Abstract
Objective
We conducted this study to evaluate the clinical impact of early enteral nutrition (EN) on in-hospital mortality and outcome in patients with critical hypertensive intracerebral hemorrhage (ICH).
Methods
We retrospectively analyzed 123 ICH patients with Glasgow Coma Scale (GCS) score of 3-12. We divided the subjects into two groups : early EN group (< 48 hours, n = 89) and delayed EN group (≥ 48 hours, n = 34). Body weight, total intake and output, serum albumin, C-reactive protein, infectious complications, morbidity at discharge and in-hospital mortality were compared with statistical analysis.
Results
The incidence of nosocomial pneumonia and length of intensive care unit stay were significantly lower in the early EN group than in the delayed EN group (p < 0.05). In-hospital mortality was less in the early EN group than in the delayed EN group (10.1% vs. 35.3%, respectively; p = 0.001). By multivariate analysis, early EN [odds ratio (OR) 0.229, 95% CI : 0.066-0.793], nosocomial pneumonia (OR = 5.381, 95% CI : 1.621-17.865) and initial GCS score (OR = 1.482 95% CI : 1.160-1.893) were independent predictors of in-hospital mortality in patients with critical hypertensive ICH.
Conclusion
These findings indicate that early EN is an important predictor of outcome in patients with critical hypertensive ICH.
Keywords: Enteral nutrition, Intracerebral hemorrhage, Mortality
INTRODUCTION
Nutritional support of critically ill patients is very important and should be considered a conerstone in critical care. Patients with brain injury commonly suffer from metabolic alterations that trigger increased energy and protein expenditure than patients without brain injury. Enteral nutrition (EN) decreases the catabolic response to injuries17) and reduces infection rate and hospital stay in patients with such injuries11,13,23,27,28). However, there are only a few studies specifically addressing the influence of early EN on outcome of hypertensive intracerebral hemorrhage (ICH). We investigated the clinical implication of early EN in critical patients with ICH.
MATERIALS AND METHODS
We retrospectively analyzed on 123 consecutive patients harboring critical hypertensive ICH with Glasgow Coma Scale (GCS) score of 3-12. All these patients had been admitted to our institution during the recent 6 years between January 2004 and December 2009. ICH was initially diagnosed by brain computed tomography (CT) scans in the emergency setting, and all of them were admitted to the intensive care unit (ICU). Parental fluids were supplied in traditional manners, and after vital signs became stabilized, enteral nutritional support was provided as soon as possible.
The subjects were classified as early EN group (< 48 hours) and delayed EN group (≥ 48 hours). We reviewed medical records and radiological data, and collected baseline characteristics including age, sex, locations of ICH, hematoma volume, initial GCS score, length of ICU stay, modified Rankin scale37) at discharge, 30-day mortality, in-hospital mortality. Additionally, possible confounding variables including current smoking, alcohol consumption, previous stroke, diabetes, heart diseases were retrieved. We also reviewed data regarding enteral nutritional including the time interval to EN after admission, body weight, total intake and output, feeding method, aspiration episodes during enteral feeding, serum albumin, C-reactive protein (CRP), calories and diet volume on day 4, because similar amount and calories have been provided in 2 groups after about 4 days23). We compared morbidity at discharge, in-hospital mortality and infectious complications including nosocomial pneumonia, urinary tract infection and sepsis between two groups.
For the purpose of this study, each parameters were defined as follows : Critical hypertensive ICH was defined as hypertensive ICH with GCS score of 3-12; early EN was defined as enteral feeding within 48 hours of admission; diabetes, a previous medical history of diabetes or fasting glucose value > 7.78 mmol/L at admission; previous strokes, history of cerebral infarction except for transient ischemic attack; heart diseases, only coronary artery diseases; current smoking, daily use of > 10 cigarettes during previous 6 months; alcohol consumption, ingestion of > 700 g/week of alcohol during previous 2 months or a history of chronic alcoholism; noscocomial pneumonia was defined as a compatible chest X-ray seen interpreted by chest radiologist, as well as fever and yellow sputum from the tracheal tube with positive culture; urinary tract infection was defined as a positive urine culture (more than 100,000 colony forming units/mL); sepsis was defined as systemic inflammatory response syndrome with bacteriological evidence of infection2). Infectious complications were defined as nosocomial pneumonia, urinary tract infection and sepsis. Aspiration episodes during feeding was defined as a coughing episodes during feeding or coughing episodes by vomiting during or after feeding.
