Abstract
Objective
Body dysmorphic disorder (BDD) is an often-severe disorder, but few treatment studies have been done. This pilot study explored the efficacy and safety of the antiepileptic medication levetiracetam for BDD.
Methods
Seventeen subjects with DSM-IV BDD participated in a 12-week open-label levetiracetam trial. Subjects were assessed at regular intervals with standard measures.
Results
In intention-to-treat analyses, scores on the BDD-YBOCS, the primary outcome measure, decreased from 32.5 ± 4.7 at baseline to 21.5 ± 11.0 at endpoint (p<.001). 52.9% (n=9) of subjects were responders (greater than or equal to 30% decrease on the BDD-YBOCS). The mean time to response was 4.6 ± 2.8 (range=2–10) weeks. Scores also significantly improved on the Brown Assessment of Beliefs Scale, Hamilton Depression Rating Scale, Global Assessment of Functioning Scale, and Social and Occupational Functioning Assessment Scale. Scores did not significantly improve on the Quality of Life Enjoyment and Satisfaction Questionnaire, the Beck Anxiety Inventory, or the Social Phobia Inventory. The mean endpoint dose was 2,044.1 ± 1,065.2 (range=250–3,000) mg/day. Levetiracetam was relatively well tolerated.
Conclusions
Randomized, double-blind placebo-controlled studies of levetiracetam for BDD are needed to confirm these preliminary findings.
INTRODUCTION
Body dysmorphic disorder (BDD), a distressing or impairing preoccupation with an imagined or slight defect in appearance, is an often severe and relatively common disorder, with a point prevalence of 0.7%–2.4%.1–5 Common symptoms of BDD include excessive and repetitive grooming, mirror checking, skin picking, reassurance seeking, and other behaviors. 6,7 Without appropriate treatment, BDD appears to usually be chronic.8 Nearly all individuals with BDD experience impairment in social functioning, and most have impaired academic and/or occupational functioning, because of BDD symptoms.6,7,9 Mental health-related quality of life is markedly poorer than for the general population, and is poorer than norms for patients with clinical depression (major depressive disorder and/or dysthymia).10,11 Quality of life and psychosocial functioning in BDD also appear as poor as, or even poorer than, that in OCD.12,13 Furthermore, available data indicate that individuals with BDD have high rates of lifetime suicidal ideation, suicide attempts, and completed suicide.7,14–16
Knowledge of effective pharmacotherapy for BDD has substantially increased over the past 15 years.17 Both controlled and open-label studies have shown that serotonin-reuptake inhibitors (SRIs) appear effective for a majority of patients with BDD.17,18 In a double-blind cross-over trial, clomipramine was more efficacious than desipramine for BDD.19 In a double-blind parallel-group study, fluoxetine was more efficacious than placebo.20 Four systematic open-label studies (n=15 to n=30) have found fluvoxamine, citalopram, and escitalopram to be efficacious and well tolerated.21–24 Of note, patients with delusional BDD (a type of delusional disorder, somatic type, which is double coded with BDD in DSM-IV) also often improve with SRI monotherapy.19,20,22–24 A recent open-label trial of venlafaxine (n=17) suggests that this medication may also be efficacious for BDD,25 although controlled studies are needed.
Additional treatment studies are needed, however, as relatively few pharmacotherapy studies of BDD have been done. In addition, while the above studies indicate that a majority of patients with BDD significantly improve with SRI treatment, response is often partial, and a substantial minority of patients do not improve to a clinically significant degree.17
The present study obtained pilot data on the efficacy and safety of the antiepileptic medication levetiracetam in patients with BDD. Levetiracetam is indicated as adjunctive therapy in the treatment of partial onset seizures, myoclonic seizures, and primary generalized tonic-clonic seizures. While levetiracetam’s precise mechanism of action is unknown, it has been shown to bind to the presynaptic vesicle protein SV2A, which is thought to be involved in the regulation of vesicle exocytosis.26 Levetiracetam also moderately blocks high-voltage calcium channels and opposes the activity of the negative allosteric modulators zinc and β-carbolines on GABA-A and glycine receptor-mediated responses.27
One reason to examine the efficacy of levetiracetam for BDD is that an open-label study suggested that levetiracetam may be efficacious for social phobia,28 which has similarities to BDD.29 Similarities include, in BDD, high levels of social anxiety, avoidance of social interactions (related to perceived bodily “defects”), and a tendency to misinterpret ambiguous social scenarios as threatening. 7,30–33 In addition, high rates of comorbid lifetime social phobia in BDD (37%–39%)34,35 suggest that BDD and social phobia may be related disorders. While a subsequent placebo-controlled study in social phobia did not find a statistically significant difference between levetiracetam and placebo, the sample was very small, and the effect size was .33 (small-medium; Cohen’s d).36 Another reason to examine the efficacy of levetiracetam for BDD is that antiepileptic medications more generally have a broad range of efficacy for psychiatric disorders.37 To our knowledge, this is the first investigation of an antiepileptic medication in the treatment of BDD.
