Abstract
Gonadal steroids organize the developing brain during a perinatal sensitive period and exert enduring consequences for adult behavior. In male rodents, testicular androgens are aromatized in neurons to estrogens and initiate multiple distinct cellular processes that ultimately determine the masculine phenotype. Within specific brain regions, overall cell number and dendritic morphology are principle targets of hormonal organization. Recent advances have been made in elucidating the cellular mechanisms by which the neurological underpinnings of sexually dimorphic physiology and behavior are determined. These include estradiol-mediated prostaglandin synthesis, presynaptic release of glutamate, postsynaptic changes in glutamate receptors and changes in cell adhesion molecules. Sex differences in cell death are mediated by hormonal modulation of survival and death factors such as TNFα and Bcl-2/BAX.
Sexual differentiation
The term “sexual differentiation” refers to a series of events that begins with sex determination by the SRY gene on the Y chromosome which programs the bipotential gonad to become a testis, followed by hormonally mediated cascades that direct the formation of the reproductive tract, genitalia, secondary sex characteristics and ultimately, the brain (reviewed in [1]). Production of testicular hormones during development coordinately organizes the brain and behavior in a process described by the Organizational/Activational Hypothesis of sexual differentiation [2]. This simple tenet articulates the complex notion that steroid hormones act on the developing brain to permanently organize it in a manner that directs the actions of adult hormones. Testosterone from the male testis masculinizes the brain during a perinatal sensitive period and as a result, the adult male brain is sensitive to the sex-behavior inducing effects of testosterone. Successful masculinization is evident in an adult male’s mounting, intromission and ejaculatory behavior with a sexually receptive female. The opposite of masculinization is feminization, an organizational process that occurs by default when there is insufficient gonadal steroid exposure during the critical period. Successful feminization is evident in estradiol-induced lordosis behavior that coincides with ovulation in adult females. A third and distinct organizational process is defeminization, the active removal of the female phenotype from the male brain. That this process is distinct from masculinization is evident in the unique cellular processes engaged and the separate behavioral outcome, the loss of capacity to express female sexual behavior but no induction of the capacity for male sexual behavior [3-5].
In addition to sexual differentiation of behavior, the same gonadal steroids determine the adult pattern of gonadotropin secretion. Adult males have steady pulsatile release of LH and FSH that is maintained by negative feedback control exerted on the GnRH neurons of the brain. Females retain the capacity for both positive and negative feedback control and as a result produce an LH surge that is necessary for ovulation. If females are exposed to high levels of testosterone or its metabolite, estradiol, during the perinatal critical period, both their sexual response and feedback control of LH will be masculinized, resulting in both behavioral and physiological sterility (reviewed in [6]). The discovery of androgen-induced sterility in newborn female rats was made over 50 years ago, but advances have begun to elucidate the cellular mechanisms by which these processes occur. This review will discuss these recent advances and underscore how the discovery of new and unique signal transduction pathways downstream of hormone receptor activation allow for further understanding of the unique relationship between brain structure and behavior.
Estradiol induces prostaglandin synthesis in the preoptic area to promote masculinization of sex behavior
The preoptic area (POA) is a critical brain region for the control of sexual behavior and is ideally suited for this function because it 1) coordinates behavior and endocrine responses [7], 2) robustly expresses gonadal hormone receptors and aromatase during development [8-11], 3) exhibits neural activity in the adult that correlates strongly with the expression of sex behavior [12], and 4) if damaged or destroyed, sexual behavior is diminished or lost completely [13]. Electrical stimulation of the medial POA initiates male sex behavior in non-copulating male rats [14], and the frequency of action potentials in some neurons positively correlates with both the onset and peak of male sexual behavior [15]. Moreover, when an electrophysiological approach called “kindling” is used to strengthen neurotransmission at medial preoptic area synapses, previously non-sexually active males will display robust male sex behavior [16]. Some of the largest sex differences in the brain are found in the POA and include profound differences in the size of particular subnuclei [17] and the density of excitatory synapses [18].
Prostaglandins are a class of lipid membrane-derived signaling molecules that subserve normal cellular functions relevant to reproduction, inflammation, hyperalgesia and fever (reviewed in [19]). The cyclooxygenase enzymes COX-1 and COX-2 limit the rate of prostaglandin synthesis, which includes up to eight distinct prostanoids determined by distinct synthetic enzymes from short-lived precursors [20]. Estradiol induces a two-fold increase in COX-1 and -2 and a seven-fold increase in Prostaglandin E2 (PGE2) but not other prostanoids in the preoptic area during the sensitive period for masculinization [21]. More importantly, newborn females treated with PGE2 readily exhibited male sexual behavior when given testosterone in adulthood, again suggesting PGE2 mediates estradiol signaling to organize male sexual behavior. Conversely, males treated neonatally with indomethacin, which inhibits COX-1 and COX-2, show no male sexual behavior in adulthood [21]. These behavioral changes are likely mediated by underlying changes in the density of dendritic spines, post-synaptic specializations containing synapses that propagate excitatory neurotransmission, on medial POA neurons. Normal males have twice the density of dendritic spines as females, and the density of dendritic spines on POA neurons correlates with measures of adult male sex behavior [22]. Treatment of newborn females with PGE2 increases dendritic spines to that of normal males whereas treatment of newborn males with a COX inhibitor reduces the density to that of normal females [18, 21]. The relationship between POA neuron dendritic spine density and male sexual behavior remains a correlational one, but this is true for all studies connecting synaptic plasticity with behavior. Further support for a causal relationship in the current system can be found in studies of neurological underpinnings in the behaving adult.
