Abstract
Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) has been shown to have selective antitumor activity. TRAIL induces ubiquitous pathways of cell death in which caspase activation is mediated either directly or via the release of apoptogenic factors from mitochondria; however, the precise components of the mitochondrial signaling pathway have not been well defined. Notably, mitochondria constitute an important target in overcoming resistance to TRAIL in many types of tumors. Bid is considered to be fundamental in engaging mitochondria during death receptor–mediated apoptosis, but this action is dependent on mitochondrial lipids. Here, we report that TRAIL signaling induces an alteration in mitochondrial membrane lipids, particularly cardiolipin. This occurs independently of caspase activation and primes mitochondrial membranes to the proapoptotic action of Bid. We unveil a link between TRAIL signaling and alteration of membrane lipid homeostasis that occurs in parallel to apical caspase activation but does not take over the mode of cell death because of the concurrent activation of caspase-8. In particular, TRAIL-induced alteration of mitochondrial lipids follows an imbalance in the cellular homeostasis of phosphatidylcholine, which results in an elevation in diacylglycerol (DAG). Elevated DAG in turn activates the δ isoform of phospholipid-dependent serine/threonine protein kinase C, which then accelerates the cleavage of caspase-8. We also show that preservation of phosphatidylcholine homeostasis by inhibition of lipid-degrading enzymes almost completely impedes the activation of pro-caspase-9 while scarcely changing the activation of caspase-8.
Introduction
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL), a type II transmembrane protein, is a ubiquitous member of the TNF family of ligands (i.e., Fas, TNF-α, etc.; refs. 1-7). TRAIL primarily induces apoptosis in transformed or tumor cells and hence has great promise as a selective anti-cancer agent (1, 4, 5, 7-9). Additionally, the functional expression of TRAIL has been shown on the surface of natural killer cells, dendritic cells, monocytes, and T cells that have been stimulated by cellular transformation, thus suggesting a role for TRAIL in the selective ablation of transformed cells before tumorigenesis (10-15).
TRAIL receptors, DR4 and DR5, mediate TRAIL function by recruiting, via death domain interactions, adaptor proteins, such as FADD, and apical caspases, such as pro-caspase-8. This assembly forms the death-inducing signaling complex (DISC; refs. 3-5, 16-18), which promotes the autoproteolytic activation and release of apical caspases (19, 20). Activated apical caspases then initiate two main pathways of cell death signaling (21-24): the mitochondria-independent pathway and the mitochondria-dependent pathway. In the mitochondria-independent pathway, apical caspases directly activate executioner caspases, such as caspase-3 (17, 18, 23, 25, 26). In most cells (type II cells), including many tumor cells (18), death signaling bifurcates into a mitochondria-dependent pathway in which caspases are activated downstream of the release of mitochondrial apoptogenic factors into the cytosol in a manner equivalent to the intrinsic apoptotic pathway (16-18, 21, 27).
Mitochondria are targets of proapoptotic proteins of the Bcl-2 family, such as the widespread BH3-only member, Bid (27-34), the genetic ablation of which reduces Fas-induced hepatotoxicity and mitochondrial damage (35). The caspase-cleaved form of Bid, tBid, migrates to the mitochondrial outer membrane, where it cooperates with other Bcl-2 family proteins, such as Bak or Bax, to induce the release of mitochondrial proteins into the cytosol (28, 29, 36-38). Although it is now generally accepted that tBid constitutes the fundamental link between the DISC and mitochondria (28), some observations suggest that parallel, caspase-independent signals could be delivered to the mitochondria during death receptor–mediated cell death (39-41). However, the manner by which alternative pathways emanating from TRAIL receptors reach and affect the mitochondria remains unknown.
Cardiolipin is a membrane lipid uniquely present in mitochondria that may be instrumental for the proapoptotic action of tBid and Bax (31, 37, 42). Indeed, tBid binds to a byproduct of cardiolipin degradation, monolysocardiolipin (38, 43). However, the signaling steps that connect death receptors to changes in cardiolipin remain elusive. Given that phosphatidylcholine, the dominant phospholipid present in intracellular membranes, is involved in cardiolipin remodeling (37, 44), cardiolipin changes could also reflect altered phosphatidylcholine homeostasis. Intriguingly, phosphatidylcholine homeostasis influences the cellular level of diacylglycerol (DAG), which in turn activates members of the protein kinase C (PKC) family that regulate phosphatidylcholine metabolism (45-47). Therefore, changes in the cellular content of phosphatidylcholine, DAG, and PKC activity could be involved in mediating death ligand–induced apoptosis. Fas ligand (FasL) and TNF have been shown to induce phosphatidylcholine depletion in various cell types (39, 45-48), but no study has been reported to date on the relation between TRAIL and phosphatidylcholine homeostasis.
Although the differential sensitivity of normal and tumor cells to TRAIL-induced apoptosis indicates TRAIL as a potential tumor-selective cancer therapeutic, some tumor cells are resistant to TRAIL (5, 7, 9, 14, 49). Significantly, mitochondria constitute an important target in overcoming inherent resistance to TRAIL in many types of tumors (i.e., colon carcinomas and gliomas; refs. 16, 23, 50). These observations suggest that a block in the activity of TRAIL-induced mitochondrial pathways may instigate certain tumor cells to become TRAIL resistant. Based on emerging evidence accenting the role of mitochondrial lipids in the proapoptotic action of Bid (27, 31, 40) and the lack of mechanistic knowledge on the TRAIL-induced, caspase-independent signals that regulate mitochondrial pathways, we investigated the involvement of TRAIL-induced signals in the mitochondrial release of apoptogenic factors. Here, we show that TRAIL contributes to an alteration of mitochondrial lipids in a manner that may be essential for the release of apoptogenic factors and for complete execution of cell death.
Materials and Methods
Cell culture and apoptosis assay
HeLa and Jurkat cells were cultured in DMEM and RPMI, respectively, both containing 10% fetal bovine serum, and were split into 48 single-cell clones by serial dilution. Individual clones were then grown for 24 hours in medium containing 10 ng/mL recombinant human TRAIL/TNFSF10 (R&D Systems, Minneapolis, MN), 100 ng/mL recombinant soluble human FasL (Alexis Biochemicals, San Diego, CA), or 10 ng/mL recombinant human TNF-α (R&D Systems) plus 1 μg/mL cyclohexamide (Fisher Scientific, Pittsburgh, PA). The percentage of apoptosis was evaluated by propidium iodide staining as described (51, 52) using a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). Highly sensitive clones (C1 for HeLa and C3 for Jurkat cells) were selected for the present study.
Cell fractionation and lipid analysis
In this study, cell fractionation was done using the MBL mitochondria/cytosol fractionation kit (MBL, Watertown, MA) as reported previously (51) and as per manufacturer's instructions. This kit provides unique formulation of reagents for effective isolation of a very highly enriched mitochondrial fraction. The resulting cytosolic and mitochondrial fractions were then used in Western blot analysis. Complementary fractionation studies were undertaken according to the procedure reported by Sorice et al. (53).
Lipid extraction from mitochondrial fractions of Jurkat cells was accomplished as described earlier (53) and extracts were analyzed by nano-electrospray time of flight mass spectrometry (MS) using a LCT instrument (Micromass, Manchester, United Kingdom) set in positive ion mode (43, 44, 53). A few microliters of the extracts were injected using capillary voltage at 2,250 V and sample cone at 35 V. Semiquantitative evaluation of lipid peaks was undertaken using a set of internal reference ions that maintained equivalent relationships within multiple spectra of the same sample and showed little variation between control and apoptotic samples. These reference ions included sphingomyelin at 703.4 m/z (43) and the dominant phosphatidylethanolamine species, palmitoyl,stearoyl-phosphatidylethanolamine, at 768.6 m/z.
Mitochondrial staining
The cardiolipin-sensitive probe 10-nonyl-acridine orange (NAO) was used to monitor changes in mitochondrial lipids in vivo (39, 54). Briefly, after treatment with 10 ng/mL TRAIL, 106 cells were collected by centrifugation, washed, and then resuspended in PBS containing 200 nmol/L NAO (Molecular Probes, Eugene, OR) for 30 minutes at room temperature. Fluorescence was subsequently measured by flow cytometry using the Fl-1 channel. Using a similar protocol, we also measured mitochondrial membrane potential. Washed cells (106) were resuspended in 1 mL PBS containing 25 nmol/L CMXRos or 40 nmol/L TMRE (both from Molecular Probes), and after 30 minutes of incubation at 37°C, fluorescence was measured by flow cytometry (55).