METHODS
Subjects
Seventeen outpatients participated in this 12-week prospective open-label study. The hospital Institutional Review Board approved the study, and all subjects provided written informed consent. All subjects met DSM-IV criteria for BDD, a distressing or impairing preoccupation with an imagined or slight defect in appearance that is not better accounted for by another mental disorder. Inclusion criteria were: 1) age 18–65; 2) current DSM-IV BDD or its delusional variant (delusional disorder, somatic type); 3) suitable for treatment in an outpatient setting; and 4) total score of at least 20 on the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS).38 In our experience, scores lower than 20 reflect subthreshold, rather than full-criteria, BDD as defined in DSM-IV. Exclusion criteria were: 1) unstable medical illness, including renal failure or dialysis; 2) myocardial infarction within the past 6 months; 3) current pregnancy or lactation, or inadequate contraception in women of childbearing potential; 4) a need for prn benzodiazepines, another antiepileptic medication, or an anticipated change in the dose of any concomitant psychotropic medication during the study; 5) clinically significant suicidality, including a suicide attempt within the past two months; 6) lifetime history of DSM-IV dementia, schizophrenia, or any other DSM-IV psychotic disorder that is not attributable to BDD; 7) current or recent (past 3 months) DSM-IV substance abuse or dependence; 8) initiation of ongoing psychotherapy from a mental health professional within 3 months prior to study baseline; subjects could not receive cognitive-behavioral therapy (CBT) during the study or begin any other type of psychotherapy during the study; 9) previous treatment with levetiracetam; 10) treatment with investigational medication, depot neuroleptics, or ECT within the past 3 months.
Subjects taking allowed concomitant psychotropic medications were required to have been on a stable dose for at least 3 weeks before beginning levetiracetam, and the dose could not be changed during the study. Subjects already taking an SRI were required to be on the highest dose recommended by the manufacturer or tolerated by the patient before beginning levetiracetam; a total SRI trial duration of at least 12 weeks, including at least 3 weeks on the highest SRI dose, were also required. Of the 17 participants, 5 (29.4%) took a concomitant SRI during the study: escitalopram (n=2), at doses of 30 mg/day and 40 mg/day; and fluvoxamine (n=3), at doses of 250 mg/day, 350 mg/day, and 400 mg/day (the subject taking 250 mg/day had previously taken 300 mg/day with no additional benefit). For these subjects, total SRI trial duration before beginning levetiracetam ranged from 17 weeks to 10.3 years (mean = 5.7 ± 4.4 years); time on the current SRI dose ranged from 4.3 weeks to 5.0 years (mean = 1.4 ± 2.1 years). Other concomitant psychotropic medications were venlafaxine (n=3), Adderall (n=2), lithium (n=1), buspirone (n=1), clonazepam (n=1), alprazolam (n=1), and trazodone (n=1). Two subjects received non-CBT psychotherapy during the study.
Assessments
Subjects were assessed with the Structured Clinical Interview for DSM-IV, Patient Version.39 The BDD Form, used in many previous BDD studies,e.g.,7,8 obtained data on demographic characteristics, clinical features of BDD, and treatment history. The following measures were completed at the baseline visit and periodically throughout the study (details of the assessment schedule are provided below). The Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) was the primary outcome measure. This reliable and valid 12-item semi-structured clinician-administered scale assesses BDD severity during the past week.38 Items are rated from 0 (no symptoms) to 4 (extreme symptoms), with a total score of 0 to 48. The scale assesses preoccupation with the perceived appearance defects (time occupied, interference with functioning due to the preoccupation, distress, resistance, and control), associated compulsive behaviors such as mirror checking or excessive grooming (time spent, interference with functioning, distress if the behaviors are prevented, resistance, and control), insight, and avoidance. A ≥ 30% decrease in total score indicated response.38 The Clinical Global Improvement Scale (CGI) is a widely used 7-point scale that assesses global improvement or worsening of symptoms, with ratings ranging from “very much worse” to “very much improved.”40 Ratings of much improved (score of 2) or very much improved (score of 1) defined improvement in BDD. Separate CGI ratings were done by the clinician rater and the patient. Separate CGI ratings were also done for BDD symptoms and for global psychopathology including BDD. The 7-point Clinical Global Severity Scale assessed current illness severity at study baseline (score of 1 = normal, not at all ill, and score of 7 = among the most extremely ill patients).40
The Brown Assessment of Beliefs Scale (BABS) is a 7-item semi-structured clinician-administered scale that assesses seven components of delusionality (insight) during the past week.41 Scores range from 0 to 24, with higher scores indicating greater delusionality. Beliefs are also categorized as delusional or nondelusional using an empirically derived cutpoint. The BABS is reliable, valid, and sensitive to change.41 At study baseline, patients were asked to describe their belief about the disliked body areas – for example, “I look deformed.” At study endpoint, the same belief was reassessed. The BABS could not be completed for one subject who declined to answer the questions because she found them upsetting. The Hamilton Rating Scale for Depression (24 item)(HAM-D) is a widely used reliable and valid clinician-administered measure of current severity of depressive symptoms.42 The Social Phobia Inventory (SPIN) is a 17-item self-report questionnaire that assesses fear, avoidance, and physiological arousal associated with social anxiety during the past week. This scale is reliable, valid, and sensitive to change.43 Scores range from 0 to 68, with a score of 19 or higher distinguishing patients with social phobia from both healthy controls and psychiatric controls without social phobia. No special instructions were used for the SPIN; therefore, SPIN scores reflect social anxiety from any source (e.g., BDD, comorbid social phobia). The Beck Anxiety Inventory (BAI) is a reliable, valid, and widely used 21-item self-report measure of anxiety during the past week which focuses on somatic symptoms.44 The BAI has been shown to be sensitive to change.45 Scores range from 0 to 63. On all of the above symptom measures, higher scores reflect greater symptom severity.