An estimated 95% of excitatory neurotransmission in the brain occurs at dendritic spines, and AMPA/kainate and NMDA glutamate receptors are highly enriched on the surface of these structures. In the adult brain, expression of male sexual behavior correlates with increased concentrations of the extracellular excitatory glutamate in the POA [23]. Antagonizing the NMDA receptor and consequently glutamatergic transmission in this brain region diminishes measures of male sexual behavior including the ratio of mounts to intromissions and the improvement in these measures with experience [12, 23, 24], whereas increasing synaptic glutamate has the opposite effect, enhancing male sexual performance [12].
Given the importance of glutamatergic neurotransmisstion to adult male sexual behavior, this same neurotransmitter would be predicted to be involved in PGE2-induced organization of the behavior during development. PGE2 signaling is propagated by at least one of four G-protein coupled receptors, EP1-4 [25, 26]. Of those, EP2 and EP4 are required for PGE2-mediated masculinization [22]. Both receptors recruit protein kinase A (PKA) [25, 26] which helps maintain AMPA/kainate receptor expression in the post-synaptic density of the dendritic spine [27-29]. In the developing POA, activation of PKA increases the density of dendritic spines, and inhibition of the AMPA/kainate and metabotropic glutamate receptors prevents this PKA-induced dendritic spine formation [30]. Similarly, combined neonatal inhibition of AMPA/kainate and metabotropic glutamate receptors or PKA alone prevents PGE2-induced masculinization of sex behavior. Neonatal administration of either the AMPA/kainate or metabotropic glutamate receptor agonists to females results in an adult that readily mounts and engages in intromission-like behaviors [30]. In contrast, females treated with AMPA and mGluR antagonists prior to PGE2 fail to mount or intromit [30]. The neonatal involvement of both AMPA/kainate and metabotropic glutamate receptors may function to ensure that the newly formed synapses are functionally competent and permit NMDA receptor activation in adulthood.
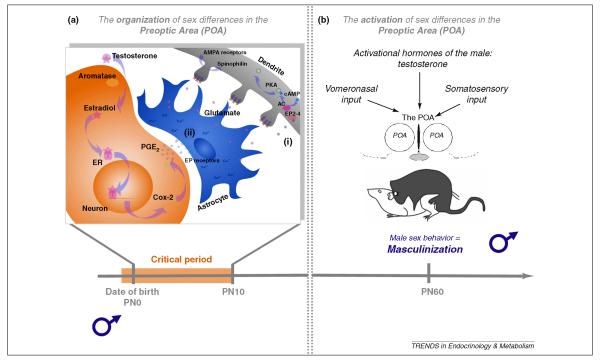
Taken together, these data suggest a working model in which estradiol increases the synthesis of COX-1 and -2 to promote PGE2 production in neurons. PGE2 then binds to EP2 and EP4 receptors, activating adenylyl cyclase, increasing cAMP and activating PKA which assures the AMPA/kainate receptors are anchored in the dendritic spine (Figure 1). In this way, estradiol initiates steps that increase the excitatory inputs onto POA neurons in order to promote male sexual behavior in adulthood. There is much that remains to be determined about this process, not the least of which is how the sex difference in the density of dendritic spines is maintained across the life span.
Figure 1. The organization of sex differences in the medial preoptic area (POA) neurons are important for the masculinization of behavior.
(a) During the perinatal critical period for sexual differentiation of the rodent brain, testosterone synthesized by the male testis is converted to estradiol within the neurons of the POA. Estradiol acts at its receptor in these neurons to up-regulate the expression of COX-2, which in turn increases the synthesis of PGE2. A working model proposes that PGE2 induces two simultaneous mechanisms; 1) activation of EP receptors located on neighboring astrocytes stimulates glutamate release, and 2) activation of EP receptors located on neurons activates PKA through the stimulation of adenlyl cyclase (AC), which supports the insertion of new glutamate receptors into the postsynaptic density of dendritic spines. The coordinated release of glutamate and clustering of AMPA receptors leads to formation and stabilization of new dendritic spines on POA neurons. (b) In adulthood, males (or females treated neonatally with estradiol) exhibit male sex behavior, and this is strongly correlated with the neural changes induced neonatally in the POA. The combination of the appropriate hormonal milieu and the detection of salient external cues from a sexually receptive female activate neuronal networks converging at the sexually differentiated POA to induce the expression of male sex behavior.
In contrast to its role as the critical downstream mediator of estradiol-induced masculinization, there is no role for prostaglandins in estradiol-mediated defeminization. In fact, females masculinized with PGE2 exhibit perfectly normal female sexual behavior, while males treated neonatally with COX inhibitors cannot be induced to exhibit lordosis when treated with appropriate hormones in adulthood [4]. Thus the cellular mechanisms for estradiol-mediated masculinization and defeminization are distinct. That there are separate signaling mechanisms for masculinization and defeminization was suggested by previous work identifying differences in the timing of the sensitive period and the relative importance of androgens versus estrogens and associated steroid receptor co-factors [31-33]. For example, estrogen can induce defeminization if given within the first week after birth, and can organize male sex behavior when given an additional three days later [34]. This divergence could be due to the very nature of sex behavior. Male sex behavior involves distinct sensory cues, muscle groups, motor plans, and motivations than female sexual behavior. The identification of PGE2 as a specific mediator of masculinization of behavior provided a valuable tool for independent exploration of the parallel process of defeminization.
