Abstract
The two main challenges facing dental composite restorations are secondary caries and bulk fracture. Previous studies developed whisker-reinforced Ca-PO4 composites that were relatively opaque. The objective of this study was to develop an esthetic glass particle-reinforced, photo-cured calcium phosphate composite. Tetracalcium phosphate (TTCP) particles were incorporated into a resin for Ca and PO4 release, while glass particles provided reinforcement. Ion release and mechanical properties were measured after immersion in solutions with pH of 7, 5.5, and 4. For the composite containing 40% mass fraction of TTCP, incorporating glass fillers increased the strength (p < 0.05). Flexural strength (mean ± sd; n = 6) at 30% glass was (99 ± 18) MPa, higher than (54 ± 20) MPa at 0% glass (p < 0.05). Elastic modulus was 11 GPa at 30% glass, compared to 2 GPa without glass. At 28 d, the released Ca ion concentration was (4.61 ± 0.18) mmol/L at pH of 4, much higher than (1.14 ± 0.07) at pH of 5.5, and (0.27 ± 0.01) at pH of 7 (p < 0.05). PO4 release was also dramatically increased at cariogenic, acidic pH. The TTCP-glass composite had strength 2-3 fold that of a resin-modified glass ionomer control. In conclusion, the photo-cured TTCP-glass composite was “smart” and substantially increased the Ca and PO4 release when the pH was reduced from neutral to a cariogenic pH of 4, when these ions are most needed to inhibit tooth caries. Its mechanical properties were significantly higher than previous Ca, PO4 and fluoride releasing restoratives. Hence, the photo-cured TTCP-glass composite may have potential to provide the necessary combination of load-bearing and caries-inhibiting capabilities.
Keywords: dental composite, tetracalcium phosphate, smart material, tooth caries inhibition, Ca and PO4 ion release
Introduction
Approximately 200 million dental restorations are placed annually in the U. S.1 Resin composites are widely used for tooth cavity restorations due to their esthetics, direct-filling capability, and improved longevity. Composites are composed of fillers in an acrylic matrix that is polymerized to form a solid restoration.2-7 Previous studies have improved the resin compositions and cure conditions, and reduced the polymerization shrinkage.8-13 In addition, extensive efforts have been undertaken to enhance the fracture and wear resistance of the composites.14-18 Despite these meritorious improvements, recent reports still show that “The two main challenges are secondary caries and bulk fracture”.19,20 Secondary caries at the tooth-restoration margins is a major reason for the replacement of existing restorations.21 Replacement of restorations accounts for 50% to 70% of all restorations.22,23 Replacement dentistry costs about $5 billion annually in the U. S. alone.24
An effective approach to addressing the caries problem was to develop calcium and phosphate (CaP) ion-releasing materials. CaP particles were filled into the resin, and the resulting composite released supersaturating levels of Ca and PO4 ions, which formed hydroxyapatite [Ca10(PO4)6(OH)2], the putative mineral in enamel and dentin.25-29 Indeed, several CaP materials were shown to effectively remineralize enamel and dentin lesions in vitro.25,28 However, while they addressed the caries problem, they did not address the restoration fracture problem. This was because these CaP materials had flexural strengths of only half that of unfilled resin.26 It was recognized that such low strengths were “inadequate to make these composites acceptable as bulk restoratives”.27
To address both the tooth caries and the restoration fracture problems, both CaP fillers and reinforcing fillers were incorporated into the resin to develop load-bearing, Ca and PO4 releasing composites.30-32 Besides the load-bearing issue, another important factor that needs to be considered is that the oral plaque pH after a sucrose rinse can decrease to 4.5 or even 4.33,34 A plaque pH of higher than 6 is considered to be the safe area, a plaque pH of 6.0 to 5.5 is the potentially cariogenic area, and pH of 5.5 to 4 is the cariogenic or danger area for cavity formation. Therefore, it is desirable for the CaP composite to be “smart”, to increase the release of caries-inhibiting ions at lower pH, when the Ca and PO4 ions are most needed. Such ion release triggered by a local pH drop may help prevent demineralization in tooth structures contiguous to the smart composite restoration. Therefore, in a recent study, tetracalcium phosphate [TTCP: Ca4(PO4)2O] composite was developed and shown to be smart to substantially increase the ion release at cariogenic pH.35 TTCP is a compound used in bone cements,36 tissue engineering scaffolds,37 and dental materials.28 TTCP is the most alkaline among all CaP compounds,36 and is promising in buffering harmful acids and inhibiting tooth caries. However, that recent study used whiskers as the reinforcing filler, resulting in an opaque paste that was not sufficiently esthetic.35 Further study is needed to develop a TTCP composite reinforced with dental glass particles that is esthetic and translucent, and the paste can be photo-cured.