Three measures assessed psychosocial functioning/quality of life. The Global Assessment of Functioning (GAF) is a global measure of symptom severity and psychological, social, and occupational functioning.46 The Social and Occupational Functioning Assessment Scale (SOFAS) is similar to the GAF but assesses only psychosocial functioning.46 Scores on both scales range from 0 to 100, with lower scores denoting more severe illness and/or poorer functioning. The Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q), a reliable and valid measure that is sensitive to change, assessed current quality of life.47 The Short Form transformed score is reported. Lower scores reflect poorer quality of life.
Procedures
After completing a urine pregnancy test (in females of childbearing potential) and the baseline evaluation, subjects received unblinded levetiracetam for 12 weeks. A fixed-flexible dosing regimen was used. The starting dose was 250 mg qhs for 1 week, which was increased to 250 mg twice a day for 1 week. The dose was then raised by 500 mg/day each week to a maximum dose of 1,500 mg twice a day, as tolerated. Subjects were evaluated with the BDD-YBOCS, CGI, and HAM-D at baseline, weekly for 4 weeks, and then every other week. The other measures were completed at baseline and endpoint. Medication compliance was assessed by tablet count. At each study visit, pulse, blood pressure, concomitant medications, and adverse events were assessed.
Data analyses
Descriptive statistics are provided for demographic variablesand baseline clinical characteristics. All primary treatment analyses are intention-to-treat with last observation carried forward. Intention-to-treat analyses include all subjects who took at least one dose of study medication, including those who withdrew early from the study. Several secondary completer analyses were also conducted where indicated. Scale scores on continuous study measures were analyzed using paired t-tests or analysis of variance with time of measurement as a within-subjects factor. The Huynh–Feldt correction was applied when the sphericity assumption was not met. The alpha level for all analyses was p<.05, and t-tests were two-tailed. For the primary outcome measure (the BDD-YBOCS), ηp2 (partial Eta squared) was calculated. Following the recommendation of Cohen,48 partial eta squared is reported as an index of effect size: large effects, ηp2 > .14; medium effects, ηp2 = .06; and small effects, ηp2 = .01. Correlations were examined with the Pearson correlation coefficient. Adverse events were tabulated by type, incidence, and likelihood that the event was related to the study medication. Reported adverse events are those considered possibly, probably, or almost certainly related to the medication in at least 10% of subjects.
RESULTS
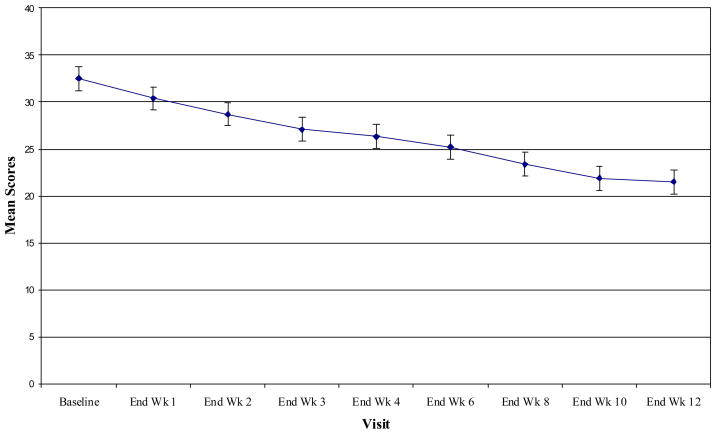
Table 1 shows the sample’s demographic and baseline clinical characteristics. Eleven (64.7%) subjects completed the study. In intention-to-treat analyses, BDD-YBOCS scores decreased from 32.5 ± 4.7 at baseline to 21.5 ± 11.0 at endpoint, F(2.71, 43.28) = 12.27, p<.001, ηp2 = .43 (see Table 2 and Figure 1). The mean decrease in BDD-YBOCS scores among the entire sample was 33.8%. On the BDD-YBOCS, 52.9% (n=9) of subjects were responders. Among subjects not taking an SRI, 58.3% (n=7/12) were responders. Among subjects who had levetiracetam added to stable ongoing SRI treatment, 40.0% (n=2/5) responded. Among responders, the mean decrease in BDD-YBOCS score was 58.9%. The mean time to response of BDD was 4.6 ± 2.8 weeks (range, 2–10 weeks). Response to levetiracetam was not predicted by baseline BDD severity, BDD duration, or current comorbid major depressive disorder.
TABLE 1.
Baseline Demographic and Clinical Features of 17 Subjects with Body Dysmorphic Disorder Treated with Levetiracetam
| Variable | % (n) or Mean ± SD |
|---|---|
| Gender (% female) | 64.7% (n=11) |
| Age | 36.8 ± 10.2 (range = 23–56) |
| Race/ethnicity (% minority) | 23.5% (n=4) |
| Marital status (% single) a | 82.4% (n=14) |
| Duration of BDD (years) | 20.6 ± 12.9 |
| Number of body areas of concern (current) | 4.5 ± 2.6 |
| Current body areas of concern (most common) | |
| Skin | 76.5% (n=13) |
| Hair | 58.8% (n=10) |
| Weight | 41.2% (n=7) |
| Teeth | 35.3% (n=6) |
| Breasts | 35.3% (n=6) |
| Number of compulsive BDD behaviors (current) | 6.3 ± 2.2 |
| Current compulsive BDD behaviors (most common) | |
| Comparing one’s appearance with that of other people | 100% (n=17) |
| Checking mirrors and other reflecting surfaces | 88.2% (n=15) |
| Camouflaging the perceived flaws (e.g., with a hat) | 88.2% (n=15) |
| Excessive grooming | 70.6% (n=12) |
| Excessive clothes changing to improve one’s appearance | 64.7% (n=11) |
| Current comorbidity | |
| Major depressive disorder | 41.2% (n=7) |
| Social phobia | 29.4% (n=5) |
| Obsessive compulsive disorder | 17.6% (n=3) |
| Panic disorder with agoraphobia | 11.8% (n=2) |
| Specific phobia | 11.8% (n=2) |
| Dysthymia | 11.8% (n=2) |
| Post-traumatic stress disorder | 5.9% (n=1) |
| Generalized anxiety disorder | 5.9% (n=1) |
Includes 12 never married and 2 divorced subjects.