Phospholipase activity assays
Activities related to phosphatidylcholine-phospholipase C (PLC) and phosphatidylcholine-phospholipase D (PLD), and possibly other lipases, were measured with the Amplex Red Assay kit (Molecular Probes). Briefly, 106 cells were treated with TRAIL and suspended in lysis buffer [20 mmol/L Tris-HCl (pH 7.4) containing 5 mmol/L EDTA, 1% NP40, 100 mmol/L NaF, 2 mmol/L sodium orthovanadate, 10 mmol/L sodium pyrophosphate, a cocktail of protease inhibitors (Roche, Indianapolis, IN)]. Reactions were carried out in the dark for 30 minutes at 37°C. Bacterial phosphatidylcholine-PLC served as a positive control. Red fluorescence was measured with a microplate reader (Perkin-Elmer, Gaithersburg, MD) and expressed in arbitrary units (a.u.).
Tricyclodecan-9-yl xanthogenate (D609; Biomol, Plymouth Meeting, PA) was employed to pharmacologically inhibit phospholipase activity (48, 56-65) and usually incubated 30 minutes with cells before other treatments.
Analysis of intracellular reduced glutathione
To ensure that D609 has no short-term antioxidative effect, cellular glutathione was measured using monochlorobimane staining (66-68). Jurkat C3 cells were collected by centrifugation and resuspended in 1 mL RPMI containing 0.5%fetal bovine serum, with or without 50 μmol/L D609, followed by TRAIL treatment. After 1-hour incubation at 37°C, the cells were washed and resuspended in 500 μL PBS containing 40 μmol/L monochlorobimane (Molecular Probes) and incubated for 5 minutes at room temperature. Fluorescence was measured immediately by flow cytometry using the Fl-1 channel.
Diacylglycerol assay
Lipid extracts of 106 PBS-washed cells were obtained by using 3 mL chloroform/methanol (1:2, v/v) supplemented with 1 mol/L NaCl. After mixing, an additional 1 mL chloroform and 1 mL of 1 mol/L NaCl were added. The samples were remixed and the organic phase was separated after centrifugation at 5,000 × g for 2 minutes. DAG levels were evaluated in the lipid extract by using the radioenzymatic Biotrak assay (Amersham, Piscataway, NJ). Specifically, DAG was converted into [32P]phosphatidic acid by exposure to Escherichia coli DAG kinase in the presence of [γ-32P]ATP. The radioactive [32P]phosphatidic acid was then extracted, separated by Amprep chromatography, and quantified by liquid scintillation counting (69).
Immunoblotting
Harvested cells were dissolved in the same lysis buffer as was used in the phosphatidylcholine-PLC assay. Samples were separated by SDS-PAGE and transferred to a polyvinylidene difluoride sheet. After blocking with 5% skim milk in TBS, the blots were incubated with anti-phospho-PKCδ (specific for Ser643 and Thr505 phosphorylation, Cell Signaling Technology, Beverly, MA), anti-PKCδ C-17, anti-actin, anti-HSP60 H-300, anti-caspase-8 p20, anti-Bid C-20, anti-caspase-9 p10 H-83, and anti-caspase-3 H-277 (all from Santa Cruz Biotechnology, Santa Cruz, CA) and monoclonal anti–cytochrome c (PharMingen, San Diego, CA). Appropriate secondary antibodies were obtained from Amersham or Jackson Immuno-Research (West Grove, PA) and blots were visualized using the enhanced chemiluminescence system (Amersham).
Statistics
All data were analyzed as continuous variables. Wilcoxon test, a rank-based statistical test for two independent samples, was used to compare treatment group medians because per group replications ranged from 3 to 5. P < 0.10 was considered statistically significant. The less restrictive significance level was selected to counterbalance the small treatment group sample sizes and to consider that the Wilcoxon test as a nonparametric procedure has, in general, greater probability relative to the t test of failing to reject a false null hypothesis (greater probability of committing type II error; refs. 70, 71). Figures depicting group means and SDs were presented to complement the statistical analyses.
Results
Tumor necrosis factor–related apoptosis-inducing ligand induces changes in mitochondrial lipids
The signaling events responsible for the changes in mitochondrial lipids that prime mitochondria for the proapoptotic action of death ligands have not been characterized yet. In this study, we investigated the link between TRAIL and mitochondrial lipids by characterizing the caspase-independent mitochondrial events that are induced in response to TRAIL and affect the homeostasis of phospholipids, including cardiolipin.
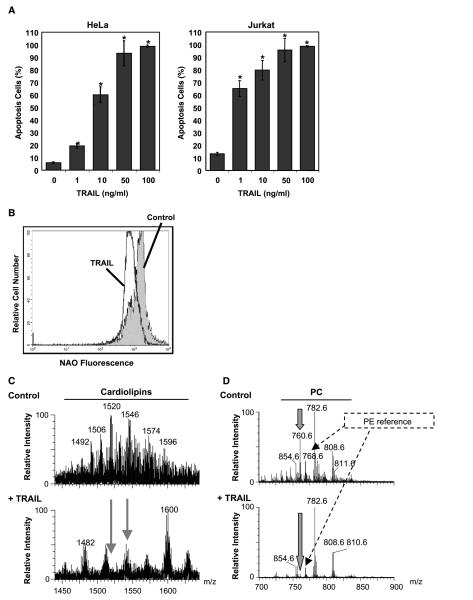
Our clones of Jurkat and HeLa cells are highly sensitive to TRAIL-induced apoptosis (Fig. 1A), which involves mitochondria in these type II cells (16, 18). After 24 hours of treatment, Jurkat and HeLa cells showed comparable sensitivity to TRAIL (80% and 60% apoptosis, respectively, Fig. 1A). To investigate early changes in mitochondrial membrane lipids, Jurkat cells were treated with TRAIL (10 ng/mL) for 1 hour. The alterations in cardiolipin elicited by TRAIL in these cells were then measured using NAO, a cardiolipin-sensitive probe. To ensure that on 1-hour treatment with TRAIL there were no significant changes in mitochondrial membrane potential that could interfere with NAO staining (cf. ref. 39), we measured membrane potential with specific mitochondrial probes like MitoTracker Red and TMRE. One-hour TRAIL treatment had negligible effects on the membrane potential and mitochondrial morphology of these cells (data not shown). At the same time, TRAIL induced a 40% decrease in NAO fluorescence as indicated by the shift in the fluorescence intensity histograms (Figs. 1B and 2E).
Figure 1.
TRAIL induces changes in mitochondrial cardiolipin. A, TRAIL-induced apoptosis: HeLa and Jurkat cells were treated with TRAIL at various concentrations for 24 hours. Cells were collected and the extent of apoptosis was determined by flow cytometry of the sub-G1 population. Columns, mean of three independent determinations; bars, SD. *, P < 0.05, statistically significant two-sided Wilcoxon test compared with control untreated cells. B, TRAIL-induced changes in cardiolipin: Jurkat cells were incubated with TRAIL (10 ng/mL) for 1 hour and then stained with NAO, the fluorescence of which was analyzed by flow cytometry. C, electrospray MS profile of mitochondrial lipid extracts: untreated (top) and TRAIL-treated Jurkat cells (10 ng/mL for 1 hour; bottom). Although the reference intensity of the dominant phosphatidylcholine species was comparatively higher for the TRAIL-treated sample than the control sample, the MS profile of the cardiolipin region was recorded to an equivalent signal-to-noise ratio to emphasize both quantitative and qualitative changes in cardiolipin species. D, electrospray MS spectra of the major mitochondrial phospholipids dominated by phosphatidylcholine (PC) species: The spectra were normalized to the intensity of the dominant palmitoyl,arachidonyl-phosphatidylcholine at 782 m/z, although this lipid showed some TRAIL-induced increase with respect to other arachidonyl-containing species (e.g., that at 808 m/z) and internal references like the major phosphatidylethanolamine (PE) species at 768 m/z. Note the TRAIL-induced severe depletion of mitochondrial palmitoyl,oleoyl-phosphatidylcholine at 760 m/z (thick arrows).
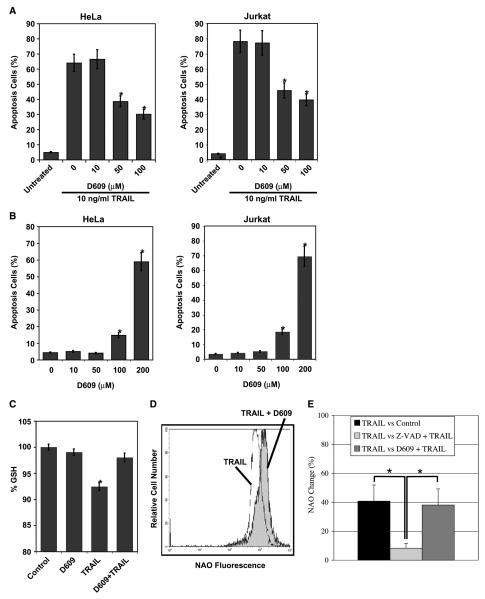
Figure 2.
