Abstract
Fatty acid synthase (FAS) and focal adhesion kinase (FAK), which are overexpressed in a variety of human epithelial tumors, play a key role in the migration and invasion of cancer cells. Hence, strategies targeted at inhibiting the FAS/FAK proteins may have therapeutic potential for cancer treatment. The goal of the present study was to determine the effect of HO-3867, a synthetic compound, on the migratory ability of ovarian cancer cells and to understand the mechanistic pathways including the involvement of FAS, FAK, and associated signaling proteins. The study was performed using two established human ovarian cancer cell lines, namely, A2780 and SKOV3. Incubation with 10-μM HO-3867 for 24 hours significantly inhibited the native as well as VEGF-mediated migration and invasion of the cells. HO-3867 significantly attenuated FAS and FAK protein levels apparently through accelerated ubiquitin-dependent degradation as shown by a clear down-regulation of isopeptidase USP2a. Exposure of cells to HO-3867 also significantly inhibited the FAS activity, mRNA levels, and a number of downstream proteins including pERK1/2, pHER1, SREBP1, VEGF, and MMP-2. Western-blot and immunohistochemical analyses of A2780 xenograft tumors in mice treated with HO-3867 showed significant reduction in FAS, FAK, VEGF, and downstream protein levels when compared to untreated control. Collectively, the results demonstrated that HO-3867 suppressed the migration and invasion of the ovarian cancer cells by inhibiting the expression/activity of FAS and FAK proteins. The study suggested that molecular targeting of FAS and FAK by HO-3867 might be a potential strategy for ovarian cancer therapy.
Keywords: human ovarian cancer, A2780, SKOV3, diarylidenylpiperidone, fatty acid synthase, focal adhesion kinase, VEGF
Introduction
Tumor progression is a complex process that includes malignant transformation, proliferation, invasion, and metastasis of cancer cells. Particularly, cancer-cell invasion and metastasis are the critical processes that define the aggressive phenotype of human cancers and pose major impediments to treatment (1, 2). While the development of anti-cancer therapy is traditionally focused on the inhibition of cancer-cell proliferation, therapeutic strategies targeted towards inhibiting the spread of cancer cells from a primary tumor to secondary sites can be valuable to treat aggressive malignancies (1). Tumor cell migration requires the concerted effort of a number of molecules such as integrins, cell adhesion molecules, soluble cytokines and growth factors, matrix-degrading proteases, and Rho GTPases (3). The migration process involves assembly and disassembly of focal adhesions and is stimulated extracellularly and initiated by integrins and intracellular signaling proteins located in focal adhesions (4). Focal adhesion kinase (FAK), a tyrosine receptor kinase, is activated in focal adhesions and is important in cell extracellular matrix (ECM) interactions that affect cell migration, proliferation, and survival (5). Many malignant human tumors exhibit increased FAK expression and tyrosine phosphorylation (6) that are correlated with the acquisition of an invasive cell phenotype and increased metastasis (7).
Ovarian carcinoma remains the most lethal among gynecological cancers due to a lack of early detection methods and effective treatments for late-stage malignancies (8). As found in many other types of human tumors, overexpression or hyperactivation of FAK and fatty acid synthase (FAS) have recently been found in most ovarian cancers, where it is highly associated with high aggressiveness and poor patient survival (4, 9-11). Increased expression of FAS occurs very early in cancer development, and is more distinct as the tumor progresses towards a more advanced stage. FAS enzyme is responsible for the de novo synthesis of fatty acids, and it has emerged as a potential therapeutic target for human cancer (10). High levels of FAS expression have been found in ovarian cancer (12) and in most human solid tumors (13). FAS plays a significant role in the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. These are raft-aggregates implicated in key cellular processes including signal transduction, intracellular trafficking, cell polarization, and cell migration. Inhibition of FAS activity is selectively cytotoxic to human cancer cells in vitro and in vivo (9, 10) including human ovarian cancer xenografts (14). However, the mechanisms linking the inhibition of FAS activity to induction of cancer-cell death and inhibition of cancer-cell migration remain an active area of investigation.
We recently reported that HO-3867, a diarylidenylpiperidone (DAP)-based synthetic compound with an interesting anti-oxidant appendage, exhibited significant growth arrest and apoptosis in a number of human cancer cell lines including breast, colon, head and neck, liver, lung, ovarian, and prostate cancer with no apparent toxicity to noncancerous cells (15, 16). We observed that the anticancer activity HO-3867 in ovarian cancer was mediated by inhibition of STAT3 phosphorylation at Tyr705 and Ser727 residues and induction of apoptotic markers cleaved caspase-3 and PARP. The protective activity of HO-3867 towards noncancerous cells was shown to be mediated by the ability of the compound to confer selective anti-oxidant protection to the healthy cells. In a subsequent in vivo study, we further demonstrated that HO-3867 significantly inhibited the growth of the ovarian xenografted tumors (A2780) in a dosage-dependent manner (17). Western-blot analyses of the xenograft tumor tissues confirmed that HO-3867 inhibited pSTAT3 (Tyr705 and Ser727) and pJAK1 and increased apoptotic markers cleaved caspase-3 and PARP.
While our previous studies clearly demonstrated the potential of HO-3867 as a safe and effective anticancer agent for ovarian cancer therapy, the possible effect and mechanism of the compound on tumor-cell migration and invasion have not been established. Accordingly, the goal of the present study was to determine the effect of HO-3867 on the migratory ability of ovarian cancer cells and to understand the mechanistic pathways including the involvement of FAS, FAK, and associated signaling proteins. The study was performed using two established human ovarian cancer cell lines, namely, A2780 and SKOV3 under in vitro as well as in vivo conditions on xenografted tumor in mice. The results clearly demonstrated that HO-3867 suppressed the migration and invasion of the ovarian cancer cells by inhibiting the expression/activity of FAS and FAK proteins. The study suggested that molecular targeting of FAS and FAK by HO-3867 might be a potential strategy for ovarian cancer therapy.