TABLE 2.
Scores on Study Measures in 17 Subjects with Body Dysmorphic Disorder Treated with Levetiracetam
| Measure | Baselinea,b | Endpointa,b | Statistic | df | Significance | |
|---|---|---|---|---|---|---|
| BDD-YBOCS | 32.5 ± 4.7 | 21.5 ± 11.0 | F=12.27 | 2.71, 43.28 | <.001 | |
| CGI (Clinician) - % Much or Very Much Improved | ||||||
| BDD | 47.1% (n=8) | |||||
| Global | 52.9% (n=9) | |||||
| CGI (Patient) - % Much or Very Much Improved | ||||||
| BDD | 47.1% (n=8) | |||||
| Global | 47.1% (n=8) | |||||
| Clinical Global Severity c | 5.2 ± 1.0 | 4.2 ± 1.6 | F = 6.08 | 3.68, 8.87 | .001 | |
| Brown Assessment of Beliefs Scale d | 14.6 ± 4.3 | 10.4 ± 5.3 | t = 3.47 | 15 | .003 | |
| Hamilton Depression Rating Scale (24 item) | 18.1 ± 6.4 | 11.4 ± 8.4 | F= 4.22 | 2.81, 44.97 | .012 | |
| Beck Anxiety Inventory | 12.4 ± 6.8 | 9.6 ± 5.5 | t = 1.50 | 16 | .154 | |
| Social Phobia Inventory | 32.9 ± 12.4 | 29.4 ± 13.8 | t = 1.37 | 16 | .189 | |
| Global Assessment of Functioning (GAF) Scale | 46.1 ± 7.8 | 55.8 ± 14.5 | t = −3.33 | 16 | .004 | |
| Social and Occupational Functioning Assessment | 47.0 ± 8.5 | 59.8 ± 18.6 | t = −3.33 | 16 | .004 | |
| Scale | ||||||
| Q-LES-QShort Form e | 49.5 ± 10.3 | 57.4 ± 14.8 | t = −1.82 | 16 | .087 | |
Abbreviations: BDD-YBOCS, Yale–Brown Obsessive Compulsive Scale Modified for Body Dysmorphic Disorder; CGI, Clinical Global Improvement; Q-LES-Q, Quality of Life Satisfaction and Enjoyment Questionnaire.
Data are presented as mean ± standard deviation, or % (n); all data presented are intention-to-treat with the last observation carried forward.
Higher scores indicate greater symptom severity on the BDD-YBOCS, Brown Assessment of Beliefs Scale, Hamilton Depression Rating Scale, Beck Anxiety Inventory, and Social Phobia Inventory. Lower scores indicate poorer functioning/quality of life on the Global Assessment of Functioning Scale, Social and Occupational Functioning Assessment Scale, and Q-LES-Q.
The mean Clinical Global Severity score for BDD at baseline was in the markedly ill (score of 5) to severely ill (score of 6) range. The endpoint score was in the moderately ill (score of 4) to markedly ill (score of 5) range.
The mean Brown Assessment of Beliefs Scale score is in the poor insight range at baseline and the fair insight range at endpoint.
Converted (transformed) scores for the Q-LES-Q Short Form are reported.
FIGURE. Mean BDD-YBOCS Scores Over 12 Weeks of Treatment with Levetiracetam.
BDD-YBOCS = Yale Brown Obsessive Compulsive Scale Modified for Body Dysmorphic Disorder. Scores over time in 17 subjects. There was a significant time effect [F(2.71, 43.28)=12.27, P<0.001]; all observations represent last observation carried forward. Bars represent 1 SE.
Among the 11 subjects who completed the full 12 weeks of levetiracetam treatment, BDD-YBOCS scores decreased from 32.9 ± 5.1 to 19.6 ± 11.8, F(3.49, 34.85) = 11.54, p<.001, ηp2 = .54. Of the 11 completers, seven (63.6%) were responders on the BDD-YBOCS.
As shown in Table 2, on the clinician BDD-CGI, BDD improved for 8 (47.1%) subjects: 7 (41.2%) were very much improved, and 1 (5.9%) was much improved. On the clinician global CGI, 9 (52.9%) of subjects improved: 4 (23.5%) were very much improved and 5 (29.4%) were much improved. Patient CGI scores were similar (Table 2).
The delusionality of appearance beliefs significantly decreased on the BABS (p=.003). Mean scores indicated poor insight at study baseline and fair insight at study endpoint. Two of 3 subjects who had delusional BDD beliefs at baseline responded to levetiracetam, and 6 of 13 subjects who had nondelusional BDD beliefs at baseline responded. Depressive symptoms significantly improved on the HAM-D (p=.012). The correlation between change in BDD-YBOCS scores and HAM-D scores was .64 (p=.006). In intention-to-treat analyses, mean scores on the HAM-D suicidal ideation item decreased at a trend level: F(4.09, 65.37) = 2.11, p=.088; scores on this item decreased significantly among study completers: F(4.45, 44.54) = 2.99, p=.025. Scores did not significantly improve on the BAI or the SPIN.
