Abstract
Objective
To investigate trends in the efficacy of praziquantel (PZQ) suggestive of the emergence of drug resistance against Schistosoma mansoni infection after 12.5 years of intense, repeated use in a small geographic area along the shores of Lake Victoria.
Methods
As part of a longitudinal study, 178 men occupationally exposed to schistosomes were repeatedly tested for S. mansoni infection at 4-6 week intervals and were treated with PZQ at each reinfection. We compared cure rates by year of study and examined factors associated with cure failure in a multivariate logistic regression model.
Results
Overall, the cure rate after a single dose of PZQ was 66%, ranging annually from 36% to 82%. In multivariate analysis, failure to cure after 1 PZQ dose was significantly associated with high intensity of infection and having fewer previous exposures to dying worms. Even after adjustment for these factors, treatments administered in 2006 were significantly more likely to result in cure failures than treatments administered in 2004, the year in which PZQ efficacy was highest. While cure rates varied over the course of 12 years, there was no consistent downward trend towards decreased efficacy over time. In years for which malacological data were available, periods of low PZQ efficacy coincide with high rates of S. mansoni infection in nearby snail populations.
Conclusion
We did not find a pattern of cure failures consistent with development of clinical resistance to PZQ in our intensely treated cohort.
Keywords: schistosomiasis, praziquantel, drug resistance
Introduction
Schistosomiasis is one of the most prevalent parasitic infections of humans, with an estimated 200 million people infected and 600 million at risk worldwide (Chitsulo et al. 2000). Presently, schistosomiasis control strategies are focused on mass administration of the drug praziquantel (PZQ), with special emphasis on treating school age children (Fenwick et al. 2003). While PZQ is safe and effective, there is concern over the reliance on a single drug for a concerted effort against such a widespread infection. While definitive evidence of clinically relevant resistance to PZQ has not been reported, field studies showing reduced efficacy of PZQ in some settings, and the ability to induce resistance in laboratory-maintained isolates through drug pressure in mice, raise concerns that PZQ resistance in human schistosomiasis could develop (Doenhoff et al. 2002).
As part of several longitudinal studies, we have been repeatedly treating a geographically defined cohort of occupationally exposed adult men for more than 12 years. If praziquantel resistance can be induced in field settings, repeated treatments in a restricted area with high numbers of individual parasites are the conditions most likely to have this result. We investigated trends in the efficacy of PZQ over time to evaluate the possible emergence of PZQ resistance in our study population as well as factors associated with failure to cure.
Methods
Patient population
All participants in this study were adult males working as car washers along the shores of Lake Victoria in Kisumu, western Kenya. Car washers are exposed to schistosomes as they stand ankle- to knee-deep in the lake to wash cars that have been driven into the shallow water at the edge of the lake. The entire car washing area under study is confined to an area approximately 30 m along the shoreline. Based on their occupation, these men likely received almost all of their exposure within this small site, which is also located within 15-25 m of the site of most fecal contamination in this area. All subjects in this current analysis were part of two longitudinal studies by our group designed to study resistance to S. mansoni reinfection (Karanja et al. 1998; Mwinzi et al. 2001; Karanja et al. 2002; Mwinzi et al. 2008). Enrollment for the first study began in June 1995 and ended in December 1999, with follow-up of willing participants that continues to the present day. Enrollment in the second cohort began in October 2003 and is currently ongoing. Since follow-up procedures regarding stool sampling and treatment are the same for both cohorts, the data were pooled for the present analysis. Thus, this analysis includes data collected from June 1995 through December 2007.
The parent studies from which the current data were obtained, including the collection of and use of all data analyzed in the current study, were approved by the institutional review boards of the University of Georgia and the Centers for Disease Control and Prevention, the Scientific Steering Committee of the Kenya Medical Research Institute, and the KEMRI/National Ethics Review Board of Kenya.