Materials & Methods
Materials
Cell-culture medium (RPMI 1640) and DMEM, fetal-bovine serum (FBS), antibiotics, sodium pyruvate, trypsin, and phosphate-buffered saline (PBS) were purchased from Gibco (Grand Island, NY). Polyvinylidene fluoride (PVDF) membrane and molecular-weight markers were obtained from Bio-Rad (Hercules, CA). Antibodies against, pHER1, HER1, FAS, pERK1/2, ERK1/2, actin, and USP2a were purchased from Cell Signaling Technology (Beverly, MA). Antibodies specific for SREBP1, FAK, MMP-2, VEGF, USP2a, and ubiquitin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced chemiluminescence (ECL) reagents were obtained from Amersham Pharmacia Biotech (GE Healthcare, Piscataway, NJ). HO-3867 was synthesized in the laboratory (18). Stock solutions of the compounds were freshly prepared in dimethylsulfoxide (DMSO). All other reagents, of analytical grade or higher, were purchased from Sigma-Aldrich.
Cell lines and cultures
A2780 and SKOV3 human epithelial ovarian cancer cell lines were used in the study. The cells were grown in RPMI 1640 and DMEM medium supplemented with 10% FBS, 2% sodium pyruvate, 1% penicillin and 1% streptomycin. Cells were grown in a 75-mm flask to 70% confluence at 37°C in an atmosphere of 5% CO2 and 95% air. Cells were routinely trypsinized (0.05% trypsin/EDTA) and counted using an automated counter (NucleoCounter, New Brunswick Scientific, Edison, NJ).
Cell migration and invasion assays
Cell-migration assay was performed by wound-healing method (1). Cells were plated at equal density and grown to 90% confluence. Wounds were created using a sterile pipette tip. Cells were then rinsed with medium and replaced with the fresh medium and incubated with HO-3867 (10 μM). Areas of wound were marked and photographed at various time-points with a phase-contrast microscope. Cell-invasive assay was measured by an in vitro Boyden chamber assay (19). Briefly, 1×105 cells in 0.5 ml of serum-free RPMI 1640 medium were added to the wells of 8-μm-diameter pore membrane Boyden chambers, either coated with (BD Biosciences, Franklin Lake, NJ) or without (Corning, Corning, NY) Matrigel. Cells were allowed to invade for 24 hours. Cells that had not penetrated the filters were removed by scrubbing with cotton swabs. Chambers were fixed in 100% methanol for 2 min, stained in 0.5% crystal violet for 2 min, rinsed in PBS and examined using a bright-field microscope. Values for invasion were obtained by counting five fields per membrane and represented as the average of three independent experiments performed over multiple days.
Fatty acid synthase activity assay
The FAS activity was determined spectrophotometrically at 37°C in particle-free supernatants by measuring the decrease of absorption at 340 nm due to oxidation of NADPH.
Immunoblot analysis
Cells in RPMI 1640 medium were treated with DMSO (control) or HO-3867 (10 μM) for 24 h. Equal volumes of DMSO (0.1% v/v) were present in each treatment. Following treatment, the cell lysates were prepared in nondenaturing lysis buffer containing 10-mM Tris-HCl (pH 7.4), 150-mM NaCl, 1% Triton X-100, 1-mM EDTA, 1-mM EGTA, 0.3-mM phenylmethylsulfonyl fluoride, 0.2-mM sodium orthovanadate, 0.5% NP40, 1-μg/ml aprotinin, and 1-μg/ml leupetin. The lysates were centrifuged at 10,000×g for 20 min at 4°C, and the supernatant was separated. The protein concentration in the lysates was determined using a Pierce detergent-compatible protein assay kit. For Western blotting, 25 to 50 μg of protein lysate per sample was denatured in 2× SDS-PAGE sample buffer and subjected to SDS-PAGE on a 10% tris-glycine gel. The separated proteins were transferred to a PVDF membrane and blocked with 5% nonfat milk powder (w/v) in TBST (10-mM Tris, 10-mM NaCl, 0.1% Tween 20) for 1 h at room temperature or overnight at 4°C. The membranes were then incubated with the primary antibodies. The bound antibodies were detected with horseradish peroxidase (HRP)-labeled sheep anti-mouse IgG or HRP-labeled donkey anti-rabbit IgG using an enhanced chemiluminescence detection system (ECL Advanced kit). Protein expressions were determined using Image Gauge version 3.45.
Reverse Transcription-PCR
Total RNA isolated from ovarian tumor tissue was prepared with TRIzol (Life Technologies, Grand Island, New York) according to the manufacturer’s instructions. RNA quantification was done using spectrophotometry. Reverse transcription (RT)-PCR analysis for the mRNA expressions in FAS, FAK, VEGF and p21 and the internal control GAPDH was carried out using a GeneAmp PCR System Veriti thermo cycler (Applied Biosystems, Foster City, CA) under the following conditions: initial denaturation at 94°C for 2 min, 35 cycles of amplification (denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 30 s), and extension at 72°C for 5 min. The PCR products were electrophoresed on 1.5% agarose gel and stained with ethidium bromide.
Ovarian cancer tumor xenografts in mice
A2780 cells (5×106 cells in 60 μl of PBS) were subcutaneously (s.c.) injected into the back of 6-week-old BALB/c nude mice from the National Cancer Institute. On the 5th day when the tumor size reached approximately 2 to 4 mm, the control groups was supplemented a normal diet (no treatment) while the experimental groups were treated using the DAP compounds mixed with the animal feed (Harlan Teklad) at 2 different levels (500 and 100 ppm). The doses were chosen based on an initial dose-response study optimized to produce an observable effect on tumor growth. The tumor tissues were then subjected to immunoblotting and immunohistochemistry.
Immunohistochemistry
Tumor tissues were fixed in formalin and embedded in paraffin. Sections (6-μm thick) were obtained and used for hematoxylin and eosin staining. For immunofluorescence staining, the tissue sections (8-μm thick) were serially rehydrated in 100%, 95%, and 80% ethanol after deparaffinization with xylene. Slides were kept in steam for 30 min and then washed in PBS (pH 7.4) three times for 5 min each. Tissue sections were incubated with 2% goat serum and 5% bovine serum albumin in PBS to reduce nonspecific binding. The sections were then incubated for 4 h with an anti-mouse anti-FAS, or anti-VEGF. The sections were then incubated with secondary antibodies (1:1000 dilutions) conjugated to horseradish peroxidase (HRP)-labeled sheep anti-mouse IgG or HRP-labeled donkey anti-rabbit IgG (Amersham Pharmacia Biotech). The tissue slides were visualized using a Nikon fluorescence microscope.