GAF and SOFAS scores significantly improved. In intention-to-treat analyses, Q-LES-Q scores improved at a trend level (p=.087); among study completers, Q-LES-Q scores significantly improved (t=−2.46, df=10, p=.034).
The mean endpoint levetiracetam dose for the entire sample was 2,044.1 ± 1,065.2 mg/day (range = 250 – 3,000 mg/day). Among study completers, the mean endpoint dose was 2,568.2 ± 791.3 mg/day (range = 750 – 3,000 mg/day). Among those subjects who responded to levetiracetam, the mean endpoint dose was 2,083.3 ± 935.4 mg/day (range = 750 – 3,000 mg/day).
Adverse events occurring in 10% or more of subjects that were considered possibly, probably, or almost certainly related to the medication were fatigue (n=8), irritability (n=4), insomnia (n=4), headache (n=3), anxiety (n=3), nausea (n=2), and dizziness (n=2). Nearly all adverse events were mild or moderate. In four patients, possible or probable side effects prevented a planned dose increase or led to a dose decrease: anxiety and dizziness in one patient, nausea and fatigue in another, fatigue in the third, and irritability in the fourth.
Two serious adverse events occurred. One subject, a 56-year-old married African American woman, was withdrawn from the study and hospitalized at week 8 due to increased depression and suicidal ideation, which appeared attributable to substantially worsening psychosocial stressors. A 20-year-old single white woman was withdrawn from the study and hospitalized before beginning levetiracetam (at her second screening assessment) because she was actively suicidal; she attributed her suicidality to BDD symptoms.
In addition to the above subject who was withdrawn from the study at week 8, five subjects dropped out of the study after the baseline visit. One subject dropped out after taking only one dose of levetiracetam because she “wanted to get better on [her] own,” and another dropped out at week 3 because she felt that “talking about [her] symptoms was making them worse.” One subject dropped out at week 6 due to lack of improvement in depressive symptoms, and another dropped out at week 10 due to a lack of improvement in depressive symptoms and worsening anxiety. Another subject discontinued the medication at week 8 because she was convinced it was making her hair fall out and look too thin; there was no objective evidence for this, and this concern was considered a symptom of BDD.
DISCUSSION
This pilot study provides preliminary evidence that levetiracetam may be efficacious for BDD. In the more conservative intention-to-treat analyses (the study’s primary analyses), BDD-YBOCS scores significantly decreased, and 52.9% of subjects responded to levetiracetam. The response rate was higher (63.6%) in a secondary analysis of those subjects who completed the study. The effect size was large for these analyses.
In published open-label studies of SRIs, response rates in intention-to-treat analyses based on the BDD-YBOCS were 73% for escitalopram (n=15),24 73% for citalopram (n=15),23 and 63% for fluvoxamine (n=30).22 However, comparisons of response rates across studies must be made very cautiously, because levetiracetam was not directly compared to an SRI and because samples were small. Compared to previous open-label SRI trials, the levetiracetam study sample had somewhat more severe BDD symptoms and poorer scores on functioning/quality of life measures at baseline, although BDD severity has not been found to predict medication response, either in the present study or in prior SRI studies.20,22 It is also possible that the levetiracetam study sample may have been relatively treatment refractory. Sixteen of the 17 subjects had received an SRI in the past; of the 18 total SRI trials received that were considered at least minimally adequate for BDD, 49 subjects reported that only 28% (n=5) were efficacious for BDD.
It is worth noting that among those subjects who were improved on the BDD-CGI, a high proportion (7 of 8) were “very much improved,” rather than only “much improved” (1 of 8). The proportion of the entire sample whose BDD was very much improved (41.2%) is in the range reported in open-label studies of SRIs (30%,22 40%,23 and 47%24). When comparing these rates, however, the small sample sizes of these studies must be kept in mind.
A placebo response cannot be ruled out in an open-label study such as this, and placebo-controlled studies are needed. In the only placebo-controlled study of BDD to date, the placebo response rate was only 18%.20 In this regard, it is worth noting that after completing the study, four patients who responded to levetiracetam continued to be treated in the first author’s clinic, all of whom stopped the medication and relapsed in terms of BDD symptoms, comorbid disorders, and (in two cases) suicidal ideation. One patient discontinued the medication twice, and both times experienced relapse of severe depression, OCD, and (on one occasion) BDD. Another patient decreased or stopped the medication three times, and each time experienced relapse of BDD, anxiety, depression, and social phobia symptoms. In all cases, symptoms worsened within several days to several months after stopping the medication. Levetiracetam was restarted following five of these discontinuations, with subsequent marked improvement in BDD and comorbid symptoms in all five cases within several days to several months. Three of four patients remained on levetiracetam, with improved symptoms and good tolerability, for up to two years. These clinical observations suggest that these patients’ improvement on levetiracetam was unlikely to reflect a placebo response.