Patient follow-up
Upon enrollment, car washers were tested for infection with S. mansoni by the modified Kato-Katz technique. Duplicate slides from 3 stool samples taken within a 2-week period were used to quantify S. mansoni eggs per gram (epg) of feces. Persons positive for S. mansoni eggs were treated with 40 mg/kg PZQ. Follow-up stool samples were taken 4 to 6 weeks later to allow clearance of already deposited eggs from the treated subjects' intestines, and those persons still excreting eggs were retreated with another dose of PZQ. Retreatment continued until stool samples were negative for S. mansoni eggs, based again on duplicate slides of 3 separate stools. After cure by these standard criteria, participants were continually followed and retested for the presence of S. mansoni eggs by 3 stool samples provided at 4-week intervals. Each time stools were found to be positive subjects were treated with PZQ until only negative stools were obtained, as above. PZQ was purchased through the Kenyan Ministry of Health and has been manufactured by Cosmos Ltd (Nairobi, Kenya) since 2002.
Participants donated blood samples for HIV-1 testing as previously described (Karanja et al. 2002; Mwinzi et al. 2008) approximately yearly for those enrolled prior to 2003 and every six months for those enrolled thereafter.
Malacological studies
Snails of the species Biomphalaria sudanica were collected at the carwash site as part of a study of the interactions between human and rodent schistosomes in the Lake Victoria region of Kenya (Steinauer et al. 2008). B. sudanica was the only species of snails found to harbor S. mansoni in this location. Briefly, snail surveys were conducted intermittently between November 2004 and February 2007 using a standard wire-mesh scoop. Snails were isolated in aged tap water in individual wells of tissue culture plates for 24-48 hours and examined for shedding cercariae. Cercariae from infected snails were used to infect mice, and adult worms perfused from mice were identified as S. mansoni by molecular techniques.
Statistical analyses
Each infection or reinfection was counted as a separate infection event. Instances in which the participant left the study or follow-up ended after PZQ treatment and before cure could be assessed or more than 4 months transpired between treatment and assessment of cure were considered censored. Observations that were censored after 1 dose of PZQ following a new reinfection were excluded from the analyses. Subjects that were censored after having received more than 1 dose of PZQ without demonstrated cure were included in the analyses with an imputed cure after the final documented dose of PZQ. Cure rate was defined as the percent of infections cleared of S. mansoni eggs after one or two doses of PZQ. Percent reduction in mean egg count after a single PZQ dose was defined as:
Logistic regression was used to examine the effect of year of treatment, intensity of infection, number of previous cures, number of years worked in the lake, and HIV status on the efficacy of a single dose of PZQ. Intensity of infection was included in the logistic regression models as a continuous variable representing the log transformation of the arithmetic mean egg count of the 6 stool slides examined. Number of previous cures and number of years worked in the lake were included in the regression models as a composite variable, as both variables are used as indicators of past exposure to dying worms, either due to PZQ or through natural worm death. Also, as a longer history of work at the lake is strongly correlated with length of time in the study and thus number of previous treatments received, inclusion of both variables would introduce collinearity into the models. Thus, past exposure to dying worms was defined as having worked in the lake at least 10 years or having 3 or more previous documented cures. Ten years was chosen as the cutoff because it represents the upper range of the mean natural life span of S. mansoni worms (Fulford et al. 1995). Because the home villages of approximately 90% of the car washers in this study were not lakeside villages endemic for schistosomiasis, it is unlikely that they received exposures to schistosomes before they began working at the car wash.
For comparisons between year of treatment, 2004 was chosen as the reference year because cure rates were high during this year and an ample number of infections occurred to make meaningful comparisons with other years. Generalized estimating equations were used to account for non-independence due to the inclusion of multiple infection episodes of the same individuals. All analyses were performed with SAS version 9.1 (Cary, NC).
Results
The study population consisted of 201 men who experienced 916 infection episodes over the course of approximately 12.5 years. Two men were treated with oxamniquine after repeatedly failing to cure with PZQ, one of whom successfully cured with oxamniquine after 7 failed PZQ treatments, and one of whom never cured despite 14 PZQ and 9 oxamniquine treatments. Both of these men were excluded from further analyses. An additional 91 infection episodes (9.9% of total) were excluded from the analyses due to censoring after 1 PZQ treatment, leaving a final sample size of 823 infections in 178 men. The mean number of infection episodes per individual was 5 (range: 1-18) and the mean follow-up time was 59.5 months (range: 1-152 months).