Data analysis
The statistical significance of the results was evaluated using Student’s t-test. A p value of less than 0.05 was considered significant.
Results
Effect of HO-3867 on ovarian cancer cell migration and invasion
The effect of HO-3867 on the motility of ovarian cancer cells was measured by wound-healing migration and Transwell cell-invasion assays. Incubation of A2780 or SKOV3 cells with HO-3867 (10 μM) for 24 hours showed significant inhibition of cell migration (Figure 1A) and invasion (Figure 1B) when compared to untreated cells. Since VEGF-induced angiogenesis is initiated by cell migration and invasion, we next determined whether HO-3867 could inhibit the cell motility-promoting effect of VEGF. We observed that HO-3867 significantly inhibited the VEGF-induced migration and invasion of both the ovarian cancer cell lines tested (Figure 1C). The results suggested that HO-3867 could not only inhibit ovarian cancer cell migration and invasion, but also block VEGF-induced angiogenesis.
Figure 1. HO-3867 inhibits cancer cell migration and invasion.
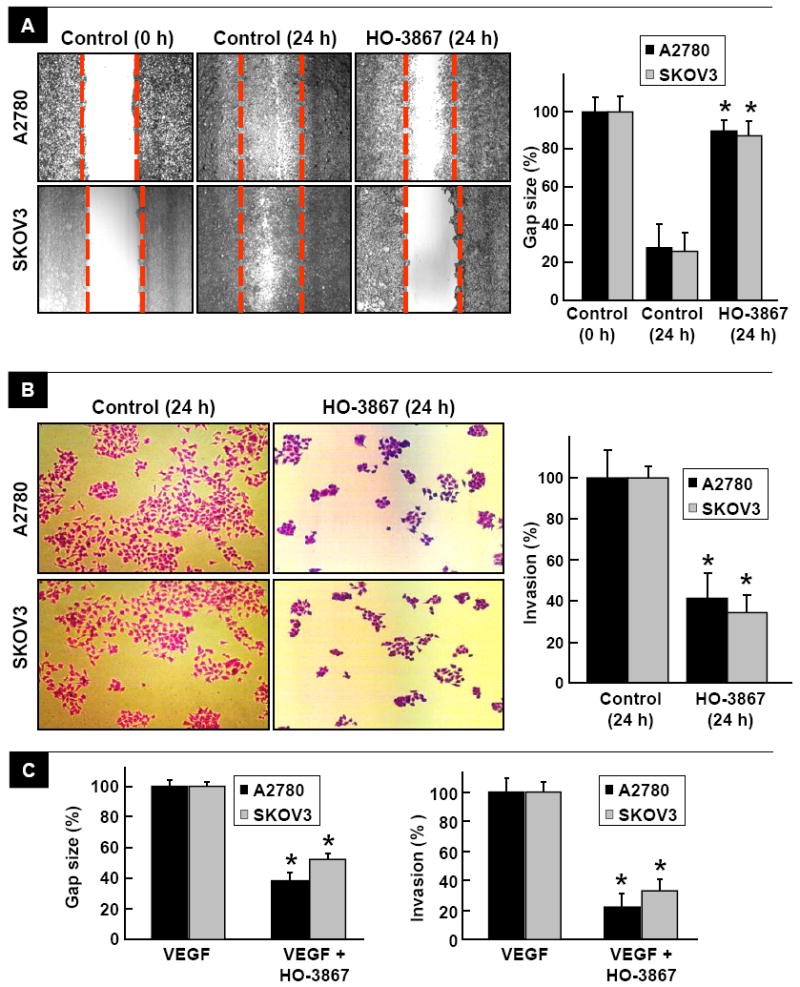
Cell-migration (wound healing) assay was performed by Transwell cell-invasion assay using A2780 and SKOV3 cancer cells at 0 and 24 h, and in the presence of HO-3867 (10 μM) at 24 h. (A) A representative image of six experiments is shown for each group. Gap size and cell invasion were quantified in the regions flanked by dotted lines. The residual gap between the migrating cells from the opposing edges is expressed as a percentage of the initial, scraped area. Data represent mean±SE (N=6); *p<0.05 versus Control (24 h). The migration results show that HO-3867 significantly inhibited the reduction in gap size caused by cell migration. (B) Inhibition of A2780 and SKOV3 cell invasion by HO-3867 (10 μM) at 24 h using a Boyden chamber migration assay. Representative images selected from six experiments are shown for each group. Quantification of cell invasion expressed as a percent of Control. Data represent mean±SE (N=6); *p<0.05 versus Control (24 h). The invasion results show that HO-3867 significantly inhibited cell invasion. (C) Quantitation of the effect of HO-3867 (10 μM; 24-h incubation) on gap-size and cell-invasion of VEGF-induced cell migration and invasion in A2780 and SKOV3 cells. Data represent mean±SE (N=6); *p<0.05 versus VEGF group. The results show that HO-3867 significantly inhibited the effect of VEGF on cell migration and invasion.
Effect of FAS and FAK on ovarian cancer cell migration and invasion
We observed that FAS and FAK proteins were significantly expressed in all six human ovarian cancer cell lines tested, including the cisplatin-resistant cancer cell line A2780R (Figure 2A). We next determined the effect of FAS and FAK inhibition by using their siRNA transfection on the migration and invasion of A2780 cells. Cells transfected with FAS siRNA or FAK siRNA exhibited significant reduction in migration and invasion when compared to control cells. The results suggested that both FAS and FAK are involved in ovarian cancer cell migration and invasion.
Figure 2. FAS and FAK are involved in cancer cell migration and invasion.