Statistical analysis
All variables were expressed as mean ± standard deviation (SD). Pearson χ2 or Fisher's exact test was used to assess proportions in nominal variables for bivariate analyses. To compare continuous variables between two groups, independent-samples t-test or Mann-Whitney U test were performed in distributions of parametric variables. Confounding variables with a value of p < 0.1 were included in the multivariate analysis model, and were categorized as quartiles for multivariate analysis. To identify the correlations between continuous variables, the Spearman correlation coefficient was used. To determine independent predictors of in-hospital mortality, multiple logistic regression analysis models (backward method) were constructed. Adjusted odds ratio (OR) with the respective 95% CI are provided. All p values are two-sided and considered as having significant difference when p < 0.05. All statistical analyses were conducted with SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
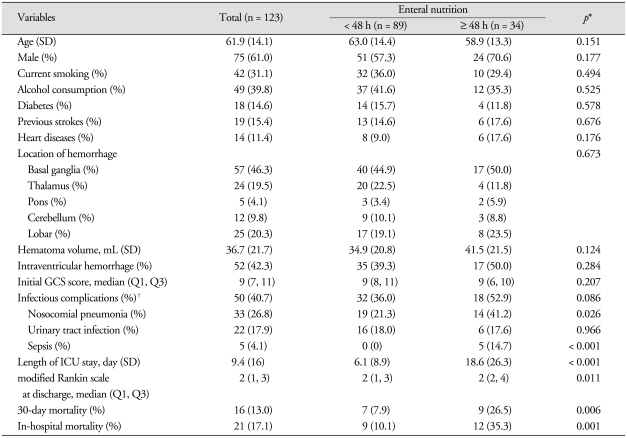
Baseline characteristics of 123 patients with ICH according to the time interval to EN were summarized in Table 1. Mean age was 61.9 (SD = 14.1) years old, and men outnumbered women by 75 (61.0%) to 48 (39.0%). The mean duration of in-hospital stay was 26.5 days (SD = 20.1). The early EN group was more predominating, in 89 patients (72.4%).
Table 1.
Baseline characteristics of 123 patients with critical intracerebral hemorrhage
*p value for differences between early enteral nutrition and delayed enteral nutrition after intracerebral hemorrahge; Pearson χ2 or Fisher's exact test, Student t-test, Mann-Whitney test as appropriate. †Infectious complications include nosocomial pneumonia, urinary tract infection and sepsis. GCS : Glasgow coma scale, ICU : intensive care unit, SD : standard deviation
There were no statistically significant differences in initial GCS score, hematoma volume and intraventricular hemorrhages on initial CT (all p > 0.05) scans between two groups. The incidence of both nosocomial pneumonia (21.3% vs. 41.2%; p = 0.026) and sepsis (0% vs. 14.7%; p < 0.001) were significantly lower in the early EN group than in the delayed EN group. Infectious complications occurred in 50 patients (40.7%), and were lower in the early EN group than in the delayed EN group (36.0% vs. 52.9%, respectively), but was not statistically significant (p = 0.086). Morbidity at discharge estimated by modified Rankin scale was significantly lower in the early EN group than in the delayed EN group (p = 0.011). In-hospital mortality was found in 21 patients (17.1%) and were significantly lower in the early EN group than in the delayed EN group (10.1% vs. 35.3%, respectively; p = 0.001). In addition, length of ICU stay was significantly shorter in the early EN group than in the delayed EN group (p < 0.001).
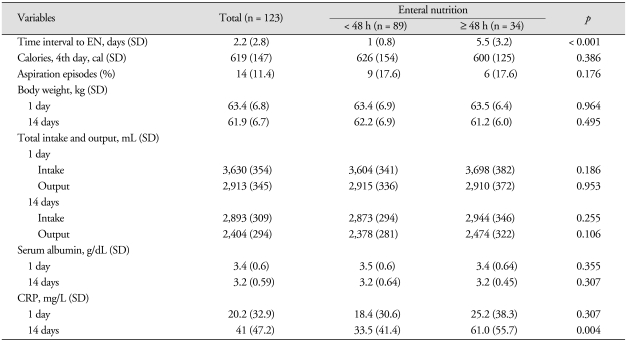
All patients were initially fed by nasogastric tube in the ICU. About 100-330 mL of formula was continuously administrated over 30 to 60 minutes q 3-6 hours. The mean time to enteral feeding after admission was 1.0 day (SD = 0.8) and 5.5 days (SD = 3.2) in the early and delayed EN groups, respectively. There were no statistically significant differences in body weight, total intake and output, total calories of intake and diet volume on 4th day between both groups (p > 0.05). Aspiration episodes during enteral feeding were not significantly different between two groups (p = 0.176). C-reactive protein checked at 14 days after admission was significantly higher in the delayed EN group (61.0 mg/L, SD = 55.7) than in the early EN group (33.5 mg/L, SD = 41.4) (p = 0.004). Table 2 shows data with reference to the enteral nutritional in 123 ICH subjects.