Estradiol induces glutamate release in the hypothalamus to promote defeminization
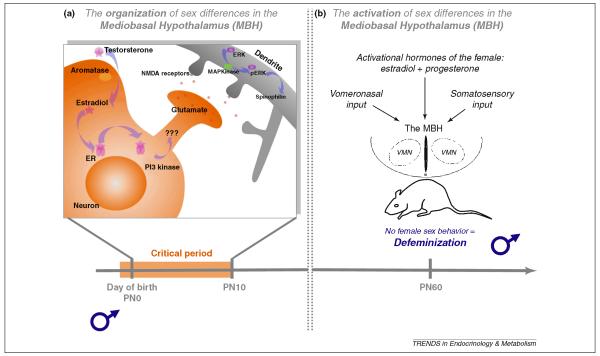
The ventromedial nucleus (VMN) located in the mediobasal hypothalamus (MBH) is a key brain region for control of female sexual behavior [35-37]. The dendrites of neurons in the male VMN branch more frequently and therefore have more overall dendritic spine synapses than females [38-40]. Estradiol induces both masculinization and defeminization, but via distinct cellular mechanisms. In the MBH, estradiol-induced defeminization begins with rapid (~1 hr) ER-mediated activation of PI3 kinase and enhanced release of presynaptic glutamate (Figure 2). The connection between PI3 kinase activation and glutamate release remains poorly characterized but is independent of protein synthesis. Increased synaptic glutamate leads to increased activation of post-synaptic NMDA receptors followed by dendritic branching and construction and stabilization of dendritic spines [41]. The latter process is dependent upon protein synthesis, but does not require ER, in the postsynaptic neuron. This means that a sequence of events leading to the sexual differentiation of the mediobasal hypothalamus begins with non-genomic effects of estradiol in a presynaptic neuron which then modifies the morphology of the postsynaptic neuron. This is achieved via the induction of glutamate release from the presynaptic neuron which binds to and activates NMDA receptors on the postsynaptic neuron. Calcium influx through the NMDA receptors leads to activation of MAP Kinase which, through an as yet to be identified signaling pathway, leads to increased transcription of genes associated with the construction and maintenance of dendritic spines. Activity-dependent dendritic growth and synaptogenesis is a common theme throughout brain development, but in the case of sexual differentiation, it is quite surprising that initiation begins with non-genomic effects of estradiol in one neuron to induce transcription and translation in the postsynaptic neuron that are independent of ER.
Figure 2. The organization of sex differences in the mediobasal hypothalamus (MBH) are important for defeminization of behavior in males.
Sexual differentiation of the male brain is a two-step process that requires both active masculinization and active removal of the female phenotype via defeminization. The same hormonal variables mediate these two processes, with testicular testosterone being converted to estradiol, but the brain region and mechanisms differ. (a) Estradiol initiates defeminization within the MBH by binding to its estrogen receptor (ER) and inducing rapid activation of PI3 kinase which enhances the release of glutamate. Increased release of glutamate activates postsynaptic glutamate receptors, in particular the NMDA receptor, leading to activation of MAP kinase and the formation/stabilization of new dendritic spines. (b) In adulthood, a male (or a female treated neonatally with estradiol) will not express female sex behavior (defeminization) even if treated with the appropriate hormones and provided the appropriate somatosensory cues.
The effects of estradiol on the presynaptic neuron are mediated by ERα, not ERβ [41]. Thus while the effects of estradiol are non-traditional, meaning non-genomic, in initiating sexual differentiation, the receptor mediating the effects are the classic ER. Moreover, these results demonstrate that estradiol-mediated sexual differentiation of the brain is not a cell autonomous process in which only ER-containing neurons change morphology in response to steroid exposure. Instead, a neurotransmitter serves as a signaling factor that elicits a morphological change in an entire network of cells, suggesting all neuronal inputs to sexually differentiated brain regions such as the POA and VMN are considered and interpreted depending on the animal’s sex, regardless of whether the incoming signals are relevant to sex or some other function of the POA. Both the POA and VMN subserve multiple other functions, including maternal behavior, temperature regulation and feeding, to name a few. Among the major challenges ahead is determining how inputs as various as olfaction and fear are processed by neurons in a sexually dimorphic way to produce the appropriate behavioral response.