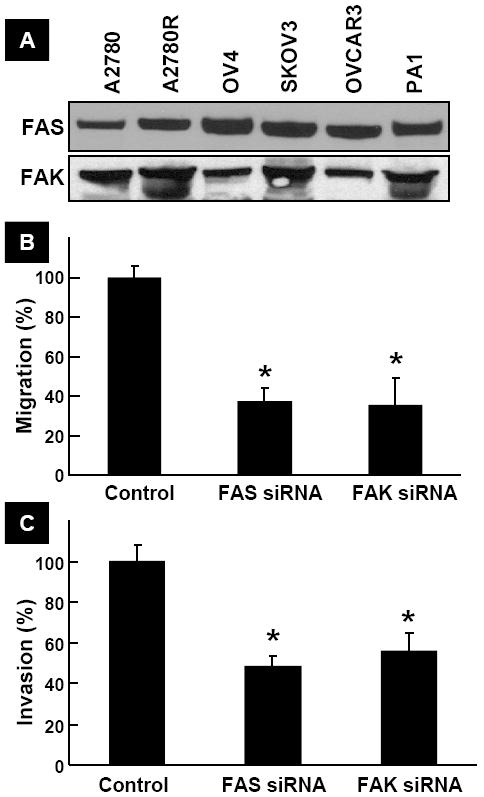
(A) Basal levels of FAS and FAK expression in human ovarian cancer cell lines. (B) Effect of silencing of FAS and FAK in A2780 cells by transfection of FAS siRNA and FAK siRNA, respectively, on cell migration expressed as a percent of Control. Data represent mean±SE (N=6); *p<0.05 versus Control. (C) Effect of silencing of FAS and FAK in A2780 cells by transfection of FAS siRNA and FAK siRNA, respectively, on cell invasion expressed as a percent of Control. Data represent mean±SE (N=6); *p<0.05 versus Control. The results show that silencing of FAS and FAK significantly inhibited cell migration and invasion.
Effect of HO-3867 on FAS and FAK expression in ovarian cancer cells
We next determined whether the inhibitory effect of HO-3867 on the ovarian cancer cell migration and invasion was due to its regulation of FAS and FAK expression levels. Incubation of A2780 or SKOV3 cells with HO-3867 (10 μM) resulted in an incubation-time-dependent inhibition of FAS and FAK, both at the protein and expression levels (Figure 3A/B). We further checked the activity of FAS in cells treated with HO-3867 for 6, 12 and 24 h. The FAS activity was significantly reduced in both the cell lines up on incubation with HO-3867 for 12 or 24 h (Figure 3C). The results suggested that HO-3867 inhibited the expression of FAS and FAK in the ovarian cancer cells.
Figure 3. HO-3867 inhibits FAS and FAK expression in ovarian cancer cells.
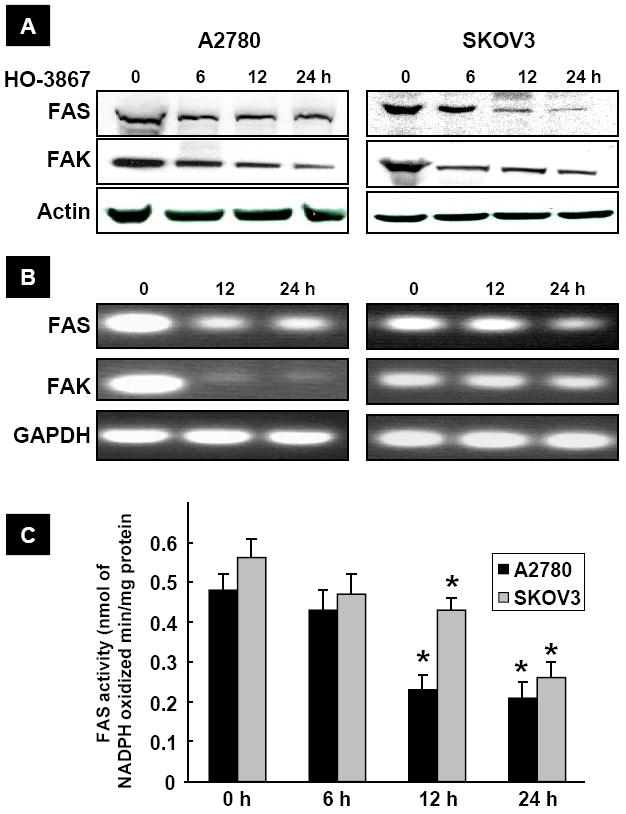
(A) Representative Western blots showing a time-dependent inhibition of FAS and FAK levels in A2780 and SKOV3 cells treated with HO-3867 (10 μM). (B) Expression levels of FAS and FAK mRNA following HO-3867 exposure (10 μm for 24 h). (C) FAS activity measured in A2780 and SKOV3 cells using NADPH by spectrophotometry. *p<0.05 versus respective control (0 h). The results show that HO-3867 significantly inhibited FAS and FAK expressions in cancer cells.
Effect of HO-3867 on FAS and FAK downregulation by proteasome pathways
It is known that the intracellular amount of rapid-turnover proteins, such as FAS and FAK, is tightly regulated by the ubiquitin-dependent proteolytic pathway (20). The fast and significant decrease in the FAS and FAK expressions observed in the HO-3867-treated cells prompted us to check any involvement of ubiquitin-dependent degradation mechanism. The isopeptidase USP2a has been shown to regulate the stability of FAS in prostate cancer (20). We analyzed the USP2a level in HO-3867-treated ovarian cancer cells and observed that it was clearly downregulated by HO-3867 (Figure 4). We further observed an enhanced polyubiquitination on FAS and FAK in the HO-3867-treated cancer cells, under the condition of co-incubation with MG-132, a proteasome inhibitor. The results strongly suggested that HO-3867 inhibited FAS/FAK through the ubiquitination and inhibited FAS stability through USP2a.
Figure 4. HO-3867 downregulates FAS and FAK levels via proteasome pathways.
