Abstract
Rationale
Craving is often assumed to cause ongoing drug use and relapse and is a major focus of addiction research. However, its relationship to drug use has not been adequately documented.
Objectives
The aim of this study was to investigate the relationship between craving and drug use in real time and in the daily living environments of drug users.
Methods
In a prospective, longitudinal, cohort design (Ecological Momentary Assessment), 112 cocaine-abusing individuals in methadone maintenance treatment rated their craving and mood at random times (two to five times daily, prompted by electronic diaries) as they went about their everyday activities. They also initiated an electronic-diary entry each time they used cocaine. Drug use was monitored by thrice-weekly urine testing.
Results
During periods of urine-verified cocaine use, ratings of cocaine craving increased across the day and were higher than during periods of urine-verified abstinence. During the five hours prior to cocaine use, ratings of craving significantly increased. These patterns were not seen in ratings of heroin craving or mood (e.g., feeling happy or bored).
Conclusions
Cocaine craving is tightly coupled to cocaine use in users’ normal environments. Our findings provide previously unavailable support for a relationship that has been seriously questioned in some theoretical accounts. We discuss what steps will be needed to determine whether craving causes use.
Keywords: craving, Ecological Momentary Assessment, cocaine, mood, addiction, psychological theory
Craving—a conscious, reportable urge—is a frequently discussed aspect of drug addiction (Lowman et al. 2000; Pickens and Johanson 1992), but its exact role in addiction, particularly its relationship with drug use and relapse, has been disputed from both theoretical and clinical perspectives. Across the spectrum of addiction theories, craving is given varying degrees of importance as a driver of drug use (Drummond 2001). For example, in their incentive-sensitization theory, Robinson and Berridge (1993) posit that excessive incentive salience (“wanting”), experienced as craving, is the central pathology in addictive behavior. In contrast, Tiffany and Carter (1998) argue that craving is an epiphenomenon of thwarted drug use, rather than a cause of drug use.
Clinical studies of the relationship between craving and drug use have had mixed results. Some studies have shown that craving before or during treatment predicts post-treatment cocaine use (Baer et al. 1989; Hartz et al. 2001; Paliwal et al. 2008; Rohsenow et al. 2007; Weiss et al. 2003), while others have shown no relationship (Kranzler et al. 1999; Walton et al. 2003; Weiss et al. 1995). In laboratory studies, the amount of cocaine craving induced by stressors in experimental sessions predicts time to resumption of cocaine use in daily life (Sinha et al. 2006); similar findings have been reported for tobacco smokers, with either stress-induced (al’Absi et al. 2005) or cue-induced craving (Waters et al. 2004). However, during an experimental session, reductions in craving do not necessarily lead to reductions in drug self-administration (Haney and Spealman 2008; Leyton et al. 2005; Sofuoglu et al. 2009).
In spite of the mixed clinical data, much addiction research—especially in the context of animal models and neuroimaging—has focused on determining the neurological bases of craving, on the assumption that craving is central to use and relapse (Belin et al. 2008; Conrad et al. 2008; Reid et al. 2008; Volkow et al. 2006). Craving has also been identified as a potential treatment target (Addolorato et al. 2005; Heilig and Egli 2006; Leyton et al. 2005), though in this context, as well, some authors have expressed skepticism (Kosten et al. 2006; Miczek and de Wit 2008). If craving is ultimately not a relevant treatment target or a critical intermediary in addiction, then ongoing research efforts should be redirected toward a more productive target.
Much of this disagreement has occurred in the absence of data on the temporal relationship between cocaine craving and cocaine use in the user’s normal environment, i.e., during daily life in the community. As Tiffany and Carter (1998) noted: “The assumption that craving drives compulsive drug use requires that moment to moment variations in drug seeking and drug administration must be tightly coupled to changes in craving. The data of immediate relevance to this proposal are those that examine relationships between particular instances of drug use and craving that occurs in conjunction with that episode of use.” Ecological Momentary Assessment (EMA) is a research methodology ideally suited to conduct such an investigation. In EMA, study participants report on handheld electronic diaries, in real time, their activities and moods in their daily environments. EMA comprises two complementary types of data collection: entries prompted at random times (e.g., to assess levels of ongoing craving), and participant-initiated entries to collect information about specific events (e.g. discrete episodes of craving or use). Using EMA, Shiffman and colleagues have repeatedly shown that cigarette craving predicts smoking (during quit attempts and ad lib smoking) and that smoking rarely occurs in the total absence of craving (Catley et al. 2000; Shiffman et al. 1997a; Shiffman et al. 2002; Shiffman et al. 1997b; Shiffman et al. 1996). Similar associations have been shown in EMA studies of alcohol-dependent (Litt et al. 2000) and MDMA (ecstasy) users (Hopper et al. 2006).
We now report associations between cocaine craving and cocaine use as assessed by EMA during outpatient treatment. We previously showed that participant-initiated reports of cocaine use are tightly coupled to prior increases in exposure to drug-associated cues and changes in mood, within a time interval of five hours or less (Epstein et al. 2009b). In the analyses reported here, from the same data set, we examine participants’ ratings of ongoing cocaine craving at randomly timed assessments, in relation to: (a) periods of urine-verified cocaine use or abstinence, and (b) reports of individual episodes of cocaine use. (Unlike most of the EMA studies cited above, we examine the time course of craving hour by hour for the five hours preceding use.) Such data do not definitively establish causation, but can prospectively show the temporal relationships between cocaine use and cocaine craving in users’ everyday environments, providing new empirical bases for refinement of theory.