Effects of estradiol on cell death in distinct subnuclei of the POA
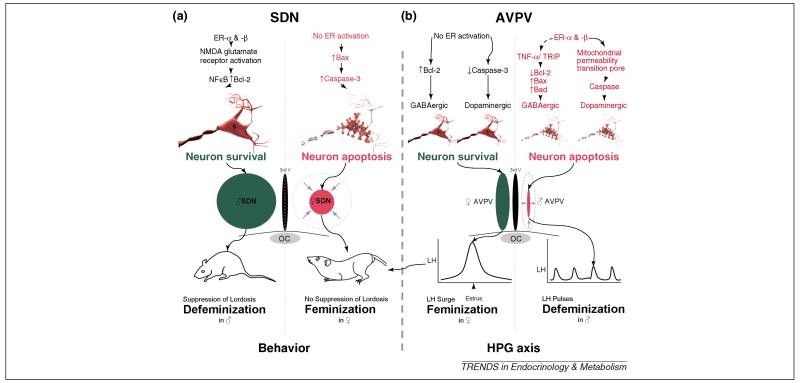
In addition to the profound sex differences in synaptic patterning, the POA is also a brain region characterized by the presence of two nuclei that differ in overall volume in males and females: the sexually dimorphic nucleus of the preoptic area (SDN-POA) and the anteroventral periventricular nucleus (AVPV). In both regions, males and females begin with the same number of neurons but there is selective cell death in one sex versus the other and in both instances, the sex difference in cell death is mediated by estradiol. The SDN is 3-5 times larger in males due to anti-apoptotic effects of estradiol while the AVPV is smaller in males due to pro-apoptotic effects of estradiol [6]. This remarkable divergence in estrogenic effects has not been fully explained but recent advances have shed light upon at least one source of the difference. TNFα is a proinflammatory cytokine that activates NFκB receptors and promotes cell survival. This pathway is constitutively active in AVPV neurons of neonatal females but repressed in male littermates. The suppression in males is the result of higher expression of an associated protein called TRIP (TNF receptor associated-associated factor 2-inhibiting protein) which inhibits both the TNFα-NFκB survival pathway and the anti-apoptotic protein, bcl-2 [42]. Whether the down regulation of TRIP in males is directly a result of estrogen action has not been established, but it has been determined that these events are exclusive to GABAergic neurons of the AVPV, and, equally importantly, Trip is not expressed in the SDN-POA, which consists largely of GABAergic neurons [43]. The selective involvement of GABA neurons of the AVPV is complemented by a parallel effect of estrogen-induced activation of caspase-dependent cell death in the dopaminergic neurons of the AVPV, reducing the number of this class of neurons in males as well [44]. The combination of estradiol effects simultaneously reduces both dopaminergic and GABAergic neurons, resulting in a significantly smaller male AVPV (Figure 3). The female AVPV is characterized by a large number of glutamatergic neurons which project to GnRH neurons [45, 46]; their stimulatory effect on those neurosecretory neurons are believed to mediate the estradiol-induced LH-surge that is essential for ovulation [47].
Figure 3. Organizational effects on cell survival are opposite in the SDN and AVPV.
(a) During the critical period for rodent brain sexual differentiation, estradiol mediates a 3-to-4 fold increase in the volume of SDN in males compared to females. NMDA glutamate receptor activation and Bcl-2 are higher in males than females, promoting neuron survival, while females have elevated pro-apoptotic proteins such as Bax and Caspase 3. (b) In contrast, in the AVPV, females experience greater dopaminergic and GABAergic neuron survival and increased AVPV volume. In males, higher levels of TNF-α and TRIP in GABAergic neurons selectively increases cell death. Exposure to estradiol also activates the mitochondrial permeability transition pore and caspases in dopaminergic neurons. The loss of GABAergic and dopaminergic neurons and consequent decrease in AVPV volume prevents males from exhibiting an LH-surge in adulthood.
The opposite effect is found in the SDN-POA, where estradiol promotes survival of neurons in males leading to a significantly larger overall volume of the nucleus (Figure 3). The mechanism here has been confirmed to also involve the apoptotic pathway, but the precise cellular mediators are not known, although several proteins of interest have been implicated including NMDA receptor glutamatergic signaling and neurotrophic proteins such as RNA binding motif protein 3 and alpha-tubulin [48-50]. The functional significance of a larger SDN remains a point of some contention but a consensus is emerging that it plays a role in defeminization of sexual behavior [51] and/ or partner preference [52]. In males demasculinized by treatment with COX inhibitors, the SDN is unaffected and these males are fully defeminized [4]. Lesioning the SDN in adult males can unmask a latent lordosis behavior [51]. In sheep, a small percentage of rams prefer to engage in sexual behavior with other rams, and the SDN in these males is smaller than that of rams that prefer ewes [52]. A similar pattern has been found for some hypothalamic nuclei in human male homosexuals versus heterosexuals [53], but it remains difficult to explain a highly complex human social behavior by variation in the number of a small collection of neurons.
It is worth nothing another system in which hormonally-mediated cell death is a major contributor to the establishment of a functional sex difference, the spinal nucleus of the bulbocavernosus (SNB). This collection of motor neurons in the lumbar spinal cord innervates the levator ani and bulbocavernosus muscles that control the penis. In a parallel manner to that seen for the SDN and AVPV, males and females begin life with the same number of SNB motor neurons but due to a lack of androgens during a critical developmental window, the majority of the cells die in females [54). In this system, the primary hormonal mediators are androgens, not estrogens. The mechanisms of androgen action are complex and involve both direct effects on the motor neurons and indirect effects via the muscles, but the essential principle of cell death as the regulated endpoint demonstrates the ubiquitousness of this strategy for establishment of sex differences (reviewed in [55]).
Challenges inherent in the study of feminization
Understanding the cellular basis of feminization is difficult on multiple levels. The definition of a feminized brain is one that supports the expression of female reproductive behavior and estradiol initiated positive feedback control of LH release. While the female brain is the default, it is not merely the absence of masculinization. The fact that defeminization is a distinct and active process negates the notion that the female brain is an undifferentiated brain. But how do we gain an understanding of what cellular processes mediate feminization? One approach is to identify factors that are inhibited or suppressed by the hormones mediating masculinization, such as testosterone and estradiol. A novel set of signaling molecules implicated as potential mediators of feminization come from studies on the roles of focal adhesion kinase (FAK) and its associated protein, paxillin. Both these proteins regulate cell adhesion and interaction with the extracellular matrix and are therefore essential for dendritic and axonal growth and branching [56] and dendritic spine morphology [57]. Newborn females have higher hypothalamic FAK and paxillin content than their male counterpoints [58], which is associated with reduced dendritic branching [59]; furthermore, treatment of females with estradiol drives down levels to that of males (Figure 4). Thus feminization could involve cellular processes that actively suppress neural arborization and consequently prevent the synapse formation off dendrites in brain areas that would otherwise suppress female sex behavior. Complete feminization no doubt involves many other signaling pathways and cellular changes which have yet to be identified and will be important for a complete understanding of the process of brain sexual differentiation.