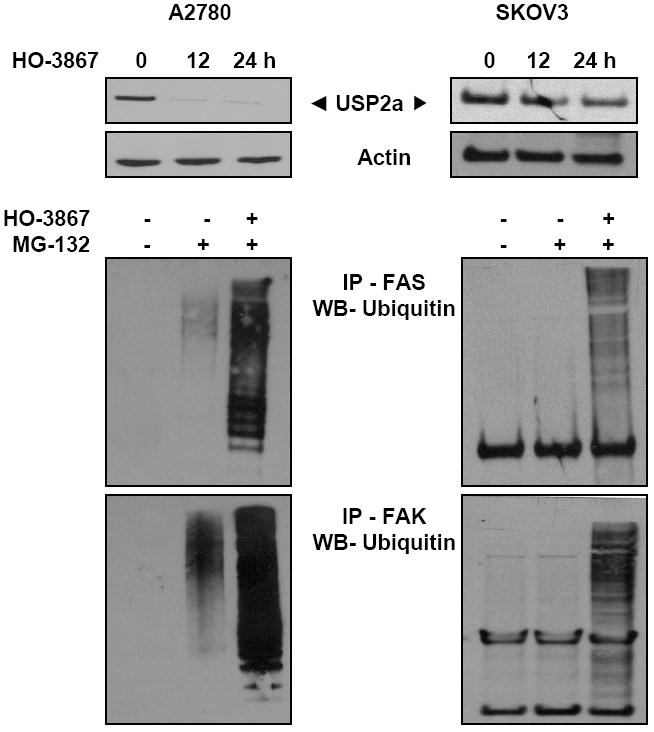
To catch any ubiquitinated proteins in the cell lysates, agarose beads coated with domains having affinity to ubiquitin were incubated in the lysates at 4°C for 2 hours. After washing the beads, the ubiquitinated proteins were subjected to immunoblot for FAS and FAK and blotted by the ubiquitin antibody. A predominant ubiquitination of FAS and FAK is seen in the HO-3867-treated A2780 and SKOV3 cells under proteasomal inhibition using MG-132 (50 μM). The results show that HO-3867 clearly downregulated the FAS stability protein of USP2a in a time-dependent manner.
Effect of HO-3867 on FAS/FAK regulatory genes
We determined the effect of HO-3867 on the FAS and FAK-regulatory cell-migration and invasion genes. HO-3867 inhibited pHER2, pERK1/2, SREBP1, MMP-2, and VEGF in A2780 and SKOV3 cells (Figure 5). The results suggested that HO-3867 not only inhibited FAS and FAK expression, but also blocked their regulating genes in the two ovarian cancer cell lines tested.
Figure 5. HO-3867 downregulates FAS/FAK regulatory genes.
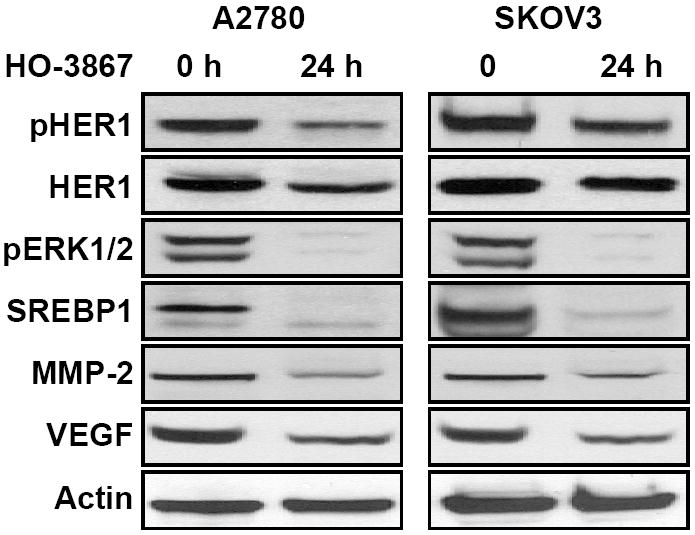
A2780 and SKOV3 cells were treated with HO-3867 (10 μM; 24 h) followed by blotting of FAS/FAK-regulating proteins HER1, SREBP1, ERK1/2, MMP-2 and VEGF. The results show that HO-3867 at 24-h incubation clearly down-regulated the FAS/FAK-regulating proteins in both ovarian cancer cell lines.
Effect of HO-3867 on FAS/FAK levels in tumor tissues
Finally, we analyzed the FAS, FAK and their regulatory protein expression levels in an in vivo xenograft mouse model of ovarian cancer. We observed, in concert with the decreased expression of both FAS and FAK, a clear down-regulation in the target gene products of FAS and FAK, namely SREBP1, MMP-2, and VEGF in the xenografted tumor tissues of the HO-3867-treated mice, in a dose-dependent manner (Figure 6A/B). We further determined the FAS and VEGF levels by immunocytochemistry. HO-3867 treatment significantly inhibited the protein levels of FAS and VEGF in tumor-bearing mice (Figure 5C). The in vivo results suggested that administration of HO-3867 inhibited tumor-growth through the inhibition of migration- and invasion-regulatory genes such as FAS and FAK in ovarian cancer xenografts in mice.
Figure 6. HO-3867 suppresses FAS/FAK and VEGF levels in tumor tissues.
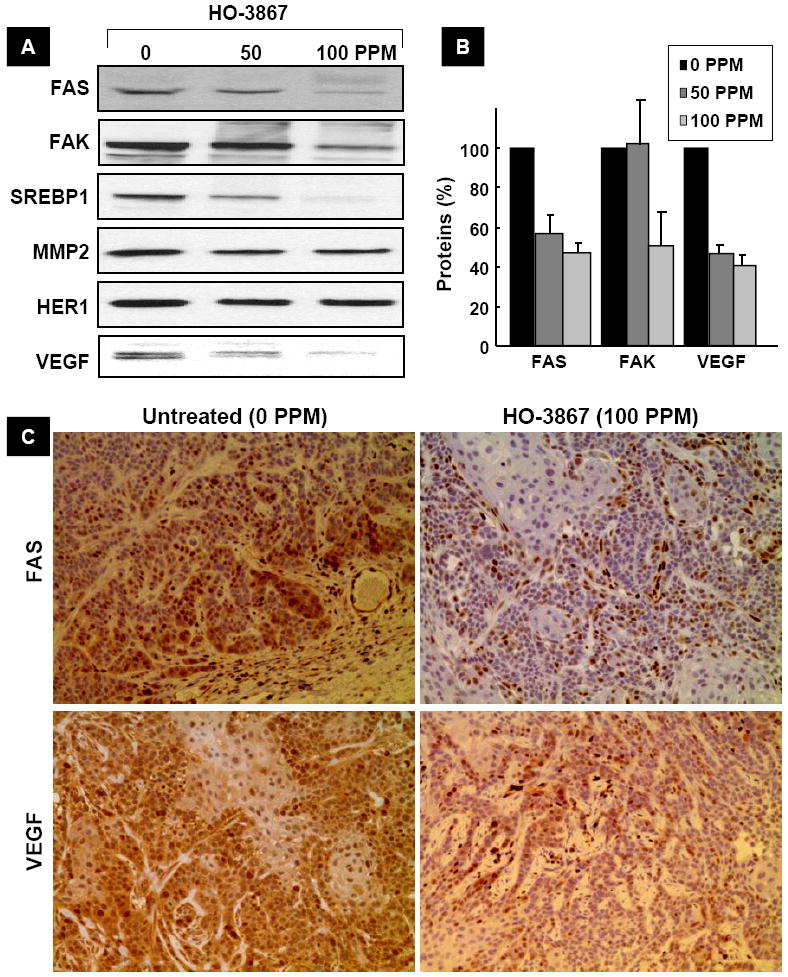
(A) Tissue lysates containing 50 mg protein of A2780 xenograft tumors from mice treated with 50 or 100 ppm HO-3867, containing 50-μg protein each, were subjected to immunoblot analyses. The decreased expression of FAS, FAK, and VEGF levels are noted in the HO-3867-treated tumor lysates, in a dose-dependent manner. (B) Quantitative results of FAS, FAK and VEGF bands by densitometric analysis. Data represent mean±SE (N=3). *p<0.05 versus untreated (0 ppm) group. (C) Immunohistochemistry showing decreased expression of FAS and VEGF levels in the tumor tissues. The results show that HO-3867-treatment to mice suppressed FAS, FAK, and VEGF levels in the tumor.