It is worth highlighting that study participants had markedly poor functioning and quality of life at study baseline. Baseline scores on the GAF and SOFAS indicated “serious” symptoms or “serious” impairment in social, occupational, or school functioning. Q-LES-Q scores were 2.1 standard deviation units poorer than published norms and were poorer than scores reported in treatment studies of anxiety and depressive disorders.50 In intention-to-treat analyses, GAF and SOFAS scores significantly improved, and Q-LES-Q scores improved at a trend level. However, scores on these measures at study endpoint reflected continued impairment. This study was only 12 weeks long, and more time than this may be needed for patients to make life changes such as getting a job or starting to socialize. Future studies are needed to determine whether functioning/quality of life might further improve with longer-term treatment.
While levetiracetam’s precise mechanism of action is unknown, it has been shown to enhance the effects of GABA by opposing activity of the negative allosteric modulators zinc and β-carbolines on GABA-A receptors. 27 This is interesting in light of very preliminary data showing an association for the GABA-A-γ2 (5q31.1-q33.2) receptor gene in a preliminary candidate gene study in a different BDD sample.51 Despite its GABAergic effects, levetiracetam did not significantly improve anxiety as measured by the BAI or social anxiety as measured by the SPIN (although among levetiracetam responders the decrease in SPIN scores was statistically significant [p=.039] and BAI scores decreased at a trend level [p=.080]). Other preliminary open-label and retrospective chart-review studies suggest that levetiracetam may be efficacious for anxiety symptoms and certain anxiety disorders.28,52–55 Anxiety is an understudied aspect of BDD that needs to be examined in future treatment studies. In the present study levetiracetam significantly improved depressive symptoms, as was found in an uncontrolled study of patients with epilepsy and concomitant depressive symptoms.53
Levetiracetam was relatively well tolerated. Only one subject dropped out of the study in part because of an adverse event (increased anxiety). In four subjects, however, the dose was reduced or not raised as planned because of symptoms that were considered possible or probable side effects. It is possible that fewer adverse events may have occurred with slower dose titration or a lower final target dose.
Only 64.7% of participants who took at least one dose of levetiracetam completed this 12-week study; this dropout rate is similar to or somewhat higher than that in other BDD pharmacotherapy studies. As detailed in the results section, subjects dropped out for a variety of reasons, which in two cases was due to BDD symptoms themselves (distress caused by discussing BDD symptoms in one case and an erroneous belief that the medication made the patient look uglier in another). In our experience it can be challenging to engage and retain some patients with BDD in treatment because of BDD symptoms themselves.
This study has a number of limitations, most notably its lack of a comparison/control group, unblinded assessment of treatment outcome, and small sample size. Given these limitations, and the fact that this is to our knowledge the only study of levetiracetam in BDD, these findings should be considered preliminary. Additional studies – in particular, placebo-controlled studies -- are needed to more definitively evaluate the efficacy of levetiracetam both as monotherapy and as an SRI augmentation agent for BDD.
CONCLUSION
BDD is an often-severe mental illness that is associated with high rates of disability and suicidality, and efficacious treatments are greatly needed. However, treatment studies are still very limited. This pilot study provides preliminary data suggesting that levetiracetam may diminish BDD symptoms, BDD-related delusionality, and depressive symptoms, and may also improve psychosocial functioning/quality of life (although findings for the latter were mixed). Levetiracetam was generally well tolerated. Given this study’s small sample size and open-label design, these findings are preliminary and need to be confirmed in randomized, double-blind placebo-controlled studies.
Focus Points.
Serotonin-reuptake inhibitors are currently considered the first-line medication for body dysmorphic disorder (BDD), but additional treatment strategies are needed for this often-severe disorder.
Levetiracetam, a novel antiepileptic medication, was efficacious for BDD symptoms in 9 of 17 patients (52.9%) and was generally well tolerated in this open-label pilot study.
Randomized, double-blind, placebo-controlled studies are needed to further investigate the efficacy of levetiracetam for BDD.
Learning Objectives.
Upon reading this articles, readers will be able to:
Define body dysmorphic disorder and list common symptoms.
List medications that have been shown in previous controlled or open-label studies to be efficacious for body dysmorphic disorder.
List symptoms that significantly improved in this pilot study of levetiracetam.
Needs Assessment
Although body dysmorphic disorder (BDD) is a relatively common and often-severe disorder, treatment data are limited. This paper reports the results of a pilot study on a novel treatment -- the antiepileptic medication levetiracetam -- for BDD.
Acknowledgments
This study was funded by an unrestricted educational grant from UCB Pharma.
Footnotes
Disclosure: In the past year, Dr. Phillips has received salary and research support from the National Institute of Mental Health; she has received research funding from the FDA, the American Foundation for Suicide Prevention, and Forest Laboratories (medication only for an NIMH-funded study). She has received publication/speaking honoraria or royalties from the Merck Manual, academic institutions, and consumer advocacy organizations. Future royalties may potentially be received from Oxford University Press, Guilford Publications, and The Free Press. She has received travel and expense reimbursement from various academic, professional, and consumer advocacy organizations. In the past year, Mr. Menard has received support from the National Institute of Mental Health and the American Foundation for Suicide Prevention.