Methods and materials
Participants and Setting
Participants were polydrug-using (cocaine and heroin) individuals seeking outpatient treatment for opioid dependence. Inclusion criteria were: (1) age between 18 and 65 years; (2) physical dependence on opioids; and (3) evidence of cocaine and opiate use (by self-report and urine drug testing). Exclusion criteria were: (1) current psychotic disorder (by DSM-IV criteria); history of bipolar disorder; current major depressive disorder; (2) current dependence on alcohol or any sedative-hypnotic (by DSM-IV criteria); (3) cognitive impairment severe enough to preclude informed consent or valid self-report; and (4) medical illness that would compromise study participation. Psychiatric diagnoses were based on the Diagnostic Interview Schedule -version IV (Robins et al. 1995), a structured interview administered by a trained research technician. All participants also had a history and physical examination administered by a physician assistant who documented physical dependence on opioids. Subsequent chart review by a physician confirmed eligibility for methadone maintenance and for the study. All participants met DSM-IV dependence criteria for heroin and cocaine, although these were not inclusion requirements.
Upon enrollment, participants began receiving methadone maintenance therapy at a treatment-research clinic in Baltimore, MD. All participants received the same treatment, including daily oral methadone (target dose 100 mg/day), weekly individual drug counseling, and 12 weeks of abstinence reinforcement. Participants attended clinic 7 days a week for up to 28 weeks; urine drug screens were conducted three times per week. During the abstinence-reinforcement portion of treatment (weeks 7–18), all participants received vouchers in exchange for urine specimens negative for cocaine, opiates, or both; up to $2310 in vouchers were available for participants continuously abstinent from cocaine and opiates, as described previously (Epstein et al. 2003).
The Institutional Review Board (IRB) of the NIDA Intramural Research Program approved this study. Each participant gave written informed consent before being enrolled.
Study Design
The study was designed to assess the natural history of craving and lapse against a background of methadone maintenance and abstinence reinforcement (Epstein et al. 2009b). At the end of the third week of the study, each participant was trained to use and was issued a PDA (personal digital assistant, i.e. Palm Zire or Palm Zire 21, Palm, Inc., Sunnyvale, CA) running our Transactional Electronic Diary (TED) software (Vahabzadeh et al. 2004). Participants were instructed to make two types of entries: randomly prompted and event-contingent entries. At each entry, participants reported where they were, whom they were with, and what they were doing and answered questions about their mood. Random prompts were triggered 5 times per day for 5 weeks, then 2 times per day for 20 weeks, and were timed to occur only during each participant’s typical waking hours, which were programmed separately for each day of the week. Event-contingent entries were initiated by participants whenever they used cocaine or heroin or both drugs or craved drug without using.
At each random prompt, participants rated their ongoing cocaine craving and mood on a four-point scale: “NO!! no?? yes?? YES!!” These response anchors, based on psychometric work by Meddis (1972) and have provided useful information in several prior EMA studies (O’Connell et al. 1998; Shiffman et al. 2007; Shiffman et al. 2000; Shiffman et al. 2002; Shiffman et al. 1997c; Shiffman et al. 1996). Craving items were worded: “Right now, do you crave cocaine?” and “Right now, do you crave heroin?” Mood items were worded: “Right now, do you feel happy?,” “Right now, do you feel relaxed?,” “Right now, do you feel tired?,” “Right now, do you feel irritated?,” “Right now, do you feel stressed?,” and “Right now, do you feel bored?” Thus, these items were framed differently from those analyzed in our prior report from the same data set (Epstein et al. 2009b), in which the questions were worded “In the past hour, I felt…” and answered yes or no.
A total of 130 participants (84 men, 46 women) enrolled in the study; 112 (71 men, 41 women) attended clinic long enough to be issued a PDA and provided sufficient data for the main analyses reported here. These 112 participants did not differ significantly demographically from the other 18 in terms of their demographic characteristics or drug-use history (variables shown in table 1).
Table 1.
Participant Characteristics
| Participants | |
|---|---|
| N | 112 |
| Sex | 71 men, 41 women |
| Age | mean 40.7 (SD 8.1, range 20–58) years |
| Education | mean 11.8 years (SD 1.5, range 7–15) |
| Race/ethnicity | 61% African-American, 37% white, 2% Hispanic |
| Employment | 38% unemployed, 34% employed full-time, 27% employed part-time |
| Heroin Use at intake | |
| Days used in past 30 – mean 29.3 days (SD 3.3, range 5–30; the participant reporting 5 days of use had transferred from a community methadone program) | |
| Main route of administration - intravenous (IV, 61%) or intranasal (39%) | |
| Cocaine Use at intake | |
| Days used in last 30 – mean 20.0 days (SD 9.2, range 4–30) | |
| Main route of administration - smoked (48%), intravenous (42%), intranasal (8%) | |
A total of 102 participants (63 men, 39 women) provided random-prompt data during the five hours preceding an episode of cocaine use. (Each report of use was generally associated with no more than one or two random-prompt assessments in the preceding five hours; data were aggregated so that each of the five hours was represented.) They did not differ from the other 28 participants in terms of demographics or drug use history. Data on their use of the electronic diaries have been reported previously (Epstein et al. 2009b).