Figure 4. Feminization is an active but poorly understood process.
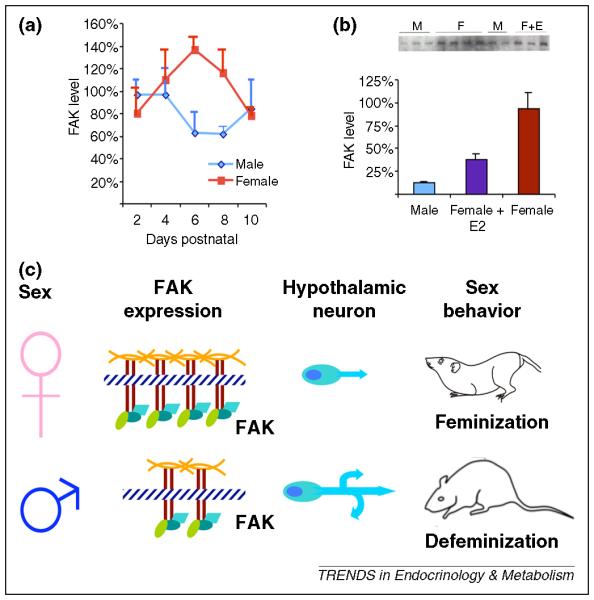
Challenges to understanding the mechanism(s) mediating feminization include the lack of an identified endocrine or genetic signal, and the conceptual distinction from defeminization. One approach is to identify factors that are elevated in the female brain but suppressed by the hormones mediating masculinization. (a) Focal-Adhesion Kinase (FAK) is a protein associated with the extracellar matrix and integral to neurite extension and branching. FAK is elevated in the female MBH compared to males, and neurons of this brain region exhibit a sexually dimorphic pattern of dendritic branching. (b) Treatment of females with estradiol suppresses FAK levels in the MBH, mimicking the levels found in males. (c) Increased FAK inhibits neurite outgrowth, which may contribute to the reductions in dendrite length and branching exhibited in the hypothalamic neurons of females.
Future directions
Fifty years ago the Organizational/Activational Hypothesis postulated that early hormonal effects are sufficiently enduring to be considered permanent and thereby constrain hormonal influences in adulthood. The organizational concept meshed well with the perception of the brain as a largely static organ in adulthood in which no new cells were born and neurons were connected via hard-wired circuits. Developmental hormonal effects on cell survival or synaptic connectivity would therefore de facto be permanent. But the concept of the brain as a plastic and dynamic organ that undergoes frequent cytoarchitectural reorganization has gained increasing credence. Adult neurogenesis and minute-to-minute formation and retraction of synapses are just two of the means by which a mature brain learns and responds to a changing environment. Even in those brain regions outside the classic neurogenic sites, such as the subventricular zone and subgranular proliferative zone of the dentate gyrus, new cells continue to be born into adolescence and beyond, including in the SDN, amygdala and BNST [60, 61]. Moreover, dendrites grow, retract and grow again, while synapses are formed and eliminated in stunningly short periods of time. In light of this dynamism, how can we explain the enduring organizational effects of developmental hormone exposure as the result of permanent changes in cell number or dendritic morphology? One way to envision organizational hormone action is it being akin to a memory or an imprint of a particular pattern, be it the rate of cell proliferation and death or the number and density of dendritic branches and spine synapses. This imprint would bias the brain to future plasticity that would regulate behavior in the adult. One feasible mechanism for establishing and maintaining such a memory is epigenetic changes to the genetic code following developmental hormone exposure. Control of gene expression both across the life span and in some instances across generations is regulated by the degree of methylation of specific nucleotides or histones on the associated chromatin, as well as acetylation of histones. Steroid receptors affiliate closely with enzymes controlling acetylation and are potent regulators of epigenetic changes in peripheral tissues (see for review [62]). In the brain, histone acetylation induced by neonatal testosterone has been implicated in the differential cell death that leads to sex differences in the principle nucleus of the bed nucleus of the stria terminalis [63]. Whether epigenetic changes induced by steroids underlie more of the many diverse and enduring effects of steroids is an area ripe for future exploration.
The field of neuroendocrinology is making considerable advances in the mechanistic basis of sex differences in brain areas relevant to reproduction, i.e. the preoptic area, hypothalamus and spinal cord, but we are lacking a similar level of achievement regarding other brain areas. Resolving this deficit is important for two reasons. First is the generalized assumption that the magnitude and impact of sex differences is equal in non-reproductive brain areas to that seen in the POA and hypothalamus, leading to the equally false assumption that sex differences in cognition and emotionality are robust and enduring, when in fact there is considerable evidence to the contrary [64]. Preliminary evidence suggests the mechanisms mediating sex differences in non-reproductive brain areas are distinct from those established in the reproductive axis, beginning with the observation of a sex difference in cell birth, not death, in the developing hippocampus [65]. Secondly, sex differences outside of the reproductive axis probably offer some explanation of the large gender bias in the frequency of neurological diseases and disorders of mental health, with boys being at substantially higher risk of autism and related spectrum disorders, attention deficit hyperactivity disorder and early onset schizophrenia, while women suffer disproportionately from major depression, anxiety, panic and feeding disorders (reviewed in [61]). That male-biased disorders largely have their origins in development and female-biased disorders are generally post-pubertal in onset offers important clues into the nature of the gender bias and highlights the importance of understanding how hormones impact on the developing brain.