References
- 1.Phillips KA. Body dysmorphic disorder: the distress of imagined ugliness. Am J Psychiatry. 1991;148:1138–1149. doi: 10.1176/ajp.148.9.1138. [DOI] [PubMed] [Google Scholar]
- 2.Faravelli C, Salvatori S, Galassi F, Aiazzi L, Drei C, Cabras P. Epidemiology of somatoform disorders: a community survey in Florence. Soc Psychiatry Psychiatr Epidemiol. 1997;32:24–29. doi: 10.1007/BF00800664. [DOI] [PubMed] [Google Scholar]
- 3.Bienvenu OJ, Samuels JF, Riddle MA, et al. The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biol Psychiatry. 2000;48:287–293. doi: 10.1016/s0006-3223(00)00831-3. [DOI] [PubMed] [Google Scholar]
- 4.Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spect. 2008;13:316–322. doi: 10.1017/s1092852900016436. [DOI] [PubMed] [Google Scholar]
- 5.Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychol Med. 2006;36:877–885. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- 6.Perugi G, Giannotti D, Frare F, Di Vaio S, Valori E, Maggi L, Cassano GB, Akiskal HS. Prevalence, phenomenology and comorbidity of body dysmorphic disorder (dysmorphophobia) in a clinical population. Int J Clin Pract. 1997;1:77–82. doi: 10.3109/13651509709024707. [DOI] [PubMed] [Google Scholar]
- 7.Phillips KA, Diaz S. Gender differences in body dysmorphic disorder. J Nerv Ment Dis. 1997;185:570–577. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Phillips KA, Pagano ME, Menard W, Stout RL. A 12-month follow-up study of the course of body disorder. Am J Psychiatry. 2006;163:907–912. doi: 10.1176/appi.ajp.163.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Didie ER, Menard W, Stern AP, Phillips KA. Occupational functioning and impairment in adults with body dysmorphic disorder. Compr Psychiatry. 2008;49:561–569. doi: 10.1016/j.comppsych.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Phillips KA. Quality of life for patients with body dysmorphic disorder. J Nerv Ment Dis. 2000;188:170–175. doi: 10.1097/00005053-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Phillips KA, Menard W, Fay C, Pagano M. Psychosocial functioning and quality of life in body dysmorphic disorder. Compr Psychiatry. 2005;46:254–260. doi: 10.1016/j.comppsych.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Didie ER, Walters MM, Pinto A, et al. Comparison of quality of life and psychosocial functioning in obsessive-compulsive disorder and body dysmorphic disorder. Ann Clin Psychiatry. 2007;19:181–186. doi: 10.1080/10401230701468685. [DOI] [PubMed] [Google Scholar]
- 13.Frare F, Perugi G, Ruffolo G, Toni C. Obsessive-compulsive disorder and body dysmorphic disorder: a comparison of clinical features. Eur Psychiatry. 2004;19:292–298. doi: 10.1016/j.eurpsy.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Veale D, Boocock A, Gournay K, et al. Body dysmorphic disorder: a survey of fifty cases. Br J Psychiatry. 1996;169:196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- 15.Phillips KA, Coles M, Menard W, Yen S, Fay C, Weisberg RB. Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry. 2005;66:717–725. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- 16.Phillips KA, Menard W. Suicidality in body dysmorphic disorder: a prospective study. Am J Psychiatry. 2006;163:1280–1282. doi: 10.1176/appi.ajp.163.7.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips KA, Hollander E. Treating body dysmorphic disorder with medication: evidence, misconceptions, and a suggested approach. Body Image: Int J Res. 2008;5:13–27. doi: 10.1016/j.bodyim.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Collaborating Centre for Mental Health. National Clinical Practice Guideline Number 31. London: British Psychiatric Society and Royal College of Psychiatrists; 2006. Obsessive Compulsive Disorder: Core Interventions in the Treatment of Obsessive Compulsive Disorder and Body Dysmorphic Disorder. [PubMed] [Google Scholar]
- 19.Hollander E, Allen A, Kwon J, et al. Clomipramine versus desipramine crossover trial in body dysmorphic disorder: selective efficacy of a serotonin reuptake inhibitor in imagined ugliness. Arch Gen Psychiatry. 1999;56:1033–1039. doi: 10.1001/archpsyc.56.11.1033. [DOI] [PubMed] [Google Scholar]
- 20.Phillips KA, Albertini RS, Rasmussen SA. A randomized placebo controlled trial of fluoxetine in body dysmorphic disorder. Arch Gen Psychiatry. 2002;59:381–388. doi: 10.1001/archpsyc.59.4.381. [DOI] [PubMed] [Google Scholar]
- 21.Perugi G, Giannotti D, Di Vaio S, Frare F, Saettoni M, Cassano GB. Fluvoxamine in the treatment of body dysmorphic disorder (dysmorphophobia) Int Clin Psychopharmacol. 1996;11:247–254. doi: 10.1097/00004850-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Phillips KA, Dwight MM, McElroy SL. Efficacy and safety of fluvoxamine in body dysmorphic disorder. J Clin Psychiatry. 1998;59:165–171. doi: 10.4088/jcp.v59n0404. [DOI] [PubMed] [Google Scholar]
- 23.Phillips KA, Najjar F. An open-label study of citalopram in body dysmorphic disorder. J Clin Psychiatry. 2003;64:715–720. doi: 10.4088/jcp.v64n0615. [DOI] [PubMed] [Google Scholar]
- 24.Phillips KA. An open-label study of escitalopram in body dysmorphic disorder. Int Clin Psychopharmacol. 2006;21:177–179. doi: 10.1097/01.yic.0000194378.65460.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen A, Hadley SJ, Kaplan A, et al. An open-label trial of venlafaxine in body dysmorphic disorder. CNS Spect. 2008;13:138–144. doi: 10.1017/s1092852900016291. [DOI] [PubMed] [Google Scholar]