Phospholipase activity mediates TRAIL-induced apoptosis. A, inhibition of phospholipase activity suppresses TRAIL-induced apoptosis: HeLa and Jurkat cells were preincubated with D609 at various concentrations for 30 minutes followed by addition of 10 ng/mL TRAIL for 24 hours and apoptosis was evaluated by flow cytometry after propidium iodide staining. Columns, mean of three independent determinations; bars, SD. *, P < 0.05, statistically significant two-sided Wilcoxon test compared with control cells (0 μmol/L D609 and 10 ng/mL TRAIL). B, D609 is not cytotoxic at 50 μmol/L concentration: Jurkat and HeLa cells were treated with increasing concentrations of the pharmacologic inhibitor of phospholipase activity, D609, for 24 hours as in (A). There is not a significant difference between untreated cells and cells that are treated with 10 or 50 μmol/L D609 (P = 0.144). C, D609 does not promote short-term antioxidant activity: Jurkat cells were pretreated with 50 μmol/L D609 for 1 hour. The antioxidant effect of D609 was evaluated by measuring glutathione depletion with monochlorobimane using flow cytometry. All measurements were done in triplicates. *, P < 0.05, statistically significant two-sided Wilcoxon test compared with control cells. D, suppression of phospholipase activity inhibited TRAIL-induced changes in NAO staining: Jurkat cells were preincubated with 50 μmol/L D609 for 30 minutes followed by incubation with TRAIL (10 ng/mL) for 1 hour. E, quantitative measurements of percent change in NAO fluorescence in Jurkat cells that are treated with TRAIL, TRAIL and z-VAD, and TRAIL and D609. Columns, mean of three independent experiments each done in duplicate; bars, SD.
Mass spectroscopy (MS) analysis of the mitochondrial lipid extract from TRAIL-treated Jurkat cells revealed substantial cardiolipin alteration (Fig. 1C; cf. ref. 53). The major species of cardiolipin [e.g., that at 1,520 m/z (dioleoyl,dilineoyl-cardiolipin-3Na)] were severely depleted after TRAIL treatment (Fig. 1C; cf. ref. 43). Conversely, minor species of cardiolipin with either saturated acyl chains (e.g., tristearoyl,palmitoyl-2Na at 1,482 m/z) or long unsaturated acyl chains [e.g., that at 1,600 m/z (a mixture of chains corresponding to a total of 78 carbons and 7-9 double bonds)] seemed to remain unchanged or even increase in relative proportion to oleoyl-rich species in extracts of TRAIL-treated cells (Fig. 1C). Consequently, induction of apoptosis not only depleted mitochondria of the overall content of cardiolipin but also had modified the fatty acid distribution of the remaining cardiolipin species. This loss of cardiolipin corresponded with the TRAIL-induced changes in NAO staining (Fig. 1B and C). We have observed a comparable trend of qualitative changes in cardiolipin after Fas stimulation in mouse liver (43) as well as Jurkat cells,4 with the additional observation of increased levels of monolysocardiolipin species.
The change in the acyl chain composition of cardiolipin that was induced by TRAIL clearly indicated that apoptosis signaling severely perturbed the remodeling of this lipid, which is synthesized in mitochondria in precursor forms like the species at 1,482 m/z (40, 44). Given that phosphatidylcholine is the major donor of unsaturated acyl chains to remodel cardiolipin precursors into their highly unsaturated forms (40, 44), we studied next the MS profile of mitochondrial phosphatidylcholine species (Fig. 1D). It became immediately apparent that TRAIL treatment resulted in the severe depletion of a single major species of phosphatidylcholine, palmitoyl,oleoyl-phosphatidylcholine, at 760 m/z in its protonated form (Fig. 1D, thick arrow). Once normalized to internal references, including the dominant phosphatidylethanolamine species, the level of palmitoyl,oleoyl-phosphatidylcholine decreased 6-fold after 1-hour TRAIL treatment (Fig. 1D; data not shown). Correspondingly, there was a relative increase in palmitoyl,arachidoyl-phosphatidylcholine at 782 m/z, whereas other species, such as oleoyl,arachidoyl-phosphatidylcholine (at 808 m/z), remained substantially unaltered (Fig. 1D). Hence, the TRAIL-induced changes in cardiolipin (Fig. 1C) seemed to reflect a selective depletion of palmitoyl,oleoyl-phosphatidylcholine accompanied by an overall redistribution of acyl chains favoring enrichment in arachidonate. Similar, but not identical, changes were observed in the relative levels of phosphatidylcholine species of mitochondria from Fas-treated cells (data not shown).
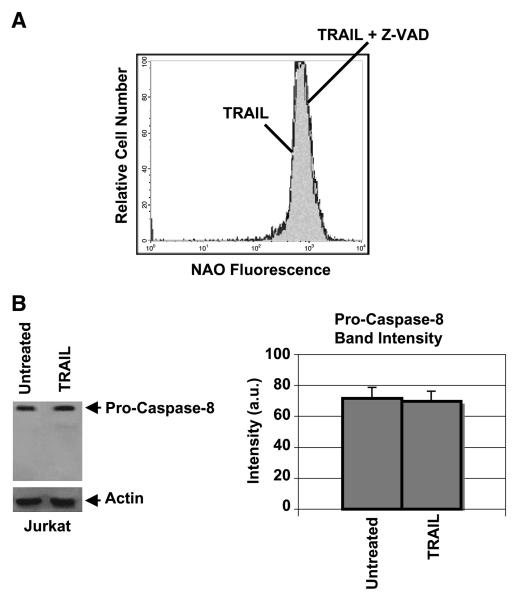
Interestingly, the TRAIL-induced changes in mitochondrial lipids occurred independently of caspase activity as evidenced by the fact that the pan-caspase inhibitor z-VAD did not inhibit the decrease in NAO that is associated with TRAIL (Figs. 2E and 3A). Hence, TRAIL signaling provokes early changes in mitochondrial lipids that occur before, or independently of, caspase activation. Indeed, following 1-hour treatment with TRAIL, processing of caspase-8 into its signature fragments could not be detected (Fig. 3B; ref. 21). We have also observed that, similar to the TRAIL-induced changes, treatment of Jurkat cells with effective concentrations of FasL also resulted in a decrease in NAO fluorescence and cardiolipin depletion (data not shown; cf. ref. 53). These observations suggest the existence of common death receptor–mediated reactions that contribute to changes in mitochondrial membrane lipids before or parallel to activation of apical caspases.
Figure 3.
TRAIL-induced changes in NAO staining are not dependent on caspase activation. A, z-VAD does not inhibit TRAIL-induced changes in NAO staining: Flow cytometry of NAO fluorescence for Jurkat cells, which were preincubated with 100 μmol/L Z-VAD-FMK for 30 minutes followed by TRAIL (10 ng/mL) treatment for 1 hour as in Fig. 1. Data obtained in the presence of z-VAD alone were superimposable to those of the control sample (data not shown). B, pro-caspase-8 is not processed significantly after 1-hour TRAIL treatment under the same conditions as in (A).
Phospholipases are engaged in tumor necrosis factor–related apoptosis-inducing ligand-induced apoptosis
In general, cell surface receptors transduce their intracellular signals by rapid activation of lipid-degrading enzymes (45, 46, 56, 69, 72, 73). For example, a PLC activity degrading phosphatidylcholine (phosphatidylcholine-PLC) has been repeatedly reported to be elevated following stimulation of cell surface receptors, such as Fas (45, 56-62, 69, 73). However, the possible involvement of phospholipases in the mitochondrial arm of TRAIL-mediated apoptosis has not been previously studied, especially with regard to phosphatidylcholine.
Our initial approach for assessing the involvement of phosphatidylcholine-degrading enzymes in TRAIL-mediated apoptosis was to pharmacologically inhibit phospholipase activity with D609. This compound has been generally considered to inhibit phosphatidylcholine-PLC as well as PLD activity (48, 56-65) and has been shown to protect cells from Fas-induced apoptosis (48, 57-59). As shown in Fig. 2A, Jurkat and HeLa cells that were pretreated with D609 (50 μmol/L) for 30 minutes showed ~50% inhibition in cell death after 24-hour TRAIL (10 ng/mL) treatment. Similar results were obtained in cells treated with FasL (100 ng/mL; data not shown). Conversely, the same concentration of D609 (50 μmol/L) had no effect on cell viability (a small cytotoxic effect was observed only at concentrations exceeding 100 μmol/L as shown in Fig. 2B). Considering that D609 possesses antioxidant properties (68), we also evaluated the effect of this compound on cellular glutathione before and after death receptor stimulation. Concentrations of D609 that significantly protected from cell death (Fig. 2A) negligibly affected cellular glutathione (Fig. 2C) while inhibiting by 38% the changes in NAO staining that were induced by 1-hour TRAIL treatment (Fig. 2D and E). Hence, these results provide novel evidence that phospholipase activity is involved in TRAIL-induced apoptosis and affects mitochondria and their signature lipid, cardiolipin.