Table 2.
Comparison of data regarding nutrition according to the time interval to enteral nutrition
CRP : C-reactive protein, EN : enteral nutrition, SD : standard deviation
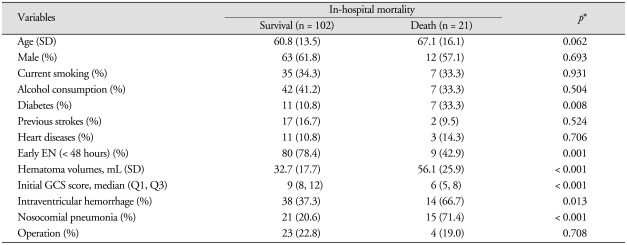
The results of univariate analysis of 123 patients according to the in-hospital mortality are presented in Table 3. Hematoma volume, initial GCS score, intraventricular hemorrhage and nosocomial pneumonia were significantly different between survival and death groups.
Table 3.
The univariate analysis of 123 patients according to in-hospital mortality after intracerebral hemorrhage
*p value for differences between survival and death group after intracerebral hemorrahge; Pearson χ2 or Fisher's exact test, Student t-test, Mann-Whitney test as appropriate. EN : enteral nutrition, GCS: Glasgow coma scale, SD : standard deviation
There was a correlation between the time interval to EN and length of ICU stay (r = 0.570, p < 0.001) and C-reactive protein 14 days after admission (r = 0.229, p = 0.004). But, there was no correlation between the time interval of EN and serum albumin 14 days after admission (r = -0.060, p = 0.510).
Multivariate analysis
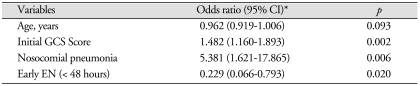
Multivariate logistic regression analysis was performed for the following variables : age (continuous variable), diabetes, early EN (< 48 hours), initial GCS score, intraventricular hemorrhage and nosocomial pneumonia. We excluded hematoma volume in multivariate logistic regression analysis because of strong correlation (r = -0.710, p < 0.001).
In multivariate analysis, early EN (OR = 0.229, 95% CI : 0.066-0.793), nosocomial pneumonia (OR = 5.381, 95% CI : 1.621-17.865) and initial GCS score (OR = 1.482, 95% CI : 1.160-1.893) were proved as independent predictors of in-hospital mortality in patients with critical hypertensive ICH (Table 4).
Table 4.
Multivariate logistic regression analysis for in-hospital mortality
*Adjusted odds ratio was obtained for in-hospital mortality using binary logistic regression analysis (backward method). CI : confidence interval, EN : enteral nutrition, GCS : Glasgow coma scale
DISCUSSION
There were few studies showing the relation between early EN and subsequent neurological outcomes in patients with hypertensive ICH, although the effect of traumatic brain injury on metabolism and nitrogen wasting have been thoroughly studied37). EN is one of the crucial outcome predictors of patients with severe traumatic brain injury13). Many well-designed clinical trials conducted in critically ill patients revealed a statistically significant reduction both in mortality and nosocomial pneumonia attributable to early EN within 24 hours of injury7,8,22,23,29,33). At present, benefits of early EN have been commonly accepted by most clinicians. In the current study, early EN (< 48 hours) and nosocomial pneumonia were independent predictors of in-hospital mortality in critical hypertensive ICH patients of GCS score 3-12. Our findings are consistent with a previous clinical trial of postoperative patients with critical hypertensive ICH that early EN improves 3-month neurological outcome38).
Hypercatabolism is a common finding in most critical illness such as surgery, trauma and stroke. Patients with acute critical illness lose on average 5-10% of skeletal muscle mass per week during their ICU stay14). Even in paralyzed patients, energy expenditure remained elevated by 20-30% in some trauma patients10). Weight reduction, negative nitrogen balance and immune dysfunction constitute a characteristic response in patients with most critical illness. This condition facilitates the onset of acute inflammation and infectious complications, and consequently results in an increased incidence of morbidity and mortality12).