Data analysis
For statistical analyses, mood and craving ratings were converted from NO!!, no??, yes??, and YES!! to values 0, 1, 2, and 3, respectively. Thrice-weekly urine drug screens were used to identify periods of sustained abstinence (1 or more weeks of consecutive cocaine-negative specimens) or of sustained use (1 or more weeks of consecutive cocaine-positive specimens).
To compare craving during periods of abstinence versus periods of use, we examined random-prompt entries in mixed regressions using Proc Mixed in SAS version 9.1 (Littell et al. 1996). Proc Mixed performs an analysis that can be functionally described as a repeated-measures regression, though the output is more like that of an analysis of variance (including F values and least-squares means). Only entries from 6:00 AM to midnight were included due to sparsity of data from postmidnight hours; data were divided into 18 time bins (6:00–7:00 AM and so on). We checked the specificity of the craving findings by also examining ratings of heroin craving and of six mood variables. Each model had one dependent variable (a craving rating or a mood rating) and three independent variables: Abstinence (a time-varying predictor that could repeatedly alternate between present and absent within each participant), Time of Day (an 18-level categorical variable), and a control variable for the number of data points that each participant contributed to the analysis. The control was included to reduce potential bias associated with differences in protocol compliance. A first-order autoregressive error structure provided the best fit to the data. The models used the between-within method to determine denominator degrees of freedom (SAS Institute 2008); thus, the denominator degrees of freedom for abstinence (the time-varying predictor) do not reflect the full sample size, even though all 112 participants were included in the analyses. The “slice” option in Proc Mixed was used to generate post hoc F tests between the use and abstinence conditions during each time bin (6:00–7:00 AM, 7:00–8:00 AM, and so on); the 18 resultant p values were Bonferroni-corrected. Contrast coefficients were used to test for linear trends across the day.
To evaluate ratings of cocaine craving associated with individual episodes of cocaine use, we examined the random-prompt entries in each of the five hours preceding each event-contingent entry of cocaine use in generalized linear mixed models (Hu et al. 1998), using the GLIMMIX macro in SAS version 9.1. GLIMMIX performs an analysis that can be functionally described as a repeated-measures logistic regression, though the output is more like that of an analysis of variance (including F values). The selection of random-prompt entries was determined by their proximity to a subsequent event-contingent cocaine use entry. Again, we checked the specificity of the craving findings by also examining ratings of heroin craving and mood. Contrast coefficients were used to test for a linear trend in Hours Before Use. For the final set of analyses (Figures 4 and 5), Hours Before Use (1, 2, 3, 4, or 5) were the within-subject independent variable. To reduce potential bias associated with differences in protocol compliance or amount of data provided, each GLIMMIX model included a control term for the number of records that each participant contributed to the dataset. A first-order autoregressive error structure provided the best fit to the data. Post-use entries were not selected because each event-contingent entry led to a one-hour suspension of random prompts, thus limiting the post-use dataset.
Fig. 4.
Real-time participant ratings of cocaine and heroin craving in the five hours of random-prompt entries preceding each episode (participant-initiated report) of cocaine use. The median number of datapoints per bar is 91 (range 84 to 101). *Significant linear increase over time in post hoc contrast.
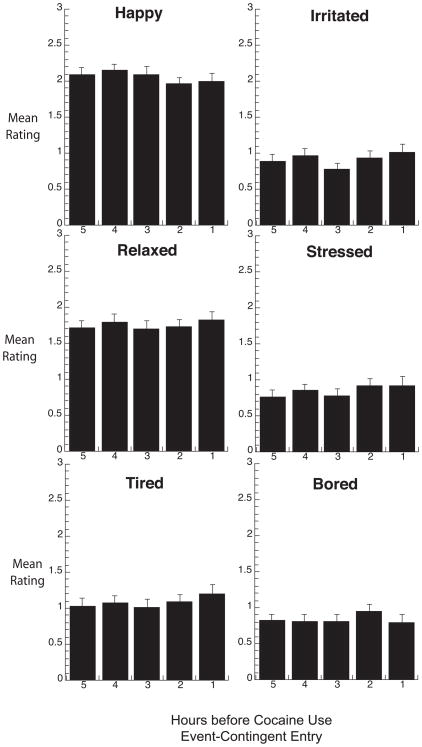
Fig. 5.
Real-time participant ratings of mood in the five hours of random-prompt entries preceding each episode (participant-initiated report) of cocaine use. Details are the same as those for Figure 4.
The criterion for significance was p ≤ .05, two-tailed. Effect sizes were expressed as correlation coefficients calculated from F values and degrees of freedom (Rosnow and Rosenthal 1996; Rosnow et al. 2000).