Table 1. Masculinization and defeminization involve signal transduction pathways activated by perinatal estrogen exposure in the male and naïve female rodent model.
Masculinization involves the developmental induction of dendritic spines and prevention of cell death in the medial POA and SDN and expression of male sexual behavior in adulthood. Defeminization involves changes in neuronal number and morphology in the AVPV and VMN, resulting in dissolution of the estrus cycle and cessation of female sex behavior.
| Process | Masculinization | Defeminization | ||||
|---|---|---|---|---|---|---|
| Brain Area | Medial POA | VMN | SDN-POA | AVPV- POA | Arcuate | |
| Signal Cascade During Critical Period |
ER-α [66, 67] | ER-α & -β [5, 66] | ER-α & -β [68] | ER-α & -β [69] | ER-α & -β [70] | |
| Increase in COX-1 & -2 [21] |
PI3Kinase [41] | pNKCC1 [71] | ||||
| PGE2 [21] | Presynaptic glutamate release [41] |
NMDA glutamate receptor [48] |
TNF-α / TRIP [42] |
Mitochondria Permeability transition [42, 44] |
Excitatory GABA [72] |
|
| EP2 & EP4 [22] | NMDA glutamate receptor [39, 41] |
↑Bcl-2 [49] | ↓Bcl-2, ↑BAD, BAX [42] |
Caspase [42, 44] | L-Type VG-Ca2+ channels [73] |
|
| PKA/AKAP [30] | MAP kinase [41] |
|||||
| AMPA/kainate & mGluR signaling [30] |
FAK and Paxilin [58] |
Caspase-3 repression [50] |
GABA-ergic cell loss |
Dopaminergic cell loss [74] |
pCREB [73] | |
|
| ||||||
| Anatomical Change |
Dendritic branching [58] |
|||||
| Dendritic spine formation [18, 21] |
Dendritic spine formation [39, 76] |
Increased SDN volume [17] |
Decrease in AVPV volume [75] |
Decrease in dendritic spine number [40] |
||
|
| ||||||
| Behavioral or Physiological Change |
Male sex behavior [21, 30] |
Loss of female sex behavior [39] |
Loss of positive-feedback induced LH surge [47, 77] |
|||
Glossary
- AMPA/kainate glutamate receptors
a subclass of glutamate receptors agonized by either kainite or alpha-amino-3-hydroxyl-5-methyl-4i-isoxazole-propionate (AMPA).
- COX
cyclooxygenase enzymes that convert arachadonic acid to short-lived precursors of the prostanoid class of paracrine molecules.
- Defeminization
the developmental cellular processes that organize the brain and prevents the ability to express adult female sexual behaviors such as lordosis in adulthood.
- Feminization
the developmental cellular processes that organize the brain regions controlling female sexual behavior so that the behaviors such as lordosis can be displayed in adulthood.
- GABA
γ aminobutyric acid, an inhibitory amino acid neurotransmitter in the central nervous system
- Intromitting
the thrusting of the male rodent pelvis to make genital to genital contact with the female.
- Lordosis
the sexually receptive posture of the female rat involving dorsoflexion of the back and elevation of the rump.
- Masculinization
the developmental cellular processes that organize brain regions controlling male sexual behavior.
- Metabotropic glutamate receptors
The subtype of glutamate receptors that are G-protein coupled.
- Mounting
sex behavior of male rodents including but not limited to the grabbing of the females haunches by the male forepaws to stimulate the lordosis of the female.
- NMDA glutamate receptors
glutamate receptors agonized by N-methyl-D-aspartic acid that are also gated by a magnesium ion in the channel and requires depolarization in addition to glutamate binding in order to conduct.
- Organizational/Activational Hypothesis of the sexual differentiation of the brain
a hypothesis codifying the two separate effects of gonadal hormones in regulating sexually dimorphic behavior and physiology. The hypothesis predicts that once the brain regions controlling sex behavior are perinatally organized by the presence or absence of gonadal hormones, subsequent exposure in adulthood activates these brain areas, causing adaptive cellular and network processes. Subsequently, an animal will express male or female sexual behavior under the correct conditions.
- PGE2
prostaglandin E2, a labile arachadonic acid metabolite produced by the serial reactions of the cyclooxygenase and prostaglandin E synthase enzymes.