Efficacy of PZQ
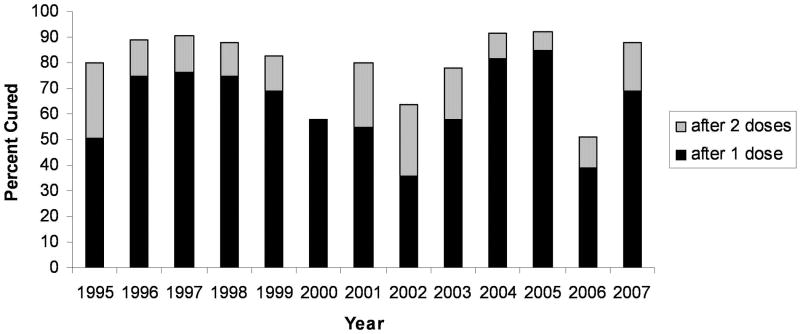
The overall efficacy of 1 and 2 doses of PZQ over the entire period of 12.5 years was 66% and 82%, respectively. Efficacy by year of treatment is shown in Figure 1. The cure rate after 1 dose of PZQ ranged yearly from 36% to 82%. The percentage of infected persons cured after 2 doses of PZQ ranged from 52% to 92%. While cure rates varied over the course of 12 years of PZQ administration, there was no consistent downward trend of decreased efficacy over time. After 2 doses of PZQ cure rates were mostly well above the generally accepted efficacies for PZQ, although during the years 2000, 2002 and 2006 efficacy was below 65% even after 2 doses.
Figure 1.
Percent of S. mansoni infections cured after 1 or 2 doses of PZQ, by year of treatment.
The percent reduction in egg count after a single dose of PZQ, by year of treatment, is given in Table 1. The overall mean percent egg reduction was 83%, and annually ranged from 62% to 91%. The overall mean number of PZQ doses needed to achieve cure was 1.8, and annually ranged from 1.4 to 3.1 (Table 1). As with cure rates, mean reductions in egg count and the number of doses needed to clear S. mansoni eggs fluctuated by year of treatment but did not follow any linear pattern over time.
Table 1.
Percent reduction in egg count after 1 dose of PZQ and mean number of doses needed to cure S. mansoni infections by year of first treatment after reinfection
| Year | Total men | Total episodes | Pre-treatment infection intensity Mean* EPG (range) |
% Reduction in egg count Mean (sd) |
Number of doses needed to cure Mean (sd) |
|---|---|---|---|---|---|
| 1995 | 74 | 91 | 432 (8-7356) | 85.5 (28.3) | 2.0 (1.7) |
| 1996 | 41 | 55 | 59 (8-1152) | 87.5 (29.3) | 1.6 (1.7) |
| 1997 | 46 | 63 | 18 (4-1160) | 81.2 (35.9) | 1.5 (1.2) |
| 1998 | 43 | 68 | 21 (4-2244) | 84.6 (32.3) | 1.6 (1.4) |
| 1999 | 32 | 48 | 41 (4-2508) | 83.4 (34.2) | 1.7 (1.8) |
| 2000 | 17 | 19 | 72 (12-3204) | 80.0 (35.2) | 2.1 (1.6) |
| 2001 | 22 | 22 | 68 (4-1092) | 77.4 (38.8) | 2.5 (2.5) |
| 2002 | 22 | 25 | 107 (4-2094) | 73.8 (37.1) | 2.7 (2.1) |
| 2003 | 39 | 52 | 153 (4-5907) | 89.4 (24.2) | 1.8 (1.4) |
| 2004 | 59 | 120 | 15 (3-2349) | 88.7 (27.3) | 1.4 (1.2) |
| 2005 | 64 | 100 | 11 (3-1422) | 91.2 (25.2) | 1.4 (1.2) |
| 2006 | 65 | 95 | 21 (3-3541) | 61.8 (42.9) | 3.1 (2.4) |
| 2007 | 45 | 65 | 20 (3-1991) | 81.0 (36.0) | 1.5 (1.0) |
EPG = eggs per gram of feces
Geometric mean
Factors associated with cure failure
Overall, 34% of infections failed to clear after a single dose of PZQ. The frequencies of cure failures after 1 dose of PZQ, stratified by year of treatment, number of previous cures, years worked in the lake, intensity of infection, and HIV status are given in Table 2. Cure failures were highest in the years 1995, 2000-2003 and 2006, among those subjects with no previous cures (and thus treatment naïve), those with less than 10 years of lake exposure, those with highest egg counts, and those who were HIV negative.