The gastrointestinal tract is known to play an important role in the immune response aside digesting and absorbing functions. The gut is another intricate immune system that consists of three main components : the epithelium, the mucosal immune system and the commensal normal flora9). In the early stages of critical illness, immune function of the gut is compromised through increased intestinal permeability, a reduction of lymphoid tissue and immunoglobulin A secretion and mucosal atrophy24,25). The normal florae of the gut decreases, whereas pathogenic bacteria increase9). Translocation of indigenous bacteria or their products from the gut into the circulation, due to increased permeability of the intestinal mucosa, is a well-known feature of the metabolic response in trauma patients. This translocation of pathogenic bacteria from the gut leads to stimulation of systemic cytokine release, and resulting in an increase in infectious susceptibility. The resultant cytokine storm drives the critically ill patients towards uncontrollable multiple organ dysfunction syndrome, thus increasing the risk of mortality6,9). Two recent studies highlights the role of EN in ameliorating these changes24,25). Intestinal alkaline phosphatase (iAP) is an important brush-border protein expressed exclusively in villus-associated enterocytes, and is thought to actively remove bacterial lipopolysaccharide (LPS) and decrease bacterial translocation15,36). The expression and function of iAP is decreased in the presence of starvation in patients with critical illness, but is maintained with the provision of EN15). It is likely that the iAP dysfunction during fasting is an important component of the gut mucosal immune compromise seen in critically ill patients15).
EN is one of preferred modes of nutritional support, as it is more physiologic, less invasive and less expensive than total parenteral nutrition19,21). An ideal EN has not yet been developed, but it is generally believed that formulas containing trophic effects for the intestinal mucosa, immune-enhancing nutrients or ingredients to decrease the inflammatory acute phase reaction would be valuable30). However, decreased incidence of nosocomial pneumonia in injured patients is probably not a consequence of formula composition but rather a result of early initiation of EN5,22). Early EN has also been reported as an infection-reducing factor even when administered below the daily requirements4,30).
Nasogastric tube feeding is the preferred, universal method for early feeding of injured patients, because it is easy and therefore not time consuming to institute. However, gastric EN is blamed for inducing silent pulmonary aspiration and the resulting pneumonia in ventilated patients suffering from upper digestive intolerance16,18,32). A main factor implicated in aspiration associated with enteral feeding in critically ill patients is the size and location of the feeding tube3). Continuous postpyloric feeding has been recommended to avoid upper digestive intolerance, but in a review of delivery of enteral feed, only two of 193 patients were fed jejunally1,20). The reason may be that transpyloric tube passage is time-consuming and does not necessarily protect from pulmonary aspiration26). However, recent clinical trials revealed that early EN can lead to a decreased risk of nosocomial pneumonia5,22). In the present study, aspiration episodes in early EN group were not more frequent than in delayed EN group, and incidence of nosocomial pneumonia was significantly decreased in early EN group. It is likely that early EN may promote gut motility, decreases gastric residuals and reduces microaspiration and vomiting, thus reducing the incidence of nosocomial pneumonia.
Some limitations of our retrospective case-control study may have been existed. First, some possibilities of selection bias may exist in this study because of small number of subjects of study group. Second, EN may also be a useful prophylaxis against stress ulceration31,34,35). But, it was not analyzed in our present study. Last, the reason for not instituting early EN in some patients was not commented although this proportion was not significant. Ultimate patient's outcome would have been influenced by delayed EN, irrespective of their causes.
CONCLUSION
Our findings suggest that early EN may be an important predictor of outcome of patients with critical hypertensive ICH. Early EN is not likely to increase occurrence of respiratory complications, and may be an useful method of nutritional support in patients with critical hypertensive ICH. Further prospective study is needed to determine the impact of early EN in patients with hypertensive ICH.
