical Press; 1981. pp. 51–55. [Google Scholar]
- 36.Eyre DR. Science. 1980;207:1315–1322. doi: 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- 37.Vater C, Harris E, Jr, Siegel R. Biochem. J. 1979;181:639–645. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidgwick NV. The Chemical Elements and Their Compounds. Oxford: Clavendon Press; 1950. pp. 1213–1216. [Google Scholar]
- 39.Sneed MC, Maynard JL, Brasted RC, editors. Comprehensive Inorganic Chemistry. New York: Van Nostrad Co., Inc.; 1954. pp. 152–155. [Google Scholar]
- 40.Downs AJ, Adams CJ. In: Comprehensive Inorganic Chemistry. Bailar JC Jr, Emeleus HJ, Nyholm R, Trotman-Dickenson AF, editors. Oxford, United Kingdom: Pergamon Press; 1973. pp. 1400–1408. [Google Scholar]
- 41.Witko V, Nguyen AT, Descamps-Latscha B. J. Clin. Lab. Anal. 1992;6:47–53. doi: 10.1002/jcla.1860060110. [DOI] [PubMed] [Google Scholar]
- 42.Wasserman HH, Larsen DL. J. Chem. Soc. Chem. Commun. 1972;5:253–255. [Google Scholar]
- 43.Steinbeck MJ, Khan AU, Appel WH, Jr, Karnovsky MJ. J. Histochem. Cytochem. 1993;41:1659–1667. doi: 10.1177/41.11.8292156. [DOI] [PubMed] [Google Scholar]
- 44.Deby-Dupont G, Deby C, Mouithys-Mickalad A, Hoebeke M, Mathy-Hartert M, Jadoul L, Vandenberghe A, Lamy M. Biochim. Biophys. Acta. 1998;1379:61–68. doi: 10.1016/s0304-4165(97)00083-4. [DOI] [PubMed] [Google Scholar]
- 45.Brodsky B, Eikenberry EF. Methods Enzymol. 1982;82:127–174. doi: 10.1016/0076-6879(82)82062-4. [DOI] [PubMed] [Google Scholar]
- 46.Piez KA. In: Extracellular Matrix Biochemistry. Piez KA, Reddi AH, editors. New York: Elsevier Science Publishing Co., Inc.; 1984. pp. 1–39. [Google Scholar]
- 47.SanAntonio JD, Lander AD, Karnovsky MJ, Slayter HS. J. Cell Biol. 1994;125:1179–1188. doi: 10.1083/jcb.125.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 49.Udenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, Weigele M. Science. 1972;178:871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- 50.Bohlen P, Stein S, Dairman W, Udenfriend S. Arch. Biochem. Biophys. 1973;155:213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- 51.Steinbeck MJ, Hegg GG, Karnovsky MJ. J. Biol. Chem. 1991;266:16336–16342. [PubMed] [Google Scholar]
- 52.Brealey GJ, Kasha M. J. Am. Chem. Soc. 1955;77:4462–4468. [Google Scholar]
- 53.Berger RR, Karnovsky ML. Fed. Proc. 1966;25:840–845. [PubMed] [Google Scholar]
- 54.Khan AU, Kasha M. J. Chem. Phys. 1963;39:2105–2106. [Google Scholar]
- 55.Khan AU, Kasha M. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12362–12364. doi: 10.1073/pnas.91.26.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Held AM, Halko DJ, Hurst JK. J. Am. Chem. Soc. 1978;100:5732–5740. [Google Scholar]
- 57.Latimer WM. In: The Oxidation States of the Elements and Their Potentials in Aqueous Solutions. Latimer WM, editor. New York: Prentice-Hall Inc.; 1938. p. 48. [Google Scholar]
- 58.Thomas EL, Grisham MB, Jefferson MM. J. Clin. Invest. 1983;72:441–454. doi: 10.1172/JCI110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Straight RC, Spikes JD. In: Singlet O2. Frimer AA, editor. Vol. 4. Boca Raton, FL: CRC Press, Inc.; 1985. pp. 91–143. [Google Scholar]
- 60.Cech P, Lehrer RI. Blood. 1984;63:88–95. [PubMed] [Google Scholar]
- 61.Segal AW, Geisow M, Garcia R, Harper A, Miller R. Nature. 1981;290:406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- 62.Lukacs GL, Rotstein OD, Grinstein S. J. Biol. Chem. 1990;265:21099–21107. [PubMed] [Google Scholar]
- 63.Hazen SL, Hsu FF, d’Avignon A, Heinecke JW. Biochemistry. 1998;37:6864–6873. doi: 10.1021/bi972449j. [DOI] [PubMed] [Google Scholar]
- 64.Domigan NM, Charlton TS, Duncan MW, Winterbourn CC, Kettle AJ. J. Biol. Chem. 1995;270:16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 65.Eyre DR, Paz MA, Gallop PM. Annu. Rev. Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 66.Albrich JM, McCarthy CA, Hurst JK. Proc. Natl. Acad. Sci. U. S. A. 1981;78:210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cervera J, Levine RL. FASEB J. 1988;2:2591–2595. doi: 10.1096/fasebj.2.10.2898411. [DOI] [PubMed] [Google Scholar]
- 68.Giulivi C, Pacifici RE, Davies KJA. Arch. Biochem. Biophys. 1994;311:329–341. doi: 10.1006/abbi.1994.1245. [DOI] [PubMed] [Google Scholar]
- 69.Pacifici RE, Kono Y, Davies KJA. J. Biol. Chem. 1993;268:15405–15411. [PubMed] [Google Scholar]
- 70.Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJA. J. Biol. Chem. 1990;265:11919–11927. [PubMed] [Google Scholar]
- 71.Davies KJA, Lin SW, Pacifici RE. J. Biol. Chem. 1987;262:9914–9920. [PubMed] [Google Scholar]
- 72.Hormel S, Eyre D. Biochim. Biophys. Acta. 1991;1078:243–250. doi: 10.1016/0167-4838(91)90565-h. [DOI] [PubMed] [Google Scholar]