Table 2.
Frequency of cure failure of S. mansoni infections after 1 dose of PZQ, stratified by year of treatment, number of previous cures, years worked in the lake, intensity of infection, and HIV status
| Variable | Frequency of Cure Failure % (# failures / total N) |
|---|---|
| Year | |
| 1995 | 49.5 (45 / 91) |
| 1996 | 25.5 (14 / 55) |
| 1997 | 23.8 (15 / 63) |
| 1998 | 25.0 (17 / 68) |
| 1999 | 31.3 (15 / 48) |
| 2000 | 42.1 (8 / 19) |
| 2001 | 45.5 (10 / 22) |
| 2002 | 64.0 (16 / 25) |
| 2003 | 42.3 (22 / 52) |
| 2004 | 18.3 (22 / 120) |
| 2005 | 15.0 (15 / 100) |
| 2006 | 61.1 (58 / 95) |
| 2007 | 30.8 (20 / 65) |
| # of previous cures | |
| 0 | 58.1 (100 / 172) |
| 1-2 | 31.3 (71 / 227) |
| 3+ | 25.0 (106 / 424) |
| Years worked in lake | |
| <10 | 38.5 (156 / 405) |
| ≥10 | 21.2 (49 / 231) |
| Egg category | |
| <100 epg | 24.9 (151 / 607) |
| 100-1000 epg | 54.7 (75 / 137) |
| >1000 epg | 64.9 (50 / 77) |
| HIV status | |
| positive | 26.5 (59 / 223) |
| negative | 36.6 (169 / 462) |
EPG = eggs per gram of feces
In univariate analyses (Table 3, left-hand column), infections treated during the years 1995, 1999-2003 and 2006 were significantly more likely to be cure failures than infections treated in 2004, with odds ratios ranging from 2.4 to 8.8. Infections in subjects who had worked in the lake at least 10 years or had previously experienced 3 or more PZQ-induced cures had 0.4 times reduced odds of cure failure as infections in subjects with fewer previous exposures to dying worms. Each 1-unit increase in log egg count increased the odds of cure failure by 1.5 (95 % CI [1.4, 1.6]). Infections in HIV positive men were less likely to be cure failures than infections among HIV negative men (OR=0.6 [0.4, 0.8]).
Table 3.
Results of univariate and multivariate analyses of associations between failure to cure after 1 dose of PZQ and year of treatment, previous exposure to dying worms, intensity of infection, and HIV status
| Variable | Unadjusted OR (95% CI) | Adjusted* OR (95% CI) |
|---|---|---|
| Year | ||
| 1995 | 4.5 (2.4, 8.6)† | 0.7 (0.3, 1.7) |
| 1996 | 1.6 (0.7, 3.7) | 0.5 (0.2, 1.4) |
| 1997 | 1.5 (0.7, 3.1) | 1.1 (0.5, 2.5) |
| 1998 | 1.7 (0.9, 3.2) | 1.2 (0.5, 2.5) |
| 1999 | 2.4 (1.1, 5.1)† | 1.0 (0.4, 2.6) |
| 2000 | 3.8 (1.4, 10.3)† | 1.6 (0.4, 6.8) |
| 2001 | 3.6 (1.3, 9.8)† | 1.7 (0.3, 7.9) |
| 2002 | 8.8 (3.7, 21.3)† | 2.6 (0.8, 7.8) |
| 2003 | 3.4 (1.8, 6.5)† | 0.9 (0.3, 2.5) |
| 2004 | Ref | Ref |
| 2005 | 0.7 (0.4, 1.4) | 0.4 (0.1, 1.0)† |
| 2006 | 6.9 (3.7, 12.9)† | 4.2 (2.1, 8.3) † |
| 2007 | 1.7 (0.8, 3.9) | 1.6 (0.6, 4.4) |
| Past exposure to dying worms‡ | ||
| Yes | 0.4 (0.3, 0.5)† | 0.5 (0.4, 0.8)† |
| No | Ref | Ref |
| Log epg (continuous) | 1.5 (1.4, 1.6)† | 1.5 (1.3, 1.8) † |
| HIV status | ||
| Positive | 0.6 (0.4, 0.8)† | 1.1 (0.7, 1.6) |
| Negative | Ref | Ref |
OR = Odds ratio
CI = Confidence interval
EPG = eggs per gram of feces
Adjusted for all other variables in table
p < 0.05
Past exposure to dying worms is defined as having worked in the lake at least 10 years or having at least 3 prior documented cures
Results of the multivariate analysis of factors associated with PZQ failure are also shown in Table 3 (right-hand column). Many of the differences in cure failures by year disappeared after adjustment for intensity of infections, previous cure history, and HIV status. However, even after adjustment for these factors, the odds of cure failure for infections treated in 2006 were 4.2 times greater (95 % CI [2.1, 8.3]) than the odds of cure failure in 2004. Infections treated in 2005 were significantly less likely to be cure failures relative to infections treated in 2004 (OR=0.4 [0.1, 1.0]). HIV status was no longer associated with cure failure after adjustment for other factors, while the association between cure failure and intensity of infection and previous exposure to dying worms remained virtually unchanged.