References
- 1.Adam S, Batson S. A study of problems associated with the delivery of enteral feed in critically ill patients in five ICUs in the UK. Intensive Care Med. 1997;23:261–266. doi: 10.1007/s001340050326. [DOI] [PubMed] [Google Scholar]
- 2.Balk RA. Pathogenesis and management of multiple organ dysfunction or failure in severe sepsis and septic shock. Crit Care Clin. 2000;16:337–352. doi: 10.1016/s0749-0704(05)70113-5. [DOI] [PubMed] [Google Scholar]
- 3.Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125:1446–1457. doi: 10.1378/chest.125.4.1446. [DOI] [PubMed] [Google Scholar]
- 4.Braga M, Gianotti L, Cestari A, Vignali A, Pellegatta F, Dolci A, et al. Gut function and immune and inflammatory responses in patients perioperatively fed with supplemented enteral formulas. Arch Surg. 1996;131:1257–1264. doi: 10.1001/archsurg.1996.01430240011001. discussion 1264-1265. [DOI] [PubMed] [Google Scholar]
- 5.Caparrós T, Lopez J, Grau T. Early enteral nutrition in critically ill patients with a high-protein diet enriched with arginine, fiber, and antioxidants compared with a standard high-protein diet. The effect on nosocomial infections and outcome. JPEN J Parenter Enteral Nutr. 2001;25:299–308. doi: 10.1177/0148607101025006299. discussion 308-309. [DOI] [PubMed] [Google Scholar]
- 6.Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. Arch Surg. 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 7.Chiarelli A, Enzi G, Casadei A, Baggio B, Valerio A, Mazzoleni F. Very early nutrition supplementation in burned patients. Am J Clin Nutr. 1990;51:1035–1039. doi: 10.1093/ajcn/51.6.1035. [DOI] [PubMed] [Google Scholar]
- 8.Chuntrasakul C, Siltharm S, Chinswangwatanakul V, Pongprasobchai T, Chockvivatanavanit S, Bunnak A. Early nutritional support in severe traumatic patients. J Med Assoc Thai. 1996;79:21–26. [PubMed] [Google Scholar]
- 9.Clark JA, Coopersmith CM. Intestinal crosstalk : a new paradigm for understanding the gut as the "motor" of critical illness. Shock. 2007;28:384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifton GL, Robertson CS, Choi SC. Assessment of nutritional requirements of head-injured patients. J Neurosurg. 1986;64:895–901. doi: 10.3171/jns.1986.64.6.0895. [DOI] [PubMed] [Google Scholar]
- 11.Falcão de Arruda IS, de Aguilar-Nascimento JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci(Lond) 2004;106:287–292. doi: 10.1042/CS20030251. [DOI] [PubMed] [Google Scholar]
- 12.Field CJ, Johnson I, Pratt VC. Glutamine and arginine: immunonutrients for improved health. Med Sci Sports Exerc. 2000;32:S377–S388. doi: 10.1097/00005768-200007001-00002. [DOI] [PubMed] [Google Scholar]
- 13.Formisano R, Voogt RD, Buzzi MG, Vinicola V, Penta F, Peppe A, et al. Time interval of oral feeding recovery as a prognostic factor in severe traumatic brain injury. Brain Inj. 2004;18:103–109. doi: 10.1080/0269905031000149470. [DOI] [PubMed] [Google Scholar]
- 14.Gamrin L, Andersson K, Hultman E, Nilsson E, Essén P, Wernerman J. Longitudinal changes of biochemical parameters in muscle during critical illness. Metabolism. 1997;46:756–762. doi: 10.1016/s0026-0495(97)90119-0. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RF, Austen WG, Jr, Zhang X, Munene G, Mostafa G, Biswas S, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105:3551–3556. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen TS, Larsen K, Engberg AW. The association of functional oral intake and pneumonia in patients with severe traumatic brain injury. Arch Phys Med Rehabil. 2008;89:2114–2120. doi: 10.1016/j.apmr.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA, et al. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998;352:772–776. doi: 10.1016/S0140-6736(98)02007-8. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs S, Chang RW, Lee B, Bartlett FW. Continous enteral feeding : a major cause of pneumonia among ventilated intensive care unit patients. JPEN J Parenter Enteral Nutr. 1990;14:353–356. doi: 10.1177/0148607190014004353. [DOI] [PubMed] [Google Scholar]