Results
During periods of abstinence and use, craving data were collected on 3,476 participant-days (mean 31.0, SEM 4.7 days) and 7,305 participant-days (mean 65.2, SEM 5.1 days), respectively. Of the 112 participants, 64 consistently tested positive and, thus, contributed data only during periods of cocaine use; 14 consistently tested negative and contributed data only during periods of cocaine abstinence, and 34 had periods of at least one week of testing positive and negative during the study and contributed data during periods of both use and abstinence.
Trends across the day during urine-verified periods of cocaine use or abstinence
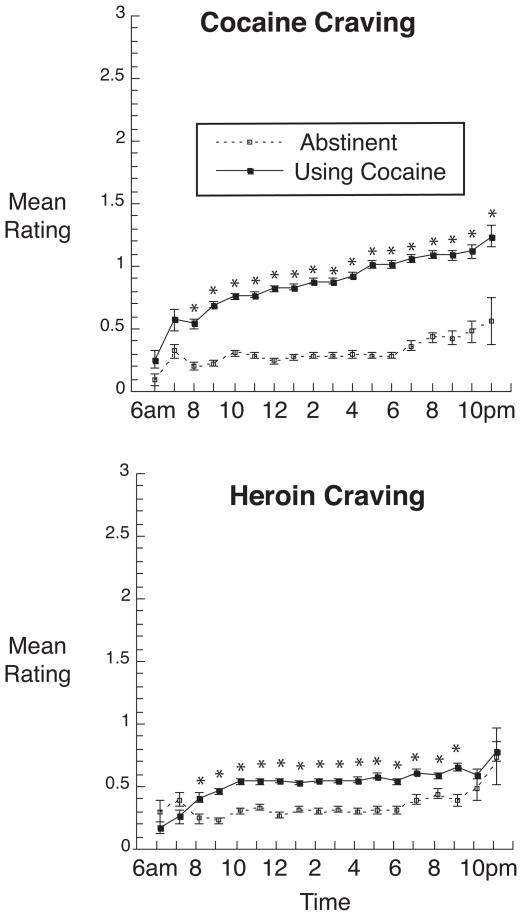
Figure 1 shows ratings of cocaine and heroin craving across the day. Cocaine-craving ratings were significantly higher during periods of use (least-squares mean = 0.85 ± .01) than during periods of abstinence (least-squares mean = 0.33 ± .02) [main effect of abstinence: F(1,33) = 396.28, p < .0001; effect-size r = 0.96]. Ratings of cocaine craving increased across the day during periods of use [Abstinence × Time interaction: F(17,306 = 5.09, p < .0001; linear trend across the day during periods of use: F(1,1191) = 135.52, p < .0001, effect-size r = 0.32; linear trend during periods of abstinence: F(1,538) = 1.36, n.s., effect-size r = 0.05]. Heroin-craving ratings were also significantly higher during periods of cocaine use (least-squares mean = 0.54 ± .02) than during periods of abstinence (least-squares mean = 0.36 ± .02) [main effect of abstinence: F(1,33) = 51.04, p < .0001; effect-size r = 0.78], but there was no interaction with time and no linear trend across the day [effect-size r = 0.02 during use, 0.04 during abstinence].
Fig. 1.
Time course of cocaine craving and heroin craving during periods of 1 or more weeks of cocaine abstinence or cocaine use across the day from 6 AM to 12 PM. Data shown are mean ratings on a four point (0 to 3 scale) collected from 112 participants. Brackets indicate SEM. Abstinence/use was a time-varying predictor; thus, 34 of the 112 participants contributed data to both the “abstinence” line and the “use” line. For cocaine abstinence, the median number of datapoints per symbol is 496 (range 24 to 789; all values over 100 from 8:00 am through 9:00 pm); for cocaine use, the median number of datapoints per symbol is 1,058 (range 63 to 1,579; all values over 100 from 7:00 am onward). *Significant difference between use and abstinence at this time point in Bonferroni-corrected post hoc F tests (“slice” option in SAS Proc Mixed).