Malacological survey results
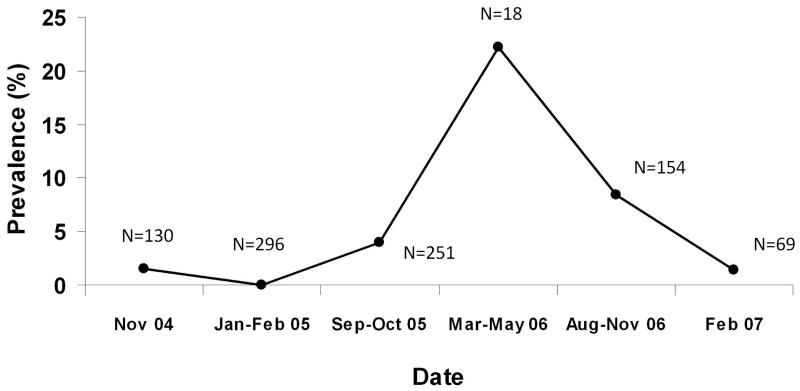
As an indicator of potential for S. mansoni transmission in this area, the prevalence of S. mansoni infection among the intermediate snail hosts was investigated. Prevalence in B. sudanica snails collected at the carwash site between November 2004 and February 2007 is shown in Figure 2. Malacological surveys were not conducted at regular intervals during this time, but time periods in which surveys were done over consecutive months are grouped together. The percentage of snails infected with S. mansoni ranged from 0% (0/296) in early 2005 to 22% (4/18) in the period from March-May 2006. After mid-2006, prevalence of infected snails decreased to 1.4% (1/69) in early 2007.
Figure 2.
Prevalence of S. mansoni in Biomphalaria sudanca snails collected at the carwash site from the period between November 2004 – February 2007. N indicates number of snails represented in each time period.
Discussion
While we observed substantial year-to-year variation in the efficacy of PZQ against S. mansoni infection, we saw no evidence of the development of clinically detectable resistance to PZQ after more than 12 years of consistent administration in this relatively closed population in a confined geographical area. Similar results were reported for the efficacy of PZQ against S. haematobium in coastal Kenya, where after repeated PZQ treatment in a group of children followed from 1984-1991, cure rates after a single dose varied from 65% in 1986 to 96% in 1990 but did not predictably decline by year (King et al. 2000). Reduced efficacy against S. japonicum was also not found in the Dongting Lake region of China after 14 years of annual mass chemotherapy with PZQ (Yu et al. 2001). However, ours is the first study to report on intense, multiple retreatments of the same individuals in a small area over the course of many years.
Two instances in the literature of mass PZQ use in the treatment of schistosomiasis are frequently cited as evidence that PZQ resistance or tolerance in schistosome infections of humans may be possible. A 1994 study in Egypt of five villages in which PZQ had been used for approximately 10 years found that 1.6% of those infected with S. mansoni were not cured after 3 doses of PZQ, and worms from those infections had reduced responsiveness to PZQ in vivo and in vitro. However, a follow-up study in the same villages after 10 additional years of continued PZQ use found cure rates after 1 dose of PZQ to be within normal ranges and no patients failed to cure after 3 successive doses (Ismail et al. 1996; Botros et al. 2005).