Phospholipase activity increases in response to tumor necrosis factor–related apoptosis-inducing ligand
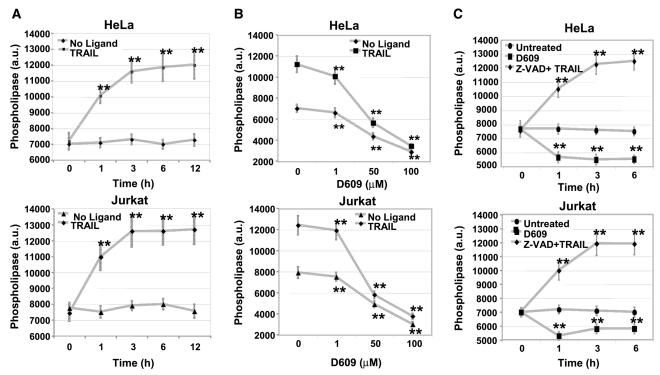
The above results imply that TRAIL-induced apoptosis requires the activity of D609-sensitive phospholipases as reported previously for Fas and TNF (45, 48, 57). To address the possibility that TRAIL directly induces phospholipase activation, we treated Jurkat and HeLa cells with 10 ng/mL TRAIL for 1 hour and measured the activities of phosphatidylcholine-PLC and phosphatidylcholine-PLD to assess the level of phospholipase activity (Molecular Probes; ref. 74). As shown in Fig. 4, phospholipase activity increased rapidly and after 3-hour treatment with TRAIL reached a maximum of 11,590 ± 701 (a.u.) in Jurkat cells and 12,590 ± 974 (a.u.) in HeLa cells. We then tested the capacity of D609 to inhibit cellular phospholipase activity by pretreating the cells with D609 before a 3-hour treatment with TRAIL (to account for maximal phospholipase activity). D609 inhibited TRAIL-induced increase in phospholipase activity in both cell lines in a concentration-dependent manner (Fig. 4B). Hence, TRAIL stimulates the activity of phosphatidylcholine-degrading phospholipases, thus providing a link between perturbation of phosphatidylcholine homeostasis and the antiapoptotic effect of D609 inhibition.
Figure 4.
Phospholipase activity increases in response to TRAIL. A, TRAIL induces activation of phospholipases: HeLa and Jurkat cells were exposed to TRAIL (10 ng/mL) for the indicated times and phospholipase activities were measured using Amplex Red fluorescence (a.u.). Points, mean of three independent experiments; bars, SD. **, 0.05 < P < 0.10, statistically significant two-sided Wilcoxon test comparing 0 hour with 1, 3, 6, and 12 hours. B, TRAIL-induced phospholipase activity is inhibited by D609: HeLa and Jurkat cells were preincubated with D609 at various concentrations for 30 minutes followed by TRAIL treatment as in (A). Points, mean of three independent experiments each done in duplicate; bars, SD. **, 0.05 < P < 0.10, statistically significant two-sided Wilcoxon test compared with control cells (no D609 treatment). C, TRAIL-induced phospholipase activity (A) is not inhibited by z-VAD: HeLa and Jurkat cells were preincubated with 100 μmol/L pan-caspase inhibitor z-VAD for 30 minutes followed by TRAIL (10 ng/mL) treatment for 1, 3, and 6 hours. Phospholipase activity was measured as for TRAIL in (A).
Phospholipase activity mediates the tumor necrosis factor–related apoptosis-inducing ligand–induced mitochondrial apoptotic pathway
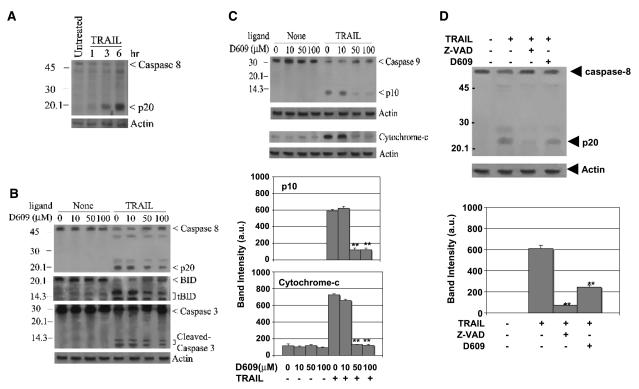
Having established that TRAIL induces early activation of phospholipases, we investigated whether this activity influenced the DISC-mediated arm of caspase cleavage or the mitochondria-mediated amplification of caspase cleavage downstream of the DISC (25, 26, 33, 34, 75, 76). To dissect these possibilities, we measured the cleavage of the key apical caspases in the extrinsic pathway (caspase-8) and in the intrinsic pathway (caspase-9) as well as the cleavage and activity of caspase-3, which is the major executioner protease for both pathways. Cleavage of caspase zymogens is instrumental to the activation of caspase-3 and directly follows proximity-induced activation of apical caspases, thus contributing to their sustained activity (21, 77). We complemented these studies with an evaluation of Bid cleavage and parallel measurements of the cytosolic relocation of mitochondrial cytochrome c. It is important to note that although Bid is a preferred substrate of caspase-8 it can also be processed via the intrinsic pathway and caspase-3 (33, 78). We additionally compared the effects of increasing concentrations of D609 on the cleavage profile of caspases and Bid after TRAIL treatment (Fig. 5). Figure 5 depicts data obtained with HeLa cells following TRAIL treatment; similar results were obtained with Jurkat cells (data not shown).
Figure 5.
Phospholipase activity mediates the mitochondrial pathway of TRAIL-induced apoptosis. A, TRAIL induces caspase-8 processing in a time-dependent manner: HeLa cells were subjected to TRAIL (10 ng/mL) treatment for the indicated times and their lysates were immunoblotted for caspase-8 (p20 antibody, Santa Cruz Biotechnology) and actin as loading control. Bottom, densitometric analysis of the p20 band data from three independent experiments. B and C, phospholipase activity does not block Bid and caspase-8 processing but affects cytochrome c release and caspase-9 processing: HeLa cells were incubated first with increasing concentrations of D609 for 30 minutes and then treated with TRAIL (10 ng/mL) for 6 hours. Whole cell lysates were immunoblotted for caspase-8, Bid, caspase-9, and caspase-3, whereas cytosolic fractions were blotted for cytochrome C; protein loading was evaluated with actin re-blots (bottom). Densitometric analysis of the bands for p10 and cytochrome C was done using data from three independent experiments (C, bottom). **, 0.05 < P < 0.10, statistically significant two-sided Wilcoxon test compared with TRAIL alone treatment. D, z-VAD is more potent than D609 in inhibiting TRAIL-induced caspase-8 processing: Lysates of HeLa cells were preincubated with 100 μmol/L z-VAD or 50 Amol/L D609 for 30 minutes as indicated and then treated with TRAIL (10 ng/mL). Densitometric analysis of the p20 band was done using data from three independent experiments (bottom). **, 0.05 < P < 0.10, statistically significant two-sided Wilcoxon test compared with TRAIL treatment alone.
On stimulation of apoptosis by TNF family of ligands, procaspase-8 is proteolytically cleaved to generate the p20 signature fragment (3, 5, 17, 23, 79). Following 3-hour TRAIL treatment (10 ng/mL), the p20 cleavage fragment of caspase-8 was observed by immunodetection and reached a maximum after 6 hours (Fig. 5A). Pretreatment with increasing concentrations of D609 had only a slight effect on the cleavage profile (Fig. 5B) and activity (data not shown) of caspase-8. Similarly, cleavage of caspase-3 was hardly affected by D609 (Fig. 5B), even at the concentrations that reduced TRAIL-induced death by ~50% (Fig. 2). Although cleavage of Bid was induced by TRAIL, as evidenced by the appearance of truncated Bid fragments, this cleavage was affected only partially by D609 concentrations that were effective in inhibiting phospholipase activity (Fig. 5B). Hence, sustained activation of caspase-8, and the consequent direct cleavage of its preferred substrates, caspase-3 and Bid, persisted when phospholipase activity was extensively inhibited by D609 (Figs. 2, 4, and 5).