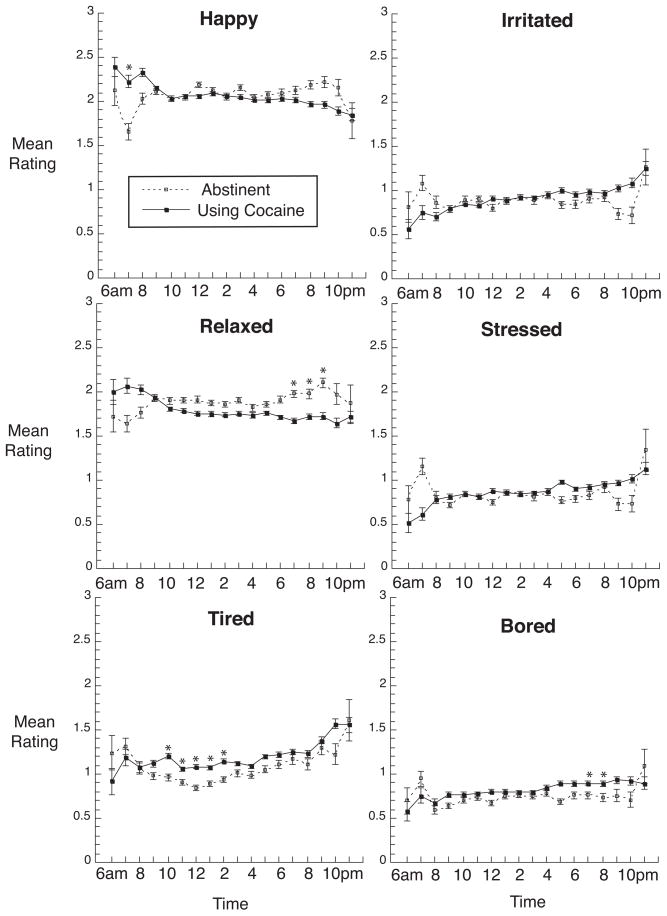
In contrast, three of the six mood ratings (Figure 2) were not significantly different during periods of use and abstinence [happy: F(1,33) = 1.46, p = .24, effect-size r = 0.21; irritated: F(1,33) = 0.37, p = .55, effect-size r = 0.11; stressed: F(1,33) = 0.10, p = .76, effect-size r = 0.05]. Periods of cocaine use were associated with higher ratings of boredom [F(1,33)=7.22, p = .01, effect-size r = 0.42], lower ratings of relaxation [F(1,33) = 11.09, p = .002, effect-size r = 0.50], and higher ratings of tiredness [F(1,33) = 4.15, p < .05, effect-size r = 0.33]. During periods of cocaine use, linear trends across the day were significant for tiredness, irritation, and stress [effect-size r’s = .10, .12, and .08, respectively]; during periods of cocaine abstinence, linear trends across the day were significant for tiredness and relaxation [effect-size r’s = .10 and .11, respectively].
Fig. 2.
Time course of mood ratings during periods of 1 or more weeks of cocaine abstinence or cocaine use across the day from 6 AM to 12 PM. Details are the same as those for Figure 1.
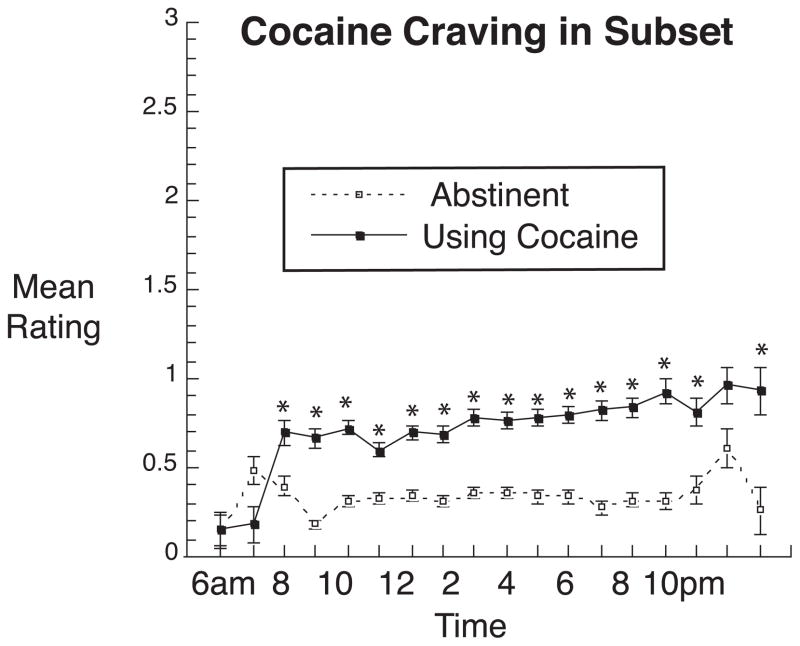
Although the above mixed regressions accounted for each participant’s pattern of contributions to the overall findings, we performed an additional mixed regression using only the subset of 34 participants who had periods of abstinence and periods of use lasting at least one week. For these 34 participants, craving ratings were significantly higher during periods of use (least-squares mean = 0.65 ± .02) than during periods of abstinence (least-squares mean = 0.32 ± .02) [F(1,33) = 107.09, < = .0001; effect-size r = 0.87] (Figure 3).
Fig. 3.
Identical to the top panel of Figure 1, with analyses restricted to the 34 participants who provided craving data during periods of both abstinence and use of cocaine. For cocaine abstinence, the median number of data points per symbol is 244 (range 13 to 400; all values over 100 from 9:00 am through 8:00 pm); for cocaine use, the median number of data points per symbol is 279.5 (range 15 to 457; all values over 100 from 8:00 am through 9:00 pm).
*Significant difference between use and abstinence at this time point in Bonferroni-corrected post hoc “slice” F tests.
Trends across the hours preceding specific reported episodes of cocaine use
In the five hours preceding individual reports of cocaine use, ratings of cocaine craving showed a significant linear increase (F(1,124) = 8.66, p=.004; Fig 4, top panel) whose effect size was r = .27. Ratings of heroin craving (Figure 4, bottom panel) and mood (Figure 5) did not change in the hours preceding cocaine use.