Primary resistance to PZQ was thought to be seen in a newly endemic area of northern Senegal when studies conducted between 1991-1993 yielded cure rates between 18-39%, the lowest cure rates reported at the time and well below the expected cure rates of 70-90% (Cioli 2000; Gryseels et al. 2001). However, the notion of PZQ resistance in this population has been largely dismissed in subsequent literature. The initial low cure rates have instead been attributed to three factors: high intensity of infection, high transmission of infection, and the lack of previous immunologic exposure to schistosomes in this population. Because the intensity of infection was so high among the participants of these studies, many researchers speculate that even if PZQ was >90% effective, enough worm pairs could remain alive to continue to excrete detectable eggs. Secondly, PZQ is largely ineffective against larval stages of worms. Due to the high intensity of transmission in this area, many people were likely infected with immature worms at the time of treatment, which would have matured and begun excreting eggs at the time of the follow-up 12 weeks later (Cioli 2000; Gryseels et al. 2001). Thirdly, though the exact mechanism of action of PZQ is unknown, efficacy in mice has been shown to be dependent on appropriate humoral immune responses in the host. Researchers have postulated that PZQ acts by exposing surface antigens on the worms and rendering them susceptible to elimination by relevant immune effector mechanisms in the host (Harnett & Kusel 1986; Brindley & Sher 1987; Doenhoff et al. 1987; Modha et al. 1990). These necessary immune responses were possibly not yet fully developed in this recently exposed Senegalese population.
The aforementioned factors for low PZQ efficacy may also explain the yearly variations in cure rates in our study population. As seen by others (Van Lieshout et al. 1999; Kabatereine et al. 2003; Raso et al. 2004), we found that higher intensities of infection were significantly associated with the inability to completely cure. Furthermore, after adjustment for egg count, cure rates in the years in which intensity of infections were high (1995, 2000, 2003) were similar to those years in which cure rates were >80%.
Even after adjustment for intensity of infection, cure rates during the year 2006 remained low relative to other years. However, as evidenced by the variation in the prevalence of S. mansoni in the intermediate snail hosts collected at the car wash site (Figure 2), intensity of transmission in the area surrounding the carwash is not consistent over time. The year in which cure failures were the greatest, 2006, is also the year in which S. mansoni prevalence in snails was highest of the years for which snail data are available. Although based on a small sample of snails, the 22% prevalence in snails observed in mid-2006 is extraordinarily high relative to what is often reported. For example, in coastal Kenya, a 1.2% prevalence of S. haematobium among Bulinus snails was sufficient to support a high prevalence of human S. haematobium infections in the area (Kariuki et al. 2004). Similarly, during the time period of the aforementioned King et al study of repeated treatment in school children, prevalence of S. haematobium in snails in the study areas ranged from 0.13% to 1.5% (Sturrock et al. 1990). Thus, the increase in the proportion of infected snails is consistent with increased transmission during 2006 and the possibility that subjects were harboring pre-patent infections that are not sensitive to PZQ. S. mansoni prevalence in snails decreased in 2007, corresponding to a decrease in cure failures in 2007 relative to 2006. Other researchers have reported lower-than-expected PZQ cure rates in areas of high schistosome transmission (Kabatereine et al. 2003) and reduced efficacy in sites with higher transmission relative to sites with lower transmission (Garcia et al. 2006).
Independently of differences in intensity of infection and transmission, we found that infections in men with less than ten years of lake exposure and less than three documented prior cures were more likely to be cure failures than those in men with a previous history of treatment-induced cures or a likelihood of exposure to naturally-occurring worm deaths. An immunologic component to PZQ cure involving antibodies targeted at tegumental antigens that are exposed upon death or damage to the worms but not normally exposed during the lifespan of the worm has been postulated (Harnett & Kusel 1986; Brindley & Sher 1987; Doenhoff et al. 1987; Modha et al. 1990), so possibly immune responses generated by previous exposure to dying worms add to the subsequent efficacy of PZQ to clear worms. Our results are in contrast to those of King et al (2000), who found no association between cure failure and prior PZQ treatment. However, their study included only children in a focally endemic area so may not be comparable to ours, which included only adults largely raised in non-endemic settings.
Consistent with a prior report by our group (Karanja et al. 1998), upon multivariate analysis we found no association between HIV status and PZQ cure failure. Of the 49 HIV positive men for whom CD4 data were available, only 14 had CD4 counts below 200 at any point during the course of follow-up, and only one man had a CD4 count below 100. Most HIV positive subjects had relatively normal CD4 counts throughout the duration of the study and were thus still likely sufficiently immunocompetent to make the appropriate antibodies postulated as necessary for PZQ efficacy (Karanja et al. 1998).