Accordingly, the objectives of this study were to develop a “smart” TTCP composite reinforced with esthetic dental glass particles, and to investigate the effects of solution pH on composite ion release and mechanical properties. It was hypothesized that: (1) The photo-cured TTCP-glass composite would be smart and able to increase its ion release when the pH is decreased; (2) Glass particle reinforcement would significantly increase the mechanical properties of the TTCP composite, which would be significantly stronger than previously-reported ion-releasing restoratives.
Materials and Methods
TTCP and Glass Fillers
TTCP was synthesized from a solid-state reaction between CaHPO4 and CaCO3 (Baker Chemical, Phillipsburg, NJ), which were mixed and heated at 1500 °C for 6 h in a furnace (Thermolyne, Dubuque, IA).36,37 The mixture was quenched to room temperature and ground in a blender (Dynamics Corp., New Hartford, CT). The powder was then sieved to obtain TTCP particles with sizes of 1.5 - 60 μm, with a median of 16 μm. This TTCP powder was then ground in 95% ethanol with a ball-mill (Retsch, Newtown, PA) for 24 hrs. The particle size distribution was measured via a sedimentation method with the use of a centrifugal particle analyzer (SA-CP3, Shimazu, Kyoto, Japan). This yielded a particle size range of 0.2 - 3.0 μm, with a median of 0.8 μm. This TTCP powder was moderately finer than the TTCP used in a previous study.35
The glass particles were a barium boroaluminosilicate glass (median diameter = 1.4 μm, Caulk/Dentsply, Milford, DE), selected because it is a typical dental glass filler similar to those in a hybrid composite (TPH, Cault/Dentsply). The purpose was to develop a model composite; the processing parameters can then be applied to the use of other glass fillers. The glass particles were silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine (mass %).38,39
Resin Composite Fabrication
A resin of Bis-GMA (bisphenol glycidyl dimethacrylate) and TEGDMA (triethylene glycol dimethacrylate) at 1:1 mass ratio was rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoate.
Four groups of specimens were fabricated. The purpose of Group 1 was to examine the effect of TTCP filler level on mechanical properties of the photo-cured composite. Only TTCP was used as fillers in the resin, without glass fillers. The TTCP filler levels (mass fraction) in the resin were: 0%, 20%, 40%, 60%, and 70%.
The fillers were mixed with the resin and the paste was placed into a stainless steel mold of 2 mm × 2 mm × 25 mm. No de-gas procedure was performed for the experimental composite pastes and the control pastes. All the experimental composite pastes and the control pastes were photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each side. The cured specimens were incubated at 37 °C for 24 h prior to testing.
The purpose of Group 2 was to investigate the effects of glass reinforcement on composite mechanical properties. The total fillers in the resin = TTCP + glass. An intermediate TTCP filler mass fraction of 40% was selected to leave room in the resin for glass reinforcement.
The glass filler mass fractions were: 0%, 10%, 20%, 30%, and 35%. Hence the TTCP + glass filler level = 40%, 50%, 60%, 70%, and 75%, respectively. Glass filler levels higher than 35% were not used because the composite paste became relatively dry. The specimens were photo-cured in the same manner as described for Group 1.