Discussion
These results show a significant positive relationship between cocaine craving and cocaine use during daily life in users’ normal environments. This conclusion is supported by the data in two ways. First, ratings of craving were significantly higher during weeks of urine-verified cocaine use than during weeks of urine-verified cocaine abstinence. This was true when evaluated across all 112 participants (Figure 1) and when evaluated in only those 34 participants who contributed data during periods of both abstinence and use (Figure 3). Second, during the five hours preceding specific episodes of reported cocaine use, ratings of cocaine craving at randomly timed prompts showed a significant linear increase (Figure 4). These effects were largely specific to cocaine craving: during weeks of verified cocaine abstinence versus use, ratings of heroin craving and mood did not show the same patterns of differences, or showed them to lesser degrees (Figures 1 and 2), and during the five hours preceding occasions of cocaine use, ratings of heroin craving and mood showed no systematic changes (Figures 4 and 5).
Our observational data meet the criteria proposed by Tiffany and Carter (1998) for establishing “relationships between particular instances of drug use and craving that occurs in conjunction with that episode of use.” Our data also fulfill a suggestion by Kassell and Shiffman (1992) that a longitudinal study be conducted “in which non-abstinent individuals continuously self-monitor both their craving episodes and drug use.” Our data provide previously unavailable evidence that the time courses of cocaine craving and use during daily life in a community setting are consistent with a causal effect; they do not, however, conclusively demonstrate that cocaine craving causes cocaine use. To test whether the relationship is causal, future studies might experimentally manipulate craving in daily life, as by incorporating EMA into a randomized trial of a treatment whose only direct effect is reliable blockade of craving (no such medication exists for cocaine craving) or by assigning participants randomly to craving-induction procedures coupled with EMA in daily life. The latter approach has been tried in non-treatment-seeking cigarette smokers; results showed no association between cue-induced craving and latency to the next cigarette, but the authors noted that this portion of their findings needed to be interpreted with caution because participants recorded a mean of only 56% of the number of cigarettes per day that they had reported typically smoking at study entry (Warthen and Tiffany 2009). Reinforcement for complete EMA recording, perhaps using bogus-pipeline procedures (Aguinis and Henle 2001; Sigall 1997), could improve the reliability of this approach and enable strong conclusions about causation.
One limitation of our data is that, to compare biochemically verified periods of use and abstinence (Figures 1–3), we had to rely on thrice-weekly urine-screen data, whose temporal resolution did not permit comparison of a day of use with an immediately preceding day of abstinence, as in studies of cigarette smokers that included breath monitoring (Shiffman and Waters 2004). Therefore, the time scale of this portion of our findings is relatively crude. However, when this portion of our findings is taken together with the prospectively recorded buildup in craving before specific episodes of cocaine use (Figure 4), the evidence appears to converge in support of a tight temporal coupling between craving and use.
Another limitation of our data is that all participants, despite using both cocaine and heroin during the study, were on methadone maintenance to reduce their heroin use. This was the main reason we chose to focus on cocaine rather than heroin in the analyses reported here. The fact that all participants were in treatment might also limit the generalizability of our findings. However, much of the EMA research on craving and smoking has been conducted in treatment seekers. Behavioral treatments for cocaine dependence are frequently tested against a background of methadone maintenance for opiate dependence (Griffith et al. 2000; Prendergast et al. 2006). A comparison of predictors of stimulant-use outcomes in methadone-maintained patients versus patients in community psychosocial treatment clinics showed that the single best predictor (testing cocaine-positive at study intake) was the same in both populations; other predictors were similar, though not identical (Peirce et al. 2009). We have found that methadone has no direct effect on cocaine use (Epstein et al. 2009a). It is possible that the relationship between craving and drug use is different in non-treatment-seeking users, but this is a question for future research.
Another limitation of our data is that participants were not given explicit instructions as to what constituted an episode of cocaine use; they were simply told to “make an entry whenever you use.” If use occurred in a series of bouts separated by only an hour or two, some participants might have reported this as a single episode, while others might have reported it as multiple episodes. However, the latter type of reporting was uncommon: of the 720 reports of cocaine use collected in the study overall, only 35 (fewer than 5%) occurred within an hour of a prior report of use, and only another nine (fewer than 2%) occurred within two hours; the median delay between pairs of cocaine-use reports was 2 days and 18 hours.
A limitation in our assessment of mood is that we did not use enough adjectives to represent all possible combinations of valence and arousal (Russell 1980); we have corrected this in our ongoing studies.
The random-prompt data used in the analyses of the 5 hours preceding cocaine use represented 46% of all cocaine-use reports (i.e., no random prompts occurred in the five hours prior to the other cocaine-use reports). A possible limitation of our study is that these episodes of cocaine use are not representative of all reported episodes. The most likely source of difference is time of day: because random-prompt data were only collected during pre-established wake times, use reports could have occurred at times when the preceding 5 hours fell outside of normal wake times and would, thus, not be represented in the analyses. However, the distribution across the day of uses included was similar to that of the uses not included. The only times not represented were between 3:00 AM and 9:00 AM, which accounted for only 2.8% of all cocaine-use reports (data not shown).
Another limitation is that, based on comparison of the EMA data and thrice-weekly urine screens (data not shown), we know that not all episodes of cocaine use were reported (and the temporal resolution of the urine-screen data does not allow us to determine how many uses were unreported). It is possible that the precipitants of unreported uses were different from those of reported uses, but we consider that possibility remote. However, in ongoing studies, we are attempting to reinforce accuracy in reporting. We should also note that the use entries themselves were not always made immediately after cocaine use. According to data from the use entries, 15% were made within 5 minutes of use, another 22% were made within 15 minutes of use, and another 30% were made within 30 minutes of use, leaving one-third that were made at longer delays. These delays probably reduced our power to detect temporal relationships between craving and use, thus arguably making our findings more impressive.