- 26.Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigo JM, Hans G, Nguyen L, et al. The anti-epilepetic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br J Pharmacol. 2002;136:659–672. doi: 10.1038/sj.bjp.0704766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon NM, Worthington JJ, Doyle AC, et al. An open-label study of levetiracetam for the treatment of social anxiety disorder. J Clin Psychiatry. 2004;65:1219–1222. doi: 10.4088/jcp.v65n0909. [DOI] [PubMed] [Google Scholar]
- 29.Coles ME, Phillips KA, Menard W, et al. Body dysmorphic disorder and social phobia: cross-sectional and prospective data. Depress Anxiety. 2006;23:26–33. doi: 10.1002/da.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto A, Phillips KA. Social anxiety in body dysmorphic disorder. Body Image: Int J Res. 2005;2:401–405. doi: 10.1016/j.bodyim.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly MM, Walters C, Phillips KA. Social anxiety and its relationship to functional impairment in body dysmorphic disorder. Behav Therapy. doi: 10.1016/j.beth.2009.01.005. in press. [DOI] [PubMed] [Google Scholar]
- 32.Wilhelm S, Otto MW, Zucker BG, Pollack MH. Prevalence of body dysmorphic disorder in patients with anxiety disorders. J Anxiety Disord. 1997;11:499–502. doi: 10.1016/s0887-6185(97)00026-1. [DOI] [PubMed] [Google Scholar]
- 33.Buhlmann U, Wilhelm S, McNally RJ, Tuschen-Caffier B, Baer L, Jenike MA. Interpretive biases for ambiguous information in body dysmorphic disorder. CNS Spectr. 2002;7:435–436. 441–443. doi: 10.1017/s1092852900017946. [DOI] [PubMed] [Google Scholar]
- 34.Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Compr Psychiatry. 2003;44:270–276. doi: 10.1016/S0010-440X(03)00088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips KA, Menard W, Fay C, Weisberg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with body dysmorphic disorder. Psychosomatics. 2005;46:317–332. doi: 10.1176/appi.psy.46.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Connor KM, Davidson JRT. Levetiracetam in social phobia: a placebo controlled pilot study. J Psychopharmacol. 2005;19:551–553. doi: 10.1177/0269881105056526. [DOI] [PubMed] [Google Scholar]
- 37.Grunze HC. The effectiveness of anticonvulsants in psychiatric disorders. Dialogues Clin Neurosci. 2008;10:77–89. doi: 10.31887/DCNS.2008.10.1/hcrgrunze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder. Psychopharmacol Bull. 1997;33:17–22. [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 40.National Institute of Mental Health. Rating scales and assessment instruments for use in pediatric psychopharmacology research. Psychopharmacol Bull. 1985;21:839. [PubMed] [Google Scholar]
- 41.Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: reliability and validity. Am J Psychiatry. 1998;155:102–108. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- 42.Miller IW, Bishop S, Norman WH, Maddever H. The modified Hamilton Rating Scale for depression: reliability and validity. Psychiatry Res. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 43.Connor KM, Davidson JRT, Churchill LE, Sherwood A, Foa E, Wesler R. Psychometric properties of the Social Phobia Inventory (SPIN) Br J Psychiatry. 2000;176:379–386. doi: 10.1192/bjp.176.4.379. [DOI] [PubMed] [Google Scholar]
- 44.Shear MK, Brown B, Clark DB. Anxiety disorder measures. In: Rush AJ, First MB, Blacker D, editors. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2007. pp. 529–558. [Google Scholar]
- 45.Brown GK, Beck AT, Newman CF, Beck JS, Tran GQ. A comparison of focused and standard cognitive therapy for panic disorder. J Anxiety Disord. 1997;11:329–345. doi: 10.1016/s0887-6185(97)00014-5. [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 47.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 48.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 49.Phillips KA, Albertini RS, Siniscalchi JM, Khan A, Robinson M. Effectiveness of pharmacotherapy for body dysmorphic disorder: a chart-review study. J Clin Psychiatry. 2001;62:721–727. doi: 10.4088/jcp.v62n0910. [DOI] [PubMed] [Google Scholar]
- 50.Rapaport MH, Clary C, Fayyad R, Endicott J. Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry. 2005;162:1171–1178. doi: 10.1176/appi.ajp.162.6.1171. [DOI] [PubMed] [Google Scholar]
- 51.Phillips KA, Kaye WH. The relationship of body dysmorphic disorder and eating disorders to obsessive-compulsive disorder. CNS Spectr. 2007;12:347–358. doi: 10.1017/s1092852900021155. [DOI] [PubMed] [Google Scholar]
- 52.Mula M, Pini S, Cassano GB. The role of anticonvulsant drugs in anxiety disorders. J Clin Psychopharmacol. 2007;27:263–272. doi: 10.1097/jcp.0b013e318059361a. [DOI] [PubMed] [Google Scholar]
- 53.Mazza M, Martini A, Scoppetta M, Mazza S. Effect of levetiracetam on depression and anxiety in adult epileptic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:539–543. doi: 10.1016/j.pnpbp.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Kinrys G, Worthington JJ, Wygant L, Nery F, Reese H, Pollack MH. Levetiracetam as adjunctive therapy for refractory anxiety disorders. J Clin Psychiatry. 2007;68:1010–1013. doi: 10.4088/jcp.v68n0705. [DOI] [PubMed] [Google Scholar]
- 55.Kinrys G, Wygant LE, Pardo TB, Melo M. Levetiracetam for treatment-refractory posttraumatic stress disorder. J Clin Psychiatry. 2006;67:211–214. doi: 10.4088/jcp.v67n0206. [DOI] [PubMed] [Google Scholar]