Pro-caspase-9 is the apical caspase of the intrinsic pathway and is efficiently processed within the apoptosome (21, 28). Cleavage of pro-caspase-9 was induced by TRAIL (10 ng/mL for 6 hours) as indicated by the concomitant degradation of the pro-caspase band and the formation of the p10 fragment (Fig. 5C). D609 strongly inhibited the cleavage of pro-caspase-9 (Fig. 5C). Similarly, the release of cytochrome c from mitochondria was strongly repressed by D609 (Fig. 5C). Consequently, inhibition of phospholipase activity seemed to primarily influence the mitochondrial arm of death receptor–mediated apoptosis.
Our data showed that enhanced activity of phosphatidylcholine-degrading lipases intersects the bifurcated pathway of cell death before the engagement of mitochondria but could not discriminate whether caspase-8 and phospholipases were activated in parallel or in sequence. To distinguish between these possibilities, Jurkat and HeLa cells were treated with the pan-caspase inhibitor, z-VAD, before a 3-hour treatment with TRAIL (10 ng/mL). Without affecting TRAIL-induced phospholipase activity (Fig. 4A and C), z-VAD inhibited caspase-8 cleavage by ~6-fold (Fig. 5D). Thus, death receptor stimulation leads to elevated activity of cytosolic phospholipases parallel to early activation of caspase-8.
Tumor necrosis factor–related apoptosis-inducing ligand induces rapid production of diacylglycerol and activation of protein kinase Cδ
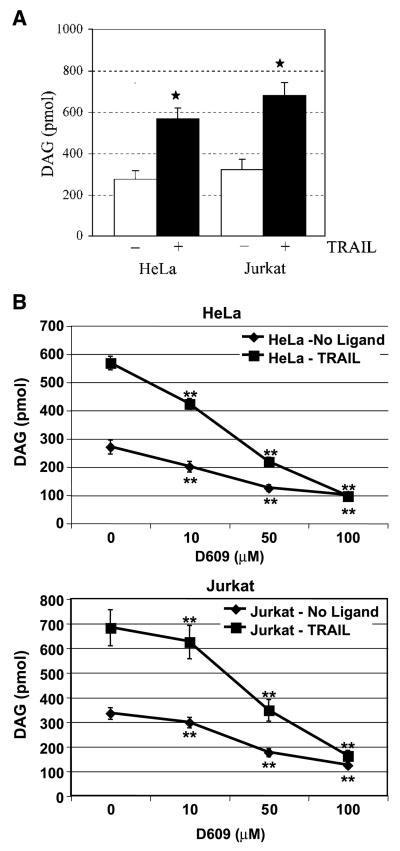
Having shown that TRAIL-induced changes in phosphatidylcholine and its degrading enzymes occur independently of caspase-8 activation, we next investigated the involvement of a key byproduct of phosphatidylcholine degradation (i.e., DAG) in the mediation of TRAIL function. It has been reported previously that phosphatidylcholine hydrolysis results in the transient accumulation of cellular DAG (45, 48), which is the direct product of phosphatidylcholine-PLC or the byproduct of the combined action of PLD and lipid phosphatases. Moreover, DAG itself can induce apoptosis as has been shown in prostate cancer cells (47). Consistent with our previous results (Figs. 2 and 4), TRAIL stimulation of Jurkat and HeLa cells (Fig. 6) induced a 2- to 3-fold elevation in intracellular DAG (Fig. 6). We additionally found that D609 inhibited both the basal levels and the TRAIL-enhanced levels of DAG in a concentration-dependent manner (Fig. 6B). These results indicated that D609 depressed the metabolic turnover of DAG in untreated and TRAIL-treated cells presumably via inhibition of the same phosphatidylcholine-degrading enzymes that contribute to the steady-state level of the lipid.
Figure 6.
TRAIL induces rapid production of DAG that is dependent on phospholipase activity. HeLa and Jurkat cells were either untreated (0 μmol/L; A and B) or preincubated with D609 (B) at various concentrations. Cells were then subjected to TRAIL (10 ng/mL) treatment for 3 hours and DAG content was measured as described in Materials and Methods. Columns, mean DAG levels of three independent experiments each done in duplicates; bars, SD. *, P < 0.05, statistically significant two-sided Wilcoxon test compared with control cells; **, 0.05 < P < 0.10, statistically significant two-sided Wilcoxon test compared with control untreated cells in HeLa and Jurkat cells.
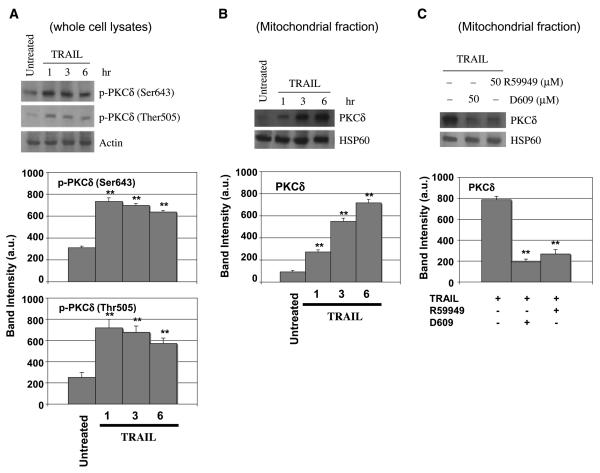
The reciprocal relationship between phosphatidylcholine and DAG homeostasis is complicated by evidence indicating that PKC regulates phospholipase activities that promote phosphatidylcholine hydrolysis (46, 80-83). PKCδ is a member of a novel PKC subfamily that is activated by DAG and relocates to the mitochondria in response to apoptosis stimuli (46, 47, 80, 82, 84-87). Although phosphorylation of PKCδ on distinct tyrosine residues is pivotal to its function during apoptosis (83), it is not known whether PKCδ is activated in response to TRAIL. Our studies showed that PKCδ is activated in response to TRAIL (Fig. 7). Treatment of HeLa cells with TRAIL (10 ng/mL) induced 3- to 4-fold increase in the phosphorylation of PKCδ at Ser643 and Thr505, which was evident as early as after 1-hour treatment (Fig. 7A). TRAIL signaling resulted also in subsequent relocation of PKCδ to mitochondria, which was substantial after 3 hours and peaked after 6 hours of TRAIL treatment (Fig. 7B). This suggests that PKCδ relocation to the mitochondria follows its phosphorylation.
Figure 7.
TRAIL induces phosphorylation and mitochondrial translocation of PKCδ that is dependent on activity of phospholipases. HeLa cells were either untreated or preincubated with D609 and R59949 (50 μmol/L) for 30 minutes and subsequently treated with TRAIL (10 ng/mL) for the indicated times (A and B) or for 6 hours (C). Whole cell lysates (A) and mitochondrial fractions (B and C) were analyzed by immunoblotting using antibodies specific for phosphorylated PKCδ, whole PKCδ, actin, and HSP60. The band intensities of phospho-PKCδ (Ser643), phospho-PKCδ (Thr505), and PKCδ were analyzed with a densitometer using data from three independent experiments (bottom). **, 0.05 < P < 0.10, statistically significant two-sided Wilcoxon test compared with untreated samples.
To show whether the TRAIL-induced activation and relocation of PKCδ was dependent on the ensuing increase in phospholipase activity, we next pretreated HeLa cells with 50 μmol/L D609 before TRAIL treatment. We additionally used R59949, a specific inhibitor of DAG kinase, which blocks metabolic conversion of DAG. R59949 produces an increase in the cellular level of DAG, which is analogous to that derived from increased phospholipase activity (85). At the standard concentration of 50 μmol/L, D609 and R59949 comparably inhibited TRAIL-induced translocation of PKCδ to mitochondria (Fig. 7C).
Altogether, these studies indicate that TRAIL signaling induces an early accumulation of DAG (45), which may derive from enhanced degradation of phosphatidylcholine via D609-sensitive phospholipases. Remarkably, early phosphorylation of PKCδ and its mitochondrial relocation is accompanied by DAG elevation that follows a caspase-independent imbalance in phosphatidylcholine homeostasis.