The data presented here only provide evidence that broad-scale PZQ resistance has not spread throughout the carwash population due to high selective drug pressure. Resistance or increased tolerance could have emerged in individual worms but not become widespread in the population. If such resistant strains exist, whether they could spread throughout the population and lead to observable clinical resistance depends on such factors as the size of the schistosome population in untreated human hosts and the fitness costs of resistance to the worms (Feng et al. 2001). Evaluation of these factors is beyond the scope of the current analysis, but ongoing population genetics studies by several groups may provide useful data on genetic population shifts under field conditions of mass drug administration.
Although we did not find broad-scale resistance to PZQ, we also did not find that repeated treatment demonstrably lessened the transmission of S. mansoni in this area. Since we do not treat everyone simultaneously, thereby eliminating worms from all human reservoirs, our individual treatment strategy leaves a constant refugium of worms in those infected but not yet diagnosed and treated. Likewise, there are sometimes others utilizing the lake in the carwash area who may be contributing to transmission. Our experience indicates that very frequent diagnosis and treatment with PZQ of schistosome-infected individuals has not lead to the widespread development of clinical resistance to PZQ, but intense individual treatment after positive diagnosis alone was also not sufficient to eliminate, or even substantially diminish, transmission in a high-intensity focus of infection.
Acknowledgments
These studies are published by permission of the Director of KEMRI. We gratefully thank all those in the Schistosomiasis Laboratory and Field Group at the Centre for Global Health Research, KEMRI for their logistical and scientific support and Drs. G. Mkoji and E. Loker for reading the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
References
- Botros S, Sayed H, Amer H, El-Ghannam G, Bennett JL, Day TA. Current status of sensitivity to praziquantel in a focus of potential drug resistance in Egypt. International Journal of Parasitology. 2005;35:787–91. doi: 10.1016/j.ijpara.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Brindley PJ, Sher A. The chemotherapeutic effect of praziquantel against Schistosoma mansoni is dependent on host antibody response. Journal of Immunology. 1987;139:215–220. [PubMed] [Google Scholar]
- Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Tropica. 2007;77:41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioli D. Praziquantel: is there real resistance and are there alternatives? Current Opinion in Infectious Diseases. 2000;13:659–663. doi: 10.1097/00001432-200012000-00014. [DOI] [PubMed] [Google Scholar]
- Doenhoff MJ, Sabah AAA, Fletcher C, Webbe G, Bain J. Evidence for an immune-dependent action of praziquantel on Schistosoma mansoni in mice. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:947–951. doi: 10.1016/0035-9203(87)90360-9. [DOI] [PubMed] [Google Scholar]
- Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:465–469. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- Feng ZL, Curtis J, Minchella DJ. The influence of drug treatment on the maintenance of schistosome genetic diversity. Journal of Mathematical Biology. 2001;43:52–68. doi: 10.1007/s002850100092. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Savioli L, Engels D, Bergquist NR, Todd MH. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends in Parasitology. 2003;19:509–515. doi: 10.1016/j.pt.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Fulford AJ, Butterworth AE, Ouma JH, Sturrock RF. A statistical approach to schistosome population dynamics and estimation of the life span of Schistosoma mansoni in man. Parasitology. 1995;110(pt 3):307–316. doi: 10.1017/s0031182000080896. [DOI] [PubMed] [Google Scholar]
- Garcia N, Isturiz G, Aular S, Incani RN. The efficacy of human schistosomide treatment may depend on the rate of transmission. Parasitology Research. 2006;98:545–549. doi: 10.1007/s00436-005-0047-1. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Mbaye A, De Vlas SJ, et al. Are poor responses to praziquantel for the treatment of Schistosoma mansoni infections in Senegal due to resistance? An overview of the evidence. Tropical Medicine and International Health. 2001;6:864–873. doi: 10.1046/j.1365-3156.2001.00811.x. [DOI] [PubMed] [Google Scholar]