Discussion
The results of the present study have shown for the first time that HO-3867 attenuates cancer cell migration and invasion through inhibition of FAS and FAK expression in ovarian cancer cell lines as well as in ovarian tumor xenografts in mice. The results confirmed that FAS and FAK are in deed necessary for the motility of the ovarian cancer cells and that HO-3867 suppresses the levels of both FAS and FAK by promoting ubiquitination-dependent degradation process and inhibiting FAS-stabilizing genes of USP2a. HO-3867 also significantly inhibited the FAS activity, mRNA levels, and downstream proteins pERK1/2, pHER1, SREBP1, VEGF, and MMP-2. The study further confirmed the inhibition of FAS, FAK, and downstream proteins in tumor tissues obtained from A2780 xenograft tumor-bearing mice treated with HO-3867. Collectively, the study established that HO-3867 is capable of suppressing the migration and invasion of the ovarian cancer cells by inhibiting the expression/activity of FAS and FAK proteins.
Cell migration and invasion are vital elements involved in numerous physiological and pathological processes, including angiogenesis and metastasis (2, 3). The high mortality rate among ovarian cancer patients is attributed not only to a lack of early detection and treatment, but also to the highly invasive (metastatic) nature of the disease (8). The poor prognosis associated with the treatment of ovarian cancer is mainly due to the late stage of disease with metastasis at presentation. Particularly, malignant ovarian surface epithelial cells primarily spread to adjacent organs by local invasion. The significant failure rate of chemotherapy in ovarian cancer patients with advanced stage of metastatic disease is also a main concern, suggesting cell motility as a potential therapeutic target for ovarian cancer treatment. In the present study, for the first time, we showed that HO-3867 acts as an effective blocker of ovarian cancer cell migration and invasion through inhibition of motility-promoting proteins including FAS, FAK, and VEGF.
Fatty acid synthase (FAS) is a metabolic enzyme involved in the synthesis of long-chain saturated fatty acids that are essential for membrane synthesis in proliferating cells. FAS is overexpressed in many human cancers including the carcinomas of the breast (21), prostate (22), stomach (23), lung (24), ovary (25, 26), and mesothelioma (27). The fact that overexpression of FAS is more pronounced in the clinically aggressive cancers (26) suggest a functional role for FAS in the progression of malignant cancer (9, 12). Inhibition of FAS activity preferentially attenuates tumor-cell growth by inducing apoptosis through inactivation of pAkt and dephosphorylation of Bad in ovarian cancer (12, 28, 29). Since FAS appears to provide a selective advantage to tumor progression, FAS has become a promising target for anticancer drug development. Several studies have shown that blocking of FAS activity using pharmacologic inhibitors of FAS such as Cerulenin and C75 attenuated carcinomas of the breast (29, 30), renal (31), colon (32), and liver (33). The present study shows that HO-3867 is capable of inhibiting both the expression and activity of FAS in A2780 and SKOV3 cells resulting in the attenuation of their ability to migrate and invade.
FAK is a focal adhesion-associated protein kinase involved in cellular adhesion and spreading processes. It serves as a key protein in the regulation of focal adhesion dynamics (34). FAK is a critical mediator of integrin adhesion turnover that promote cell migration (35). Several studies have shown the requirement of FAK-signaling in promoting invasiveness of cancer cells (36, 37). Like FAS, overexpression of FAK has also been found in most ovarian tumors, where it is shown to be associated with high aggressiveness and poor patient survival (4, 11). A recent report has highlighted a possible inverse correlation between FAK expression and clinical outcome (5). Therefore, FAK is an attractive target for ovarian cancer therapeutics/preventions. In the present study, we observed that HO-3867 was capable of inhibiting FAK expression in the ovarian cancer cell lines tested.
Although the precise mechanism of FAS degradation have not yet been fully understood, it is evident that ubiquitination is involved in the pHO-3867-mediated proteasomal degradation of FAS. A recent report showed that the isopeptidase USP2a is a preproteasomal, androgen-regulated isopeptidase and is a key regulator of prostate cancer cell survival through the stabilization of FAS (20). FAS has been shown to colocalize and physically interact with USP2a in cancer cells suggest that this isopeptidase rescues FAS from degradation and thereby prevents apoptosis (38). Of interest, the rapid downregulation in FAS was consistent with accelerated ubiquitin-dependent degradation. This effect was further confirmed by the observation that the proteasome inhibitor MG132 blocked the HO-3867-induced FAK degradation. There are also several evidences in the published reports that shows FAK is tightly regulated by ubiquitin pathways (39, 40). In addition, we observed that HO-3867 significantly suppressed the FAS-regulating genes such as, pHER, SREBP1, pERK1/2, VEGF, and MMP-2 expression. Similar to our results, a coordinated regulation of SREBP1 and FAS has been observed in clinical breast cancer (41).
The present results clearly indicated an increased degradation and decreased transcription of FAS/FAK up on treatment with HO-3867. While degradation of FAS/FAK proteins occurred in 6-12 hours of treatment (6-hour data not shown), inhibition of FAS/FAK-regulating genes HER1, SREBP1 and ERK1/2 was observed only at 24 hours suggesting that HO-3867 works by inducing degradation FAS/FAK proteins as well as by inhibiting FAS/FAK expression, but at different time points.