Tumor necrosis factor–related apoptosis-inducing ligand–induced activation of protein kinase Cδ is involved in mediating apoptosis and cooperates with death-inducing signaling complex–induced caspase activation
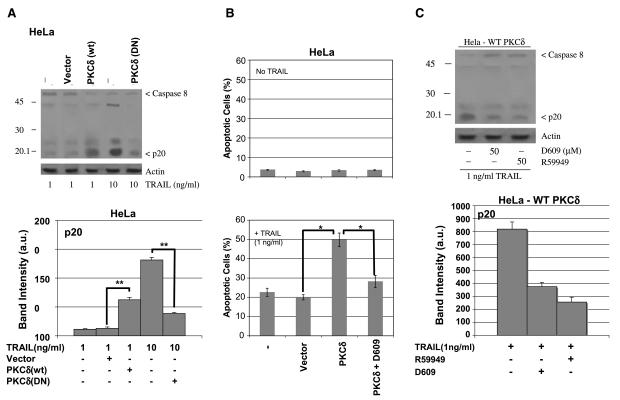
Having showed that TRAIL induces activation of PKCδ, we next investigated whether PKCδ played a role in mediating apoptosis. In fact, ectopic expression of a dominant-negative mutant of PKCδ [PKCδ (K376A); ref. 86] inhibited processing of caspase-8 (4-fold) in HeLa cells that had been treated with TRAIL (10 ng/mL) for 6 hours (Fig. 8A). Conversely, ectopic expression of wild-type PKCδ in HeLa cells enhanced TRAIL-induced caspase-8 processing (5-fold) and apoptosis (Fig. 8A and B). These data suggest that TRAIL-induced PKCδ activation cooperates with the DISC in the activation of caspase-8. In support to this possibility, R59949 also caused a 4-fold inhibition of TRAIL-induced processing of caspase-8 in HeLa cells ectopically expressing wild-type PKCδ (Fig. 8C).
Figure 8.
TRAIL-induced activation of PKCδ mediates apoptosis and cooperates with the DISC-induced activation of caspase-8. HeLa cells with ectopic expression of wild-type (WT) and dominant-negative (DN) mutant of PKCδ [PKCδ (K376A)] were generated. These cells were treated with TRAIL as indicated (1 and 10 ng/mL). A, PKCδ (K376A) inhibited and wild-type PKCδ enhanced the processing of pro-caspase-8. B, PKCδ-expressing HeLa cells were more sensitive to TRAIL-induced apoptosis and pretreatment of these cells with D609 abolished the increased sensitivity to TRAIL. *, P < 0.05, statistically significant two-sided Wilcoxon test compared with compared with control cells (no TRAIL treatment). C, DAG kinase inhibitor, R59949, inhibited TRAIL-induced activation of caspase-8 in HeLa cells ectopically expressing PKCδ (wild-type). A and B, the intensity of p20 band was analyzed as in Fig. 5 (statistical results are shown in the bottom).
In sum, these studies indicate that activated PKCδ participates in TRAIL-induced signaling by regulating or fine-tuning the cleavage of apical caspase-8. This role seems to depend on early caspase-independent reactions that affect DAG levels, indicating a possible synergistic loop between caspase-independent and caspase-dependent reactions elicited by TRAIL signaling.
Discussion
TRAIL remains a promising cancer therapeutic due to its ability to selectively kill tumor and transformed cells but not normal cells (5, 7-9, 15). Herein, we have investigated the involvement of early caspase-independent events in TRAIL-induced apoptosis in type II cells, which require mitochondria for mediating cell death (16, 18, 23). It is thought that early death receptor–mediated signaling alters mitochondrial membranes, thereby priming mitochondria for the release of apoptogenic factors like cytochrome c (40, 78, 86, 87). This priming most likely originates from changes in the lipid composition of mitochondria. Indeed, we report here that TRAIL induces caspase-independent changes in the mitochondrial lipid composition, encompassing both cardiolipin (Fig. 1B and C) and phosphatidylcholine (Fig. 1D).
Mitochondrial cardiolipin biosynthesis is coordinated with phosphatidylcholine homeostasis (39, 40) and an imbalance in phosphatidylcholine homeostasis would reduce the quantity of acyl donors that is required for normal remodeling of cardiolipin. This process is substantially more rapid than cardiolipin biosyn-thesis and requires both mitochondrial and extramitochondrial enzymes (40). As a consequence of the unusual acyl chain composition of mature cardiolipin, it is likely that only specific species of phosphatidylcholine are critical for cardiolipin remodeling. Accordingly, the selective depletion of palmitoyl,oleoyl-phosphatidylcholine after 1-hour TRAIL treatment of Jurkat cells (Fig. 1D) suggests a link with cardiolipin remodeling. The imbalance in phosphatidylcholine produced by this depletion is likely to derive from enhanced activity of phosphatidylcholine-degrading enzymes, which may have specificity for 2-oleoyl-phosphatidylcholine species.
Our studies here indicate that TRAIL induces D609-sensitive reactions that contribute to a depletion of both cardiolipin and phosphatidylcholine with concomitant DAG accumulation (Figs. 2, 4, and 5). D609 has been reported previously to inhibit phosphatidylcholine-PLC (48, 56, 57, 62, 63, 65, 73, 88, 89). Recently, it has been suggested that D609 also possesses antioxidant properties (68). However, our data indicate that the antioxidant activity of D609 does not seem to contribute to observed effects (e.g., on mitochondrial lipids; Fig. 2). Additionally, D609 has been shown to inhibit Ca-dependent PLA2 (73) and sphingomyelin synthase (90) in some cellular systems. We have observed minimal TRAIL-induced changes in phospholipase A2 activity (as well Fas-induced; cf. ref. 42). Moreover, the activation of sphingomyelin synthase is thought to be an event downstream of phosphatidylcholine-PLC activity (48, 57, 89, 90). Thus, it is plausible that the phosphatidylcholine imbalance that is initiated by TRAIL derives from a combination of diverse lipase activities (45, 48, 57-62). Additional studies are required to identify signal transduction steps that may contribute to the altered balance in the synthesis and degradation of cellular phosphatidylcholine following stimulation of death receptors.
It is clear from our results that TRAIL-induced activation of phosphatidylcholine-degrading enzymes more directly affects caspase-9 cleavage and cytochrome c release than caspase-8 or caspase-3 cleavage (Fig. 5B and C). This supports the concept that phospholipases may be primarily involved in the intrinsic (mitochondria-dependent) arm of the bifurcate pathway of apoptosis mediated by death receptors. However, phospholipases alone would not be sufficient in mediating apoptosis, as blocking lipid degradation by D609 produces only a partial protection from cell death (Fig. 2). Indeed, 6 hours after TRAIL (and also FasL and TNF; data not shown) treatment, phospholipases and caspases seem to synergistically combine their effects as indicated by the cumulative effects of D609 and z-VAD in the cleavage of various caspases and Bid. It is noteworthy that a recent study of RNA interference-based phenotypic screening has revealed new proteins that modulate the intrinsic pathway of TRAIL-induced apoptosis (91). Based on our results, we predict that some of these new apoptotic effectors may influence the response of mitochondrial lipids to the early alteration in phosphatidylcholine homeostasis.
We have also shown here that TRAIL induces the phosphorylation and relocation of PKCδ to mitochondria (Fig. 7) following accumulation of DAG. Consistent with this finding, the ectopic expression of PKCδ enhances TRAIL-induced caspase activation and apoptosis in HeLa cells (Fig. 8). Interestingly, inhibition of DAG elevation, by blocking its downstream conversion or its production via D609-sensitive lipases, results in diminished TRAIL-induced activation of PKCδ (Fig. 7C). This implies that the source of DAG elevation is not fundamental to PKC activation, possibly reflecting stress responses that are wired within cell signaling. Additionally, phospholipid scramblase 3, a member of protein family responsible for translocation of phospholipids between the two mitochondrial membranes, has recently been shown to be a target of PKCδ (86, 92). Hence, enhanced PKCδ activity may facilitate cardiolipin degradation by enhancing its scramblase-mediated shuttling to the mitochondrial surface (86, 92).
What is emerging from these studies, also in the light of reports of others (93, 94), is that death receptor signaling bifurcates early into a caspase-8-mediated and rapid cell execution pathway, which in many cells like Jurkat is amplified by the release of mitochondrial apoptogenic factors. The other arm of the bifurcation is actually inhibited by caspase-8, leading to an alteration of lipid homeostasis. In this study, we have delineated, for the first time, early biochemical events in TRAIL signaling that alter the metabolism of phosphatidylcholine, the dominant phospholipid of most membranes that is required also for the maintenance of cardiolipin homeostasis. Downstream phosphatidylcholine, therefore, the caspase-independent arm contributes to a specific “priming” of mitochondrial membranes by altering their lipid composition and thus facilitating the proapoptotic action of Bid (and possibly other factors) that are activated through the caspase-8-mediated pathway. When caspase-8 is inhibited (e.g., by z-VAD), the caspase-independent arm of death signaling that alters lipid homeostasis becomes predominant. This may lead to the proliferation of membrane organelles (vacuoles, autophagosomes, and lysosomes), which slowly eat up the cytosolic constituents, thus producing an autophagic type of cell death. In fact, when phosphatidylcholine metabolism is altered (Fig. 1), there is a switch from phosphatidylcholine to phosphatidylinositol, which enhances the production of metabolites like phosphatidylinositol 3-phosphate that are fundamental for the biogenesis and maturation of autophagolysosomes (94).