- Harnett W, Kusel JR. Increased exposure of parasite antigens at the surface of adult male Schistosoma mansoni exposed to praziquantel in vitro. Parasitology. 1986;93:401–405. doi: 10.1017/s0031182000051568. [DOI] [PubMed] [Google Scholar]
- Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennett JL. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. American Journal of Tropical Medicine and Hygiene. 1996;55:214–218. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- Kabatereine NB, Kemijumbi J, Ouma JH. Efficacy and side effects of praziquantel treatment in a highly endemic Schistosoma mansoni focus at Lake Albert, Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:599–603. doi: 10.1016/s0035-9203(03)80044-5. [DOI] [PubMed] [Google Scholar]
- Karanja DMS, Boyer AE, Strand M, et al. Studies on schistosomiasis in western Kenya: II. Efficacy of praziquantel for treatment of schistosomiasis in persons coinfected with human immunodeficiency virus-1. American Journal of Tropical Medicine and Hygiene. 1998;59:307–311. doi: 10.4269/ajtmh.1998.59.307. [DOI] [PubMed] [Google Scholar]
- Karanja DMA, Hightower AW, Colley DG, et al. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet. 2002;360:592–596. doi: 10.1016/S0140-6736(02)09781-7. [DOI] [PubMed] [Google Scholar]
- Kariuki HC, Clennon JA, Brady MS, et al. Distribution patterns and cercarial shedding of Bulinus nasutus and other snails in the Msambweni area, Coast Province, Kenya. American Journal of Tropical Medicine and Hygiene. 2004;70:449–456. [PubMed] [Google Scholar]
- King CH, Muchiri EM, Ouma JH. Evidence against rapid emergence of praziquantel reistance in Schistosoma haematobium, Kenya. Emerging Infectious Diseases. 2000;6:585–594. doi: 10.3201/eid0606.000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modha J, Lambertucci JR, Doenhoff MJ, McClaren DJ. Immune dependence of schistosomicidal chemotherapy: an untrastructural study of Schistosoma mansoni adult worms exposed to praziquantel and immune serum in vivo. Parasite Immunology. 1990;12:321–334. doi: 10.1111/j.1365-3024.1990.tb00958.x. [DOI] [PubMed] [Google Scholar]
- Mwinzi PNM, Karanja DMS, Colley DG, Orago ASS, Secor WE. Cellular immune response of schistosomiasis patients are altered by Human Immunodeficiency Virus-1 co-infection. Journal of Infectious Diseases. 2001;84:488–496. doi: 10.1086/322783. [DOI] [PubMed] [Google Scholar]
- Mwinzi PNME, Ganley-Leal L, Black CL, Secor WE, Karanja DMS, Colley DG. Circulating CD23+ cell subset correlates with development of resistance to Schistosoma mansoni reinfection in occupationally exposed, multiply treated adults. Journal of Infectious Diseases. 2008 doi: 10.1086/595792. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raso G, N'Goran EK, Toty A, et al. Efficacy and side effects of praziquantel against Schistosoma mansoni in a community of western Cote d'Ivoire. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2004;98:18–27. doi: 10.1016/s0035-9203(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Steinauer ML, Mwangi IN, Maina GM, et al. Interactions between natural populations of human and rodent schistosomes in the Lake Victoria region of Kenya: a molecular epidemiological approach. PLoS Neglected Tropical Diseases. 2008;2(4):e222. doi: 10.1371/journal.pntd.0000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock RF, Kinyanjui H, Thiongo FW, et al. Chemotherapy-based control of schistosomiasis haematobia. 3. Snail studies monitoring the effect of chemotherapy on transmission in the Msambweni area, Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84:257–261. doi: 10.1016/0035-9203(90)90278-m. [DOI] [PubMed] [Google Scholar]
- Van Lieshout L, Stelma FF, Guisse F, et al. The contribution of host-related factors to low cure rates of praziquantel for the treatment of Schistosoma mansoni in Senegal. American Journal of Tropical Medicine and Hygiene. 1999;61:760–765. doi: 10.4269/ajtmh.1999.61.760. [DOI] [PubMed] [Google Scholar]
- Yu DB, Li Y, Sleigh AC, et al. Efficacy of praziquantel against Schistosoma japonicum: field evaluation in an area with repeated chemotherapy compared with a newly identified endemic focus in Hunan, China. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95:537–541. doi: 10.1016/s0035-9203(01)90032-x. [DOI] [PubMed] [Google Scholar]