- SDN POA
sexually dimorphic nucleus of the preoptic area is 5-7 times larger in males than females
- SNB
spinal nucleus of the bulbocavernosus is located in the spinal cord and contains or controls motor neurons associated with the muscles that stimulate an ejaculatory reflex.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sinclair AH, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 2.Phoenix CH, et al. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 3.Reinisch JM, et al. Hormonal contributions to sexually dimorphic behavioral development in humans. Psychoneuroendocrinology. 1991;16:213–278. doi: 10.1016/0306-4530(91)90080-d. [DOI] [PubMed] [Google Scholar]
- 4.Todd BJ, et al. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm Behav. 2006;48:512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Kudwa AE, et al. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc Natl Acad Sci U S A. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy MM, et al. New tricks by an old dogma: mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillarp NA, et al. Evidence for the participation of the preoptic area in male mating behaviour. Experientia. 1954;10:224–225. doi: 10.1007/BF02159285. [DOI] [PubMed] [Google Scholar]
- 8.MacLusky NJ, et al. The development of estrogen receptor systems in the rat brain and pituitary: postnatal development. Brain Res. 1979;178:143–160. doi: 10.1016/0006-8993(79)90094-5. [DOI] [PubMed] [Google Scholar]
- 9.Shinoda K, et al. Neuronal aromatase expression in preoptic, strial, and amygdaloid regions during late prenatal and early postnatal development in the rat. J Comp Neurol. 1994;343:113–129. doi: 10.1002/cne.903430109. [DOI] [PubMed] [Google Scholar]
- 10.Pasterkamp RJ, et al. The perinatal ontogeny of estrogen receptor-immunoreactivity in the developing male and female rat hypothalamus. Brain Res Dev Brain Res. 1996;91:300–303. doi: 10.1016/0165-3806(95)00185-9. [DOI] [PubMed] [Google Scholar]
- 11.Beyer C, et al. Sex-specific aromatization of testosterone in mouse hypothalamic neurons. Neuroendocrinology. 1993;58:673–681. doi: 10.1159/000126608. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez JM, et al. Mating activates NMDA receptors in the medial preoptic area of male rats. Behav Neurosci. 2007;121:1023–1031. doi: 10.1037/0735-7044.121.5.1023. [DOI] [PubMed] [Google Scholar]
- 13.Christensen LW, et al. Effects of hypothalamic and preoptic lesions on reproductive behavior in male rats. Brain Res Bull. 1977;2:137–141. doi: 10.1016/0361-9230(77)90010-7. [DOI] [PubMed] [Google Scholar]
- 14.Malsbury CW. Facilitation of male rat copulatory behavior by electrical stimulation of the medial preoptic area. Physiol Behav. 1971;7:797–805. doi: 10.1016/0031-9384(71)90042-4. [DOI] [PubMed] [Google Scholar]
- 15.Pfaff DW, Pfaffmann C. Olfactory and hormonal influences on the basal forebrain of the male rat. Brain Res. 1969;15:137–156. doi: 10.1016/0006-8993(69)90315-1. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez-Salazar E, et al. Facilitation of male-like coital behavior in female rats by kindling. Behav Brain Res. 2003;140:57–64. doi: 10.1016/s0166-4328(02)00280-2. [DOI] [PubMed] [Google Scholar]
- 17.Gorski RA, et al. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 18.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bos CL, et al. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Chandrasekharan NV, Simmons DL. The cyclooxygenases. Genome Biol. 2004;5:241. doi: 10.1186/gb-2004-5-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 22.Wright CL, et al. Identification of prostaglandin E2 receptors mediating perinatal masculinization of adult sex behavior and neuroanatomical correlates. Dev Neurobiol. 2008;68:1406–1419. doi: 10.1002/dneu.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominguez JM, et al. Preoptic glutamate facilitates male sexual behavior. J Neurosci. 2006;26:1699–1703. doi: 10.1523/JNEUROSCI.4176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Regan JW, et al. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol Pharmacol. 1994;46:213–220. [PubMed] [Google Scholar]
- 26.Honda A, et al. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP2 subtype. J Biol Chem. 1993;268:7759–7762. [PubMed] [Google Scholar]
- 27.Dell’Acqua ML, et al. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur J Cell Biol. 2006;85:627–633. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Snyder EM, et al. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem. 2005;280:16962–16968. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akaneya Y, Tsumoto T. Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex. J Neurosci. 2006;26:10209–10221. doi: 10.1523/JNEUROSCI.3028-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright CL, McCarthy MM. Prostaglandin E2-Induced Masculinization of Brain and Behavior Requires Protein Kinase A, AMPA/Kainate, and Metabotropic Glutamate Receptor Signaling. J Neurosci. 2009;29:13274–13282. doi: 10.1523/JNEUROSCI.3603-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brand T, Slob AK. Perinatal flutamide and mounting and lordosis behavior in adult female Wistar and Sprague-Dawley rats. Behav Brain Res. 1991;44:43–51. doi: 10.1016/s0166-4328(05)80238-4. [DOI] [PubMed] [Google Scholar]
- 32.Vreeburg JT, et al. Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocrinol. 1977;74:375–382. doi: 10.1677/joe.0.0740375. [DOI] [PubMed] [Google Scholar]
- 33.Auger AP, et al. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Vries GJ, Simerly RB. Anatomy, Development and Function of Sexually Dimorphic Neural Circuits in the Mammalian Brain. In: Pfaff D, et al., editors. Hormones, Brain and Behavior. Academic Press; 2002. pp. 137–192. [Google Scholar]
- 35.Mathews D, Edwards DA. Involvement of the ventromedial and anterior hypothalamic nuclei in the hormonal induction of receptivity in the female rat. Physiol Behav. 1977;19:319–326. doi: 10.1016/0031-9384(77)90345-6. [DOI] [PubMed] [Google Scholar]