- 19.Kattelmann KK, Hise M, Russell M, Charney P, Stokes M, Compher C. Preliminary evidence for a medical nutrition therapy protocol : enteral feedings for critically ill patients. J Am Diet Assoc. 2006;106:1226–1241. doi: 10.1016/j.jada.2006.05.320. [DOI] [PubMed] [Google Scholar]
- 20.Kearney PA, Annis K. Perioperative nutritional support in critical illness. Curr Opin Anaesth. 1994;7:305–309. [Google Scholar]
- 21.Klodell CT, Carroll M, Carrillo EH, Spain DA. Routine intragastric feeding following traumatic brain injury is safe and well tolerated. Am J Surg. 2000;179:168–171. doi: 10.1016/s0002-9610(00)00297-x. [DOI] [PubMed] [Google Scholar]
- 22.Kompan L, Kremzar B, Gadzijev E, Prosek M. Effects of early enteral nutrition on intestinal permeability and the development of multiple organ failure after multiple injury. Intensive Care Med. 1999;25:157–161. doi: 10.1007/s001340050809. [DOI] [PubMed] [Google Scholar]
- 23.Kompan L, Vidmar G, Spindler-Vesel A, Pecar J. Is early enteral nutrition a risk factor for gastric intolerance and pneumonia? Clin Nutr. 2004;23:527–532. doi: 10.1016/j.clnu.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Kudsk KA. Early enteral nutrition in surgical patients. Nutrition. 1998;14:541–544. doi: 10.1016/s0899-9007(98)00047-1. [DOI] [PubMed] [Google Scholar]
- 25.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 26.Montejo JC, Grau T, Acosta J, Ruiz-Santana S, Planas M, García-De-Lorenzo A, et al. Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Crit Care Med. 2002;30:796–800. doi: 10.1097/00003246-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Moore EE, Moore FA. Aggressive enteral feeding reduces sepsis in severely injured patients: updating the data. JCCC. 1994;1:15–20. [Google Scholar]
- 28.Moore FA, Feliciano DV, Andrassy RJ, McArdle AH, Booth FV, Morgenstein-Wagner TB, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216:172–183. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen NQ, Fraser RJ, Bryant LK, Burgstad C, Chapman MJ, Bellon M, et al. The impact of delaying enteral feeding on gastric emptying, plasma cholecystokinin, and peptide YY concentrations in critically ill patients. Crit Care Med. 2008;36:1469–1474. doi: 10.1097/CCM.0b013e31816fc457. [DOI] [PubMed] [Google Scholar]
- 30.O'Flaherty L, Bouchier-Hayes DJ. Immunonutrition and surgical practice. Proc Nutr Soc. 1999;58:831–837. doi: 10.1017/s0029665199001123. [DOI] [PubMed] [Google Scholar]
- 31.Pachler C, Plank J, Weinhandl H, Chassin LJ, Wilinska ME, Kulnik R, et al. Tight glycaemic control by an automated algorithm with time-variant sampling in medical ICU patients. Intensive Care Med. 2008;34:1224–1230. doi: 10.1007/s00134-008-1033-8. [DOI] [PubMed] [Google Scholar]
- 32.Pingleton SK. Enteral nutrition as a risk factor for nosocomial pneumonia. Eur J Clin Microbiol Infect Dis. 1989;8:51–55. doi: 10.1007/BF01964120. [DOI] [PubMed] [Google Scholar]
- 33.Pupelis G, Selga G, Austrums E, Kaminski A. Jejunal feeding, even when instituted late, improves outcomes in patients with severe pancreatitis and peritonitis. Nutrition. 2001;17:91–94. doi: 10.1016/s0899-9007(00)00508-6. [DOI] [PubMed] [Google Scholar]
- 34.Robertson CS, Goodman JC, Narayan RK, Contant CF, Grossman RG. The effect of glucose administration on carbohydrate metabolism after head injury. J Neurosurg. 1991;74:43–50. doi: 10.3171/jns.1991.74.1.0043. [DOI] [PubMed] [Google Scholar]
- 35.Schlenk F, Nagel A, Graetz D, Sarrafzadeh AS. Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2008;34:1200–1207. doi: 10.1007/s00134-008-1044-5. [DOI] [PubMed] [Google Scholar]
- 36.Tuin A, Poelstra K, Jager-Krikken A, Bok L, Raaben W, Velders MP, et al. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009;58:379–387. doi: 10.1136/gut.2007.128868. [DOI] [PubMed] [Google Scholar]
- 37.UK-TIA Study Group. United Kingdom transient ischaemic attack (UK-TIA) aspirin trial : interim results. Br Med J (Clin Res Ed) 1988;296:316–320. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang JJ, Dong WF, Gu SJ, Zhang J, Xuan HF, Xie RL. [Clinical study on the early nutrition support in postoperative patients with critical hypertensive intracerebral hemorrhage.] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:552–555. [PubMed] [Google Scholar]