Our finding that ratings of mood were not associated with cocaine use (Figure 5) may seem inconsistent with our previously reported findings from the same data set, in which reports of mood did change during the hours before cocaine use (Epstein et al. 2009b). Those findings reflected responses to the questions, “In the past hour, I felt…,” each answered yes or no, with mood items worded differently from those discussed here; the present findings reflected responses to questions with the time frame “right now,” rated on a multipoint scale. These differing results underscore the potential importance of seemingly small differences in phrasing and response anchoring. However, in the analyses reported here, different patterns of results were seen for cocaine craving and other items when each was assessed with an identically framed question.
The findings we show in Figure 4 are consistent with findings in cigarette smokers by Shiffman and colleagues (cited earlier) and are also strikingly similar to a finding in cigarette smokers by Carter and colleagues (2008), in which EMA reports of craving at random intervals were lower than those reported just before smoking. The findings we show in Figures 1 and 3 are consistent with an earlier finding from our clinic that when cocaine use was reduced by behavioral treatment, cocaine craving was also reduced (Silverman et al. 1998). The latter finding seems likely to have been secondary to reductions in use; thus, taken together, our prior and current findings are consistent with a bidirectional effect of cocaine craving and cocaine use on each other. The causes of cocaine use probably vary not only across individuals, but within each individual, so that there may be no single correct answer to the question of whether craving causes use (Epstein et al. in press). However, we think our findings support continued efforts to develop a treatment that suppresses cocaine craving. Such a treatment might also serve as a tool to enable an experimental EMA study that can directly address the question of cocaine craving’s causal effects in daily life.
Acknowledgments
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA), National Institutes of Health. We thank the NIDA IRP Archway Clinic staff for data collection.
Footnotes
Financial disclosures/Conflicts of interest: None to declare.
References
- Addolorato G, Abenavoli L, Leggio L, Gasbarrini G. How many cravings? Pharmacological aspects of craving treatment in alcohol addiction: a review. Neuropsychobiology. 2005;51:59–66. doi: 10.1159/000084161. [DOI] [PubMed] [Google Scholar]
- Aguinis H, Henle CA. Empirical assessment of the ethics of the bogus pipeline. J Applied Social Psychology. 2001;31:352–375. [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–17. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- Baer JS, Kamarck T, Lichtenstein E, Ransom CC., Jr Prediction of smoking relapse: analyses of temptations and transgressions after initial cessation. J Consult Clin Psychol. 1989;57:623–7. doi: 10.1037//0022-006x.57.5.623. [DOI] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Carter BL, Lam CY, Robinson JD, Paris MM, Waters AJ, Wetter DW, Cinciripini PM. Real-time craving and mood assessments before and after smoking. Nicotine Tob Res. 2008;10:1165–9. doi: 10.1080/14622200802163084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D, O’Connell KA, Shiffman S. Absentminded lapses during smoking cessation. Psychol Addict Behav. 2000;14:73–6. doi: 10.1037//0893-164x.14.1.73. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC. Theories of drug craving, ancient and modern. Addiction. 2001;96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: findings during treatment and across 12-month follow-up. Psychol Addict Behav. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Schmittner J, Umbricht A, Schroeder JR, Moolchan ET, Preston KL. Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend. 2009a;101:92–100. doi: 10.1016/j.drugalcdep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Preston KL. Addiction and emotion: theories, assessment techniques, and treatment implications. In: Kassel JD, editor. Substance Abuse and Emotion. American Psychological Association; Washington, D.C: in press. [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin J-L, Preston KL. Real-time electronic-diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiat. 2009b;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depend. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–19. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–76. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–76. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Su Z, Looby AR, Ryan ET, Penetar DM, Palmer CM, Lukas SE. Incidence and patterns of polydrug use and craving for ecstasy in regular ecstasy users: an ecological momentary assessment study. Drug Alcohol Depend. 2006;85:221–35. doi: 10.1016/j.drugalcdep.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. American Journal of Epidemiology. 1998;147:694–703. doi: 10.1093/oxfordjournals.aje.a009511. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Shiffman S. What can hunger teach us about drug craving? A comparative analysis of the two constructs. Adv Behav Res Ther. 1992;14:141–167. [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacol. 2006;31:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Mulgrew CL, Modesto-Lowe V, Burleson JA. Validity of the Obsessive Compulsive Drinking Scale (OCDS): does craving predict drinking behavior? Alcohol Clin Exp Res. 1999;23:108–14. [PubMed] [Google Scholar]
- Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci. 2005;119:1619–27. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Publications; 1996. [Google Scholar]