- 36.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- 38.Todd BJ, et al. Glutamate AMPA/kainate receptors, not GABA(A) receptors, mediate estradiol-induced sex differences in the hypothalamus. Dev Neurobiol. 2007;67:304–315. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz JM, McCarthy MM. The role of neonatal NMDA receptor activation in defeminization and masculinization of sex behavior in the rat. Horm Behav. 2008;54:662–668. doi: 10.1016/j.yhbeh.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mong JA, et al. Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: an electron microscopy analysis. J Comp Neurol. 2001;432:259–267. doi: 10.1002/cne.1101. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz JM, et al. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58:584–598. doi: 10.1016/j.neuron.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan S, et al. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci U S A. 2009;106:16692–16697. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Searles RV, et al. Sex differences in GABA turnover and glutamic acid decarboxylase (GAD(65) and GAD(67)) mRNA in the rat hypothalamus. Brain Res. 2000;878:11–19. doi: 10.1016/s0006-8993(00)02648-2. [DOI] [PubMed] [Google Scholar]
- 44.Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci. 2009;29:9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojeda SR, et al. Neuroendocrine mechanisms controlling female puberty: new approaches, new concepts. Int J Androl. 2006;29:256–263. doi: 10.1111/j.1365-2605.2005.00619.x. discussion 286-290. [DOI] [PubMed] [Google Scholar]
- 46.Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- 47.Adachi S, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 48.Hsu HK, et al. Gene regulation by NMDA receptor activation in the SDN-POA neurons of male rats during sexual development. J Mol Endocrinol. 2005;34:433–445. doi: 10.1677/jme.1.01601. [DOI] [PubMed] [Google Scholar]
- 49.Tsukahara S, et al. Estrogen modulates Bcl-2 family protein expression in the sexually dimorphic nucleus of the preoptic area of postnatal rats. Neurosci Lett. 2008;432:58–63. doi: 10.1016/j.neulet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Tsukahara S, et al. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol. 2006;66:1411–1419. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- 51.Hennessey AC, et al. Preoptic lesions increase the display of lordosis by male rats. Brain Res. 1986;370:21–28. doi: 10.1016/0006-8993(86)91100-5. [DOI] [PubMed] [Google Scholar]
- 52.Roselli CE, Stormshak F. The neurobiology of sexual partner preferences in rams. Horm Behav. 2009;55:611–620. doi: 10.1016/j.yhbeh.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byne W, et al. The interstitial nuclei of the human anterior hypothalamus: an investigation of variation with sex, sexual orientation, and HIV status. Horm Behav. 2001;40:86–92. doi: 10.1006/hbeh.2001.1680. [DOI] [PubMed] [Google Scholar]
- 54.Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- 55.Sengelaub DR, Forger NG. The spinal nucleus of the bulbocavernosus: firsts in androgen-dependent neural sex differences. Horm Behav. 2008;53:596–612. doi: 10.1016/j.yhbeh.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu G, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Fu WY. EphB maintains dendritic spine morphology through focal adhesion kinase. J Neurosci. 2009;29:13091–13093. doi: 10.1523/JNEUROSCI.4155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Speert DB, et al. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology. 2007;148:3391–3401. doi: 10.1210/en.2006-0845. [DOI] [PubMed] [Google Scholar]
- 59.Ivankovic-Dikic I, et al. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat Cell Biol. 2000;2:574–581. doi: 10.1038/35023515. [DOI] [PubMed] [Google Scholar]
- 60.Kashon ML, et al. Regulation of brain androgen receptor immunoreactivity by androgen in prepubertal male ferrets. Biol Reprod. 1995;52:1198–1205. doi: 10.1095/biolreprod52.5.1198. [DOI] [PubMed] [Google Scholar]
- 61.Martel MM, et al. Potential hormonal mechanisms of Attention-Deficit/Hyperactivity Disorder and Major Depressive Disorder: A new perspective. Horm Behav. 2009;55:465–479. doi: 10.1016/j.yhbeh.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCarthy MM, et al. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murray EK, et al. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Zhang JM, et al. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kudwa AE, et al. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 67.McCarthy MM, et al. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- 68.Patchev AV, et al. Differential role of estrogen receptor isoforms in sex-specific brain organization. Faseb J. 2004;18:1568–1570. doi: 10.1096/fj.04-1959fje. [DOI] [PubMed] [Google Scholar]
- 69.Bodo C, et al. Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147:415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- 70.Temple JL, et al. Estrogen receptor beta regulates sexually dimorphic neural responses to estradiol. Endocrinology. 2001;142:510–513. doi: 10.1210/endo.142.1.8054. [DOI] [PubMed] [Google Scholar]
- 71.Perrot-Sinal TS, et al. Sex differences in the chloride cotransporters, NKCC1 and KCC2, in the developing hypothalamus. J Neuroendocrinol. 2007;19:302–308. doi: 10.1111/j.1365-2826.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 72.Mong JA, et al. GABA mediates steroid-induced astrocyte differentiation in the neonatal rat hypothalamus. J Neuroendocrinol. 2002;14:45–55. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- 73.Perrot-Sinal TS, et al. Excitatory actions of GABA in developing brain are mediated by l-type Ca2+ channels and dependent on age, sex, and brain region. Neuroscience. 2003;116:995–1003. doi: 10.1016/s0306-4522(02)00794-7. [DOI] [PubMed] [Google Scholar]
- 74.Simerly RB, et al. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simerly RB, Swanson LW. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res. 1987;400:11–34. doi: 10.1016/0006-8993(87)90649-4. [DOI] [PubMed] [Google Scholar]
- 76.Matsumoto A, Arai Y. Male-female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- 77.Smith JT, et al. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150:5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]