In conclusion, these results indicate that TRAIL stimulation induces changes in lipid metabolism that affect mitochondrial membranes and can synergistically contribute to apoptosis via both caspase-8-dependent and caspase-8-independent signaling. The TRAIL-induced signaling pathways that regulate phospholipase activity and promote changes in phosphatidylcholine and cardiolipin will offer novel targets for lipophilic anticancer drugs that, by interfering with cellular lipid metabolism, could selectively kill cancer cells.
Acknowledgments
Grant support: NIH/National Heart, Lung, and Blood Institute grant HL080192 Department of Defense grant DAMD 17-02-10299 to R. Khosravi-Far; R. Khosravi-Far is an American Cancer Society Scholar. American Cancer Society research scholar award CCG-104830 (R. Khosravi-Far), American Institute for Cancer Research grant 03-146 and Biotechnology and Biological Sciences Research Council grant (M.D. Esposti), NIH grant T32 H07893 (K. Ndebele), and IARC postdoctoral fellowship IARC/R.3159 (M. Rosenquist).
We thank Dr. Ohno for PKCδ constructs, Shalini Rana for her valuable suggestions, Elizabeth Mundy for her contribution to some experiment, and Dr. Alex Toker for his suggestions.
Footnotes
Unpublished data.
References
- 1.Degli-sposti M. To die or not to die-the quest of the TRAIL receptors. J Leukoc Biol. 1999;65:535–42. doi: 10.1002/jlb.65.5.535. [DOI] [PubMed] [Google Scholar]
- 2.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 3.MacFarlane M. TRAIL-induced signalling and apoptosis. Toxicol Lett. 2003;139:89–97. doi: 10.1016/s0378-4274(02)00422-8. [DOI] [PubMed] [Google Scholar]
- 4.Abe K, Kurakin A, Mohseni-Maybodi M, Kay B, Khosravi-Far R. The complexity of TNF related apoptosis inducing ligand. Ann N Y Acad Sci. 2000;926:52–63. doi: 10.1111/j.1749-6632.2000.tb05598.x. [DOI] [PubMed] [Google Scholar]
- 5.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–44. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Golstein P. Cell death: TRAIL and its receptors. Curr Biol. 1997;7:750–3. doi: 10.1016/s0960-9822(06)90000-1. [DOI] [PubMed] [Google Scholar]
- 7.Ozoren N, El-Deiry WS. Cell surface death receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13:135–47. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 8.French LE, Tschopp J. The TRAIL to selective tumor death. Nat Med. 1999;5:146–7. doi: 10.1038/5505. [DOI] [PubMed] [Google Scholar]
- 9.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayagaki N, Yamaguchi N, Nakayama M, Eto H, Okumura K, Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression on human T cells: a novel mechanism for the antitumor effects of type I IFNs. J Exp Med. 1999;189:1451–60. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay F, Kalled SL. TNF ligands and receptors in autoimmunity: an update. Curr Opin Immunol. 2002;14:783–90. doi: 10.1016/s0952-7915(02)00407-7. [DOI] [PubMed] [Google Scholar]
- 12.Mariani SM, Krammer PH. Surface expression of TRAIL/Apo-2 ligand in activated mouse T and B cells. Eur J Immunol. 1998;28:1492–8. doi: 10.1002/(SICI)1521-4141(199805)28:05<1492::AID-IMMU1492>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Martinez LM, Alava MA, Gamen S, et al. Involvement of APO2 ligand/TRAIL in activation-induced death of Jurkat and human peripheral blood T cells. Eur J Immunol. 1998;28:2714–25. doi: 10.1002/(SICI)1521-4141(199809)28:09<2714::AID-IMMU2714>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–33. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 15.Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–80. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozoren N, El-Deiry WS. Defining characteristics of types I and II apoptotic cells in response to TRAIL. Neoplasia. 2002;4:551–7. doi: 10.1038/sj.neo.7900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 18.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–30. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Chang HY, Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell. 1998;1:319–25. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 21.Boatright KM, Renatus M, Scott FL, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–41. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 22.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 23.Khosravi-Far R, Esposti MD. Death receptor signals to mitochondria. Cancer Biol Ther. 2004;3:1051–7. doi: 10.4161/cbt.3.11.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–67. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 25.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 26.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamzami N, Kroemer G. Apoptosis: mitochondrial membrane permeabilization—the (w)hole story? Curr Biol. 2003;13:R71–3. doi: 10.1016/s0960-9822(02)01433-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 29.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63–7. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 30.Degli-Esposti M. The roles of Bid. Apoptosis. 2002;7:433–40. doi: 10.1023/a:1020035124855. [DOI] [PubMed] [Google Scholar]
- 31.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–90. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 32.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 34.Gross A, Yin XM, Wang K, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–63. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 35.Zinkel SS, Ong CC, Ferguson DO, et al. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17:229–39. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002;9:1031–42. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 37.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goonesinghe A, Mundy ES, Smith M, Khosravi-Far R, Martinou JC, Esposti MD. Pro-apoptotic Bid induces membrane perturbation by inserting selected lysolipids into the bilayer. Biochem J. 2005;387:109–18. doi: 10.1042/BJ20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degli-Esposti M. The mitochondrial battlefield and membrane lipids during cell death signalling. Ital J Biochem. 2003;52:43–50. [PubMed] [Google Scholar]
- 40.Cristea IM, Degli-Esposti M. Membrane lipids and cell death: an overview. Chem Phys Lipids. 2004;129:133–60. doi: 10.1016/j.chemphyslip.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Lockshin RA, Zakeri Z. Caspase-independent cell deaths. Curr Opin Cell Biol. 2002;14:727–33. doi: 10.1016/s0955-0674(02)00383-6. [DOI] [PubMed] [Google Scholar]
- 42.Kuwana T, Mackey MR, Perkins G, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–42. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 43.Degli-Esposti M, Cristea IM, Gaskell SJ, Nakao Y, Dive C. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Differ. 2003;10:1300–9. doi: 10.1038/sj.cdd.4401306. [DOI] [PubMed] [Google Scholar]
- 44.Schlame M, Rustow B. Lysocardiolipin formation and reacylation in isolated rat liver mitochondria. Biochem J. 1990;272:589–95. doi: 10.1042/bj2720589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schutze S, Berkovic D, Tomsing O, Unger C, Kronke M. Tumor necrosis factor induces rapid production of 1′2′diacylglycerol by a phosphatidylcholine-specific phospholipase C. J Exp Med. 1991;174:975–88. doi: 10.1084/jem.174.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Exton JH. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Bermejo ML, Leskow FC, Fujii T, et al. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCα. J Biol Chem. 2002;277:645–55. doi: 10.1074/jbc.M107639200. [DOI] [PubMed] [Google Scholar]
- 48.Cifone MG, Roncaioli P, De Maria R, et al. Multiple pathways originate at the Fas/APO-1 (CD95) receptor: sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO J. 1995;14:5859–68. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LeBlanc H, Lawrence D, Varfolomeev E, et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–81. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 50.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–94. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 51.Ferry S, Matsuda M, Yoshida H, Hirata M. Inositol hexakisphosphate blocks tumor cell growth by activating apoptotic machinery as well as by inhibiting the Akt/NFκB-mediated cell survival pathway. Carcino-genesis. 2002;23:2031–41. doi: 10.1093/carcin/23.12.2031. [DOI] [PubMed] [Google Scholar]
- 52.Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R. Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of fork-head FOXO3a transcription factor. Proc Natl Acad Sci U S A. 2003;100:6523–8. doi: 10.1073/pnas.0731871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorice M, Circella A, Cristea IM, et al. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004;11:1133–45. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]