A recent study from our laboratory has shown that HO-3867 inhibits STAT3/JAK pathway in a wide range of human cancer cells, including ovarian cancer (17, 42). Activated STAT3 has a significant role in the metastatic progression of ovarian cancer and important roles in promoting cell proliferation, cell survival, migration and invasion in human cancer (43). Inhibition of STAT3/JAK has been shown to be involved in colorectal cancer cell growth, survival, invasion, and migration through regulation of gene expression, such as Bcl-2, p21, VEGF, and MMPs (44). The FAS inhibitor C75 has been shown to induce suppression of invasiveness and migration of renal carcinoma cells through concurrent inhibition of FAS and STAT3 (31). Interestingly, HO-3867 also showed similar inhibitory effect toward FAS STAT3 in human ovarian cancer cells. However, further investigation is necessary to determine the exact mechanisms involved in the anticancer activity of HO-3867.
FAS and the fatty-acid-synthesis pathway have been explored as a potential drug target for cancer therapy (9). Cerulenin, a natural product, was the first specific inhibitor of FAS to be studied (45), while C75, a synthetic compound, was shown to be a more potent inhibitor of FAS (46 87). Both the inhibitors have been evaluated in a variety of human cancer cell lines and xenograft tumors (14, 27, 47). Despite its measurable effect on a human cancer xenograft, cerulenin was chemically unstable, thus precluding its use as a systemic anticancer drug. Further, cerulenin induced reversible weight loss in mice (14, 48). C75 too was found to induce substantial weight loss via the induction of anorexia (eating disorder) in mice (47, 48). Recently, C93, a rationally designed molecule that inhibits FAS activity without affecting fatty-acid oxidation has been reported to inhibit chemically induced lung tumor and in preclinical models of lung cancer (12, 49, 50). Very recently, we have evaluated the efficacy of HO-3867 treatment in an in vivo model of human-ovarian-tumor xenograft in mice (17). We observed a significant reduction in tumor-growth volume without any adverse effect on body weight or diet consumption suggesting that the in vivo antitumor efficacy of HO-3867 against ovarian cancer is without any apparent signs of toxicity.
The present study provides the first evidence that HO-3867 inhibits the migration and invasion of ovarian cancer cells through downregulation of FAS and FAK. The study suggested that molecular targeting of FAS and FAK by HO-3867 might be a potential strategy for ovarian cancer therapy.
Acknowledgments
The study was supported by grants NIH CA102264 (PK), Kaleidoscope of Hope Foundation Grant (KS), and Hungarian Research Fund Grant OTKA T048334 (KH)
Grant support NIH CA102264 (PK); Kaleidoscope of Hope Foundation Grant (KS) Hungarian Research Fund Grant OTKA T048334 (KH)
Abbreviations
- A2780
human ovarian cancer cell line
- DAP
diarylidenyl piperidone
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ROS
reactive oxygen species
- STAT3
signal-transducer and activator of transcription 3
- JAK
Janus kinase
- FAS
fatty acid synthase
- FAK
focal adhesion kinase
References
- 1.Eccles SA, Box C, Court W. Cell migration/invasion assays and their application in cancer drug discovery. Biotechnol Annu Rev. 2005;11:391–421. doi: 10.1016/S1387-2656(05)11013-8. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 4.Golubovskaya VM, Kweh FA, Cance WG. Focal adhesion kinase and cancer. Histol Histopathol. 2009;24:503–510. doi: 10.14670/HH-24.503. [DOI] [PubMed] [Google Scholar]
- 5.Luo M, Guan JL. Focal adhesion kinase: A prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett. 2009 doi: 10.1016/j.canlet.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 7.Kornberg LJ. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck. 1998;20:745–752. doi: 10.1002/(sici)1097-0347(199812)20:8<745::aid-hed14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 9.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 10.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 11.Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, Kamat AA, Han LY, Kim TJ, Lu C, Tari AM, Bornmann W, Fernandez A, Lopez-Berestein G, Sood AK. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–10983. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, Ronnett GV, Townsend CA, Kuhajda FP. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 13.Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005;94:1–4. doi: 10.1002/jcb.20310. [DOI] [PubMed] [Google Scholar]
- 14.Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189–1193. [PubMed] [Google Scholar]
- 15.Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK, Hideg K, Kuppusamy P. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282:28609–28618. doi: 10.1074/jbc.M703796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvendiran K, Kuppusamy ML, Bratasz A, Tong L, Rivera BK, Rink C, Sen CK, Kalai T, Hideg K, Kuppusamy P. Inhibition of vascular smooth-muscle cell proliferation and arterial restenosis by HO-3867, a novel synthetic curcuminoid, through up-regulation of PTEN expression. J Pharmacol Exp Ther. 2009;329:959–966. doi: 10.1124/jpet.108.150367. [DOI] [PubMed] [Google Scholar]
- 17.Selvendiran K, Tong L, Bratasz A, Kuppusamy ML, Ahmed S, Ravi Y, Trigg NJ, Rivera BK, Kalai T, Hideg K, Kuppusamy P. Anticancer Efficacy of a Difluorodiarylidenyl Piperidone (HO-3867) in Human Ovarian Cancer Cells and Tumor Xenografts. Mol Cancer Ther. 2010;9:1169–1179. doi: 10.1158/1535-7163.MCT-09-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalai T, Kuppusamy P, Hideg K. Synthesis, characterization and structure-activity studies with a novel class of diarylidenylpiperidones (to be published) [Google Scholar]
- 19.Yap LF, Jenei V, Robinson CM, Moutasim K, Benn TM, Threadgold SP, Lopes V, Wei W, Thomas GJ, Paterson IC. Upregulation of Eps8 in oral squamous cell carcinoma promotes cell migration and invasion through integrin-dependent Rac1 activation. Oncogene. 2009;28:2524–2534. doi: 10.1038/onc.2009.105. [DOI] [PubMed] [Google Scholar]