- Lowman C, Hunt WA, Litten RZ, Drummond DC. Research perspectives on alcohol craving: an overview. Addiction. 2000;95(Suppl 2):S45–54. doi: 10.1080/09652140050111636. [DOI] [PubMed] [Google Scholar]
- Meddis R. Bipolar factors in mood adjective checklists. Br J Soc Clin Psychol. 1972;11:178–84. doi: 10.1111/j.2044-8260.1972.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, de Wit H. Challenges for translational psychopharmacology research--some basic principles. Psychopharmacology. 2008;199:291–301. doi: 10.1007/s00213-008-1198-4. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Gerkovich MM, Cook MR, Shiffman S, Hickcox M, Kakolewski KE. Coping in real time: using Ecological Momentary Assessment techniques to assess coping with the urge to smoke. Res Nurs Health. 1998;21:487–97. doi: 10.1002/(sici)1098-240x(199812)21:6<487::aid-nur3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–9. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Roll JM, Kolodner K, Krasnansky J, Stabile PQ, Brown C, Stitzer ML. Correlates of stimulant treatment outcome across treatment modalities. Am J Drug Alcohol Abuse. 2009;35:48–53. doi: 10.1080/00952990802455444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens RW, Johanson CE. Craving: consensus of status and agenda for future research. Drug Alcohol Depend. 1992;30:127–31. doi: 10.1016/0376-8716(92)90017-7. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–60. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Reid MS, Flammino F, Howard B, Nilsen D, Prichep LS. Cocaine cue versus cocaine dosing in humans: evidence for distinct neurophysiological response profiles. Pharmacol Biochem Behav. 2008;91:155–64. doi: 10.1016/j.pbb.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM., III . The Diagnostic Interview Schedule, version IV. St. Louis, MO: Washington University; 1995. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Eaton CA, Monti PM. Cocaine craving as a predictor of treatment attrition and outcomes after residential treatment for cocaine dependence. J Stud Alcohol Drugs. 2007;68:641–8. doi: 10.15288/jsad.2007.68.641. [DOI] [PubMed] [Google Scholar]
- Rosnow RL, Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people’s published data: general procedures for research consumers. Psychol Methods. 1996;1:331–340. [Google Scholar]
- Rosnow RL, Rosenthal R, Rubin DB. Contrasts and correlations in effect-size estimation. Psychol Sci. 2000;11:446–53. doi: 10.1111/1467-9280.00287. [DOI] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. doi: 10.1037//0022-3514.79.2.286. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT 9.2 User’s Guide. SAS Institute, Inc. SAS Institute, Inc; 2008. [Google Scholar]
- Shiffman S, Balabanis MH, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Hickcox M, Paton SM. Prediction of lapse from associations between smoking and situational antecedents assessed by ecological momentary assessment. Drug Alcohol Depend. 2007;91:159–68. doi: 10.1016/j.drugalcdep.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Paty JA, Engberg J, Gwaltney CJ, Liu KS, Gnys M, Hickcox M, Paton SM. Dynamic effects of self-efficacy on smoking lapse and relapse. Health Psychol. 2000;19:315–23. doi: 10.1037//0278-6133.19.4.315. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: predicting smoking lapse from daily urge. J Abnorm Psychol. 1997a;106:104–16. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, Hickcox M, Gnys M. Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–45. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Richards T, Kassel JD. Individual differences in the context of smoking lapse episodes. Addict Behav. 1997b;22:797–811. doi: 10.1016/s0306-4603(97)00063-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. J Consult Clin Psychol. 1997c;65:292–300. doi: 10.1037/0022-006x.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Sigall H. Ethical considerations in social psychological research: is the bogus pipeline a special case? J Appl Soc Psychol. 1997;27:574–81. doi: 10.1111/j.1559-1816.1997.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol. 1998;66:811–24. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, O’Malley SS. Minocycline reduced craving for cigarettes but did not affect smoking or intravenous nicotine responses in humans. Pharmacol Biochem Behav. 2009;92:135–40. doi: 10.1016/j.pbb.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Vahabzadeh M, Epstein DH, Mezghanni M, Lin J-L, Preston KL. An Electronic Diary Software for Ecological Momentary Assessment (EMA) in Clinical Trials. Proceedings of the 17th IEEE Symposium on Computer-Based Medical Systems (CBMS); 2004. pp. 167–172. [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MA, Blow FC, Bingham CR, Chermack ST. Individual and social/environmental predictors of alcohol and drug use 2 years following substance abuse treatment. Addict Behav. 2003;28:627–42. doi: 10.1016/s0306-4603(01)00284-2. [DOI] [PubMed] [Google Scholar]
- Warthen MW, Tiffany ST. Evaluation of cue reactivity in the natural environment of smokers using ecological momentary assessment. Exp Clin Psychopharmacol. 2009;17:70–7. doi: 10.1037/a0015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Cue-provoked craving and nicotine replacement therapy in smoking cessation. J Consult Clin Psychol. 2004;72:1136–43. doi: 10.1037/0022-006X.72.6.1136. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Hufford C. Craving in hospitalized cocaine abusers as a predictor of outcome. Am J Drug Alcohol Abuse. 1995;21:289–301. doi: 10.3109/00952999509002698. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Mazurick C, Berkman B, Gastfriend DR, Frank A, Barber JP, Blaine J, Salloum I, Moras K. The relationship between cocaine craving, psychosocial treatment, and subsequent cocaine use. Am J Psychiatry. 2003;160:1320–5. doi: 10.1176/appi.ajp.160.7.1320. [DOI] [PubMed] [Google Scholar]