- 54.Kluza J, Lansiaux A, Wattez N, et al. Induction of apoptosis in HL-60 leukemia and B16 melanoma cells by the acronycine derivative S23906–1. Biochem Pharmacol. 2002;63:1443–52. doi: 10.1016/s0006-2952(02)00899-7. [DOI] [PubMed] [Google Scholar]
- 55.Matsumura H, Shimizu Y, Ohsawa Y, Kawahara A, Uchiyama Y, Nagata S. Necrotic death pathway in Fas receptor signaling. J Cell Biol. 2000;151:1247–56. doi: 10.1083/jcb.151.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Machleidt T, Kramer B, Adam D, et al. Function of the p55 tumor necrosis factor receptor “death domain” mediated by phosphatidylcholine-specific phospholipase C. J Exp Med. 1996;184:725–33. doi: 10.1084/jem.184.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-κB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–76. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 58.Han JS, Hyun BC, Kim JH, Shin I. Fas-mediated activation of phospholipase D is coupled to the stimulation of phosphatidylcholine-specific phospholipase C in A20 cells. Arch Biochem Biophys. 1999;367:233–9. doi: 10.1006/abbi.1999.1250. [DOI] [PubMed] [Google Scholar]
- 59.Exton JH. Cell signalling through guanine-nucleotide-binding regulatory proteins (G proteins) and phospholipases. Eur J Biochem. 1997;243:10–20. doi: 10.1111/j.1432-1033.1997.t01-1-00010.x. [DOI] [PubMed] [Google Scholar]
- 60.Simarro M, Pelassy C, Calvo J, Places L, Aussel C, Lozano F. The cytoplasmic domain of CD5 mediates both TCR/CD3-dependent and -independent diacylglycerol production. J Immunol. 1997;159:4307–15. [PubMed] [Google Scholar]
- 61.Li Y, Maher P, Schubert D. Phosphatidylcholine-specific phospholipase C regulates glutamate-induced nerve cell death. Proc Natl Acad Sci U S A. 1998;95:7748–53. doi: 10.1073/pnas.95.13.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pastorino JG, Simbula G, Yamamoto K, Glascott PA, Jr, Rothman RJ, Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271:29792–8. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- 63.Muller-Decker K. Interruption of TPA-induced signals by an antiviral xanthate compound: inhibition of a phospholipase C-type reaction. Biochem Biophys Res Commun. 1989;162:198–205. doi: 10.1016/0006-291x(89)91981-5. [DOI] [PubMed] [Google Scholar]
- 64.Cai H, Erhardt P, Troppmair J, et al. Hydrolysis of phosphatidylcholine couples Ras to activation of Raf protein kinase during mitogenic signal transduction. Mol Cell Biol. 1993;13:7645–51. doi: 10.1128/mcb.13.12.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JH, Kim SW, Jung PJ, Yon C, Kim SC, Han JS. Phosphatidylcholine-specific phospholipase C and RhoA are involved in the thyrotropin-induced activation of phospholipase D in FRTL-5 thyroid cells. Mol Cells. 2002;14:272–80. [PubMed] [Google Scholar]
- 66.Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–58. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- 67.Sebastia J, Cristofol R, Martin M, Rodriguez-Farre E, Sanfeliu C. Evaluation of fluorescent dyes for measuring intracellular glutathione content in primary cultures of human neurons and neuroblastoma SH-SY5Y. Cytometry. 2003;51A:16–25. doi: 10.1002/cyto.a.10003. [DOI] [PubMed] [Google Scholar]
- 68.Zhou D, Lauderback CM, Yu T, Brown SA, Butterfield DA, Thompson JS. D609 inhibits ionizing radiation-induced oxidative damage by acting as a potent antioxidant. J Pharmacol Exp Ther. 2001;298:103–9. [PubMed] [Google Scholar]
- 69.Downes CP, Michell RH. In: Molecular mechanisms of transmembrane signaling. Cohen P, Houslay MD, editors. Elsevier; Amsterdam: 1985. pp. 3–56. [Google Scholar]
- 70.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bull. 1945;1:80–3. [Google Scholar]
- 71.Glass GV, Peckham PD, Saunders JR. Consequences of failure to meet assumptions underlying the fixed effects analysis of variance and covariance. Rev Educ Res. 1972;42:239–88. [Google Scholar]
- 72.Perez CA, Huang L, Rong M, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–76. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 73.Preuss I, Kaiser I, Gehring U. Molecular characterization of a phosphatidylcholine-hydrolyzing phospholipase C. Eur J Biochem. 2001;268:5081–91. doi: 10.1046/j.0014-2956.2001.02440.x. [DOI] [PubMed] [Google Scholar]
- 74.Rauch P, Ferri EN, Girotti S, et al. A chemiluminescent flow sensing device for determination of choline and phospholipase D activity in biological samples. Anal Biochem. 1997;245:133–40. doi: 10.1006/abio.1996.9950. [DOI] [PubMed] [Google Scholar]
- 75.Reed JC, Kroemer G. Mechanisms of mitochondrial membrane permeabilization. Cell Death Differ. 2000;7:1145. doi: 10.1038/sj.cdd.4400777. [DOI] [PubMed] [Google Scholar]
- 76.Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–60. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 77.Chang DW, Ditsworth D, Liu H, Srinivasula SM, Alnemri ES, Yang X. Oligomerization is a general mechanism for the activation of apoptosis initiator and inflammatory procaspases. J Biol Chem. 2003;278:16466–9. doi: 10.1074/jbc.C300089200. [DOI] [PubMed] [Google Scholar]
- 78.Degli-Esposti M, Ferry G, Masdehors P, Boutin JA, Hickman JA, Dive C. Post-translational modification of Bid has differential effects on its susceptibility to cleavage by caspase 8 or caspase 3. J Biol Chem. 2003;278:15749–57. doi: 10.1074/jbc.M209208200. [DOI] [PubMed] [Google Scholar]
- 79.Ozoren N, El-Deiry WS. Defining characteristics of types I and II apoptotic cells in response to TRAIL. Neoplasia. 2002;4:551–7. doi: 10.1038/sj.neo.7900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase Cδ. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 81.Kikkawa U, Matsuzaki H, Yamamoto T. Protein kinase Cδ (PKCδ): activation mechanisms and functions. J Biochem (Tokyo) 2002;132:831–9. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- 82.Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol. 2000;72:12–9. [PubMed] [Google Scholar]
- 83.Sumitomo M, Ohba M, Asakuma J, Asano T, Kuroki T, Hayakawa M. Protein kinase Cδ amplifies ceramide formation via mitochondrial signaling in prostate cancer cells. J Clin Invest. 2002;109:827–36. doi: 10.1172/JCI14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fukumoto S, Nishizawa Y, Hosoi M, et al. Protein kinase Cδ inhibits the proliferation of vascular smooth muscle cells by suppressing G1 cyclin expression. J Biol Chem. 1997;272:13816–22. doi: 10.1074/jbc.272.21.13816. [DOI] [PubMed] [Google Scholar]
- 85.Meinhardt G, Eppinger E, Schmidmaier R. Effect of novel modulators of protein kinase C activity upon chemotherapy-induced differentiation and apoptosis in myeloid leukemic cells. Anti-Cancer Drugs. 2002;13:725–33. doi: 10.1097/00001813-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Liu J, Dai Q, Chen J, et al. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol Cancer Res. 2003;1:892–902. [PubMed] [Google Scholar]
- 87.Duan S, Hajek P, Lin C, Shin SK, Attardi G, Chomyn A. Mitochondrial outer membrane permeability change and hypersensitivity to digitonin early in staurosporine-induced apoptosis. J Biol Chem. 2003;278:1346–53. doi: 10.1074/jbc.M209269200. [DOI] [PubMed] [Google Scholar]
- 88.Mufson RA, Gubina E, Rinaudo M, Baxter GA. phosphatidylcholine phospholipase C inhibitor, D609, blocks interleukin-3 (IL-3)-induced bcl-2 expression but not c-myc expression in human IL-3-dependent cells. Exp Cell Res. 1998;240:228–35. doi: 10.1006/excr.1998.3932. [DOI] [PubMed] [Google Scholar]
- 89.Porn-Ares MI, Chow SC, Slotte JP, Orrenius S. Induction of apoptosis and potentiation of TNF- and Fas-mediated apoptosis in U937 cells by the xanthogenate compound D609. Exp Cell Res. 1997;235:48–54. doi: 10.1006/excr.1997.3641. [DOI] [PubMed] [Google Scholar]
- 90.Luberto C, Hannun YA. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J Biol Chem. 1998;273:14550–9. doi: 10.1074/jbc.273.23.14550. [DOI] [PubMed] [Google Scholar]
- 91.Aza-Blanc P, Cooper CL, Wagner K, Batalov S, Deveraux QL, Cooke MP. Identification of modulators of TRAIL-induced apoptosis via RNAi-based phenotypic screening. Mol Cell. 2003;12:627–37. doi: 10.1016/s1097-2765(03)00348-4. [DOI] [PubMed] [Google Scholar]
- 92.Frasch SC, Henson PM, Kailey JM, et al. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cδ. J Biol Chem. 2000;275:23065–73. doi: 10.1074/jbc.M003116200. [DOI] [PubMed] [Google Scholar]
- 93.Holler N, Zaru R, Micheau O, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–95. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 94.Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]