- 20.Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ, Lechpammer M, Huesken D, Zimmermann J, Signoretti S, Loda M. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004;5:253–261. doi: 10.1016/s1535-6108(04)00055-8. [DOI] [PubMed] [Google Scholar]
- 21.Alo PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer. 1996;77:474–482. doi: 10.1002/(SICI)1097-0142(19960201)77:3<474::AID-CNCR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 22.Epstein JI, Carmichael M, Partin AW. OA-519 (fatty acid synthase) as an independent predictor of pathologic state in adenocarcinoma of the prostate. Urology. 1995;45:81–86. doi: 10.1016/s0090-4295(95)96904-7. [DOI] [PubMed] [Google Scholar]
- 23.Kusakabe T, Nashimoto A, Honma K, Suzuki T. Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology. 2002;40:71–79. doi: 10.1046/j.1365-2559.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 24.Piyathilake CJ, Frost AR, Manne U, Bell WC, Weiss H, Heimburger DC, Grizzle WE. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol. 2000;31:1068–1073. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- 25.Alo PL, Visca P, Framarino ML, Botti C, Monaco S, Sebastiani V, Serpieri DE, Di Tondo U. Immunohistochemical study of fatty acid synthase in ovarian neoplasms. Oncol Rep. 2000;7:1383–1388. doi: 10.3892/or.7.6.1383. [DOI] [PubMed] [Google Scholar]
- 26.Gansler TS, Hardman W, 3rd, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol. 1997;28:686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 27.Gabrielson EW, Pinn ML, Testa JR, Kuhajda FP. Increased fatty acid synthase is a therapeutic target in mesothelioma. Clin Cancer Res. 2001;7:153–157. [PubMed] [Google Scholar]
- 28.Zhou W, Simpson PJ, McFadden JM, Townsend CA, Medghalchi SM, Vadlamudi A, Pinn ML, Ronnett GV, Kuhajda FP. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003;63:7330–7337. [PubMed] [Google Scholar]
- 29.Menendez JA, Vellon L, Mehmi I, Oza BP, Ropero S, Colomer R, Lupu R. Inhibition of fatty acid synthase (FAS) suppresses HER2/neu (erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad Sci U S A. 2004;101:10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alli PM, Pinn ML, Jaffee EM, McFadden JM, Kuhajda FP. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene. 2005;24:39–46. doi: 10.1038/sj.onc.1208174. [DOI] [PubMed] [Google Scholar]
- 31.Horiguchi A, Asano T, Asano T, Ito K, Sumitomo M, Hayakawa M. Pharmacological inhibitor of fatty acid synthase suppresses growth and invasiveness of renal cancer cells. J Urol. 2008;180:729–736. doi: 10.1016/j.juro.2008.03.186. [DOI] [PubMed] [Google Scholar]
- 32.Li JN, Gorospe M, Chrest FJ, Kumaravel TS, Evans MK, Han WF, Pizer ES. Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res. 2001;61:1493–1499. [PubMed] [Google Scholar]
- 33.Gao Y, Lin LP, Zhu CH, Chen Y, Hou YT, Ding J. Growth arrest induced by C75, A fatty acid synthase inhibitor, was partially modulated by p38 MAPK but not by p53 in human hepatocellular carcinoma. Cancer Biol Ther. 2006;5:978–985. doi: 10.4161/cbt.5.8.2883. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Hua ZC. FAK expression regulation and therapeutic potential. Adv Cancer Res. 2008;101:45–61. doi: 10.1016/S0065-230X(08)00403-X. [DOI] [PubMed] [Google Scholar]
- 35.Chu PY, Huang LY, Hsu CH, Liang CC, Guan JL, Hung TH, Shen TL. Tyrosine phosphorylation of growth factor receptor-bound protein-7 by focal adhesion kinase in the regulation of cell migration, proliferation, and tumorigenesis. J Biol Chem. 2009;284:20215–20226. doi: 10.1074/jbc.M109.018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 37.van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Priolo C, Tang D, Brahamandan M, Benassi B, Sicinska E, Ogino S, Farsetti A, Porrello A, Finn S, Zimmermann J, Febbo P, Loda M. The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Res. 2006;66:8625–8632. doi: 10.1158/0008-5472.CAN-06-1374. [DOI] [PubMed] [Google Scholar]
- 39.Kim B, van Golen CM, Feldman EL. Degradation and dephosphorylation of focal adhesion kinase during okadaic acid-induced apoptosis in human neuroblastoma cells. Neoplasia. 2003;5:405–416. doi: 10.1016/s1476-5586(03)80043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang YA, Morin PJ, Han WF, Chen T, Bornman DM, Gabrielson EW, Pizer ES. Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element binding protein-1c. Exp Cell Res. 2003;282:132–137. doi: 10.1016/s0014-4827(02)00023-x. [DOI] [PubMed] [Google Scholar]
- 42.Selvendiran K, Ahmed S, Dayton A, Kuppusamy ML, Tazi M, Bratasz A, Tong L, Rivera BK, Kalai T, Hideg K, Kuppusamy P. Safe and targeted anticancer efficacy of a novel class of antioxidant-conjugated difluorodiarylidenyl piperidones: differential cytotoxicity in healthy and cancer cells. Free Radic Biol Med. 2010;48:1228–1235. doi: 10.1016/j.freeradbiomed.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devarajan E, Huang S. STAT3 as a central regulator of tumor metastases. Curr Mol Med. 2009;9:626–633. doi: 10.2174/156652409788488720. [DOI] [PubMed] [Google Scholar]
- 44.Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ, Lu R, Chen YX, Fang JY. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, Pasternack GR. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci U S A. 1994;91:6379–6383. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizer ES, Thupari J, Han WF, Pinn ML, Chrest FJ, Frehywot GL, Townsend CA, Kuhajda FP. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- 48.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 49.Orita H, Coulter J, Lemmon C, Tully E, Vadlamudi A, Medghalchi SM, Kuhajda FP, Gabrielson E. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. 2007;13:7139–7145. doi: 10.1158/1078-0432.CCR-07-1186. [DOI] [PubMed] [Google Scholar]
- 50.Orita H, Coulter J, Tully E, Kuhajda FP, Gabrielson E. Inhibiting fatty acid synthase for chemoprevention of chemically induced lung tumors. Clin Cancer Res. 2008;14:2458–2464. doi: 10.1158/1078-0432.CCR-07-4177. [DOI] [PubMed] [Google Scholar]