The purpose of Group 3 was to investigate the effect of solution pH on mechanical properties of the composite immersed in the solution. The composite containing 40% TTCP plus 30% glass particles were selected, because it had a relatively high strength and ion release. The specimens were immersed for 28 days in three solutions at pH of 7, 5.5, and 4, respectively. Then the flexural strength and elastic modulus were measured, as described below.
The purpose of Group 4 was to further increase the amount of Ca and PO4 ion release, by increasing the TTCP filler level to 50% (from 40% in Groups 2 and 3). The rationale was that the previous groups showed that the TTCP-glass composite had much higher strength than a control material that had ion release, therefore there was room to slightly reduce the glass filler level, thereby increasing the TTCP level. Three composites were made, with glass co-filler levels of 20%, 25%, and 27%, respectively (Hence TTCP + glass filler mass fraction = 70%, 75% and 77%, respectively). Glass filler levels higher than 27% were not used because the composite paste became dry and difficult to mix.
A resin-modified glass ionomer (Vitremer, 3M ESPE, St. Paul, MN) was used as a control for mechanical properties of specimens immersed in solutions at different pH. Vitremer is a two-part, powder/liquid composition, and the powder is a radiopaque fluoroaluminosilicate glass. The liquid is a light sensitive, aqueous solution of a modified polyalkenoic acid (Lot # 20081028, expiration date 2011-07, Shade B2). Recommended applications include Class III and V restorations, restoration of root caries lesions, Class I and II restorations in primary teeth, and core buildup. Following manufacturer's recommendation, two scoops of powder and two drops of liquid yielded a paste that was filled into the mold. Each component was weighed, indicating a powder/liquid mass ratio (g/g) of 2.5/1. Specimens were photo-cured (Triad-2000) for 1 min on each open side of the specimen using the same 2 mm × 2 mm × 25 mm molds, then incubated at 37 °C for 24 h before immersion and mechanical testing.
Mechanical Testing
Flexural strength and elastic modulus were measured using a three-point flexural test with a 20 mm span at a crosshead-speed of 1 mm/min on a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC). The flexural strength of the composite was calculated by S = 3PmaxL/(2bh2), where Pmax is the maximum load on the load-displacement curve, L is flexure span, b is specimen width, and h is specimen thickness.40 The elastic modulus was calculated by E = (P/d)(L3/[4bh3]), where the load P divided by the corresponding displacement d is the slope of the load-displacement curve in the linear elastic region.41
Ca and PO4 Ion Release
A sodium chloride (NaCl) solution (133 mmol/L) was buffered to three different pHs: pH 4 with 50 mmol/L lactic acid, pH 5.5 with 50 mmol/L acetic acid, and pH 7 with 50 mmol/L HEPES. Following previous studies,30-32 three specimens of approximately 2 mm × 2 mm × 12 mm were immersed in 50 mL of solution at each pH, yielding a specimen volume/solution of 2.9 mm 3/mL. This compared to a specimen volume per solution of approximately 3.0 mm3/mL in a previous study.26 For each solution, the concentrations of Ca and PO4 released from the specimens were measured at the following immersion times: 1 day (d), 3 d, 7 d, 14 d, 21 d, and 28 d. At each time period, aliquots of 0.5 mL were removed and replaced by fresh solution. The aliquots were analyzed for Ca and PO4 via a spectrophotometric method (DMS-80 UV-visible, Varian, Palo Alto, CA) using known standards and calibration curves.26,28,42
One-way and two-way ANOVA were performed to detect the significant effects of the experimental variables. Tukey's multiple comparison test was used to compare the measured data at a p value of 0.05.
Results
Figure 1 plots the results from Group 1 on the effects of TTCP filler level on composite mechanical properties (mean ± sd; n = 6). No glass particles were used. Incorporation of TTCP significantly decreased the flexural strength (ranging from about 50-60 MPa), compared to the (95 ± 1) MPa of the unfilled resin (p < 0.05). Varying the TTCP level from 10% to 70% did not significantly change the strength (p > 0.1). The composite elastic modulus was significantly increased with increasing the TTCP filler level as the paste became stiffer (p < 0.05).
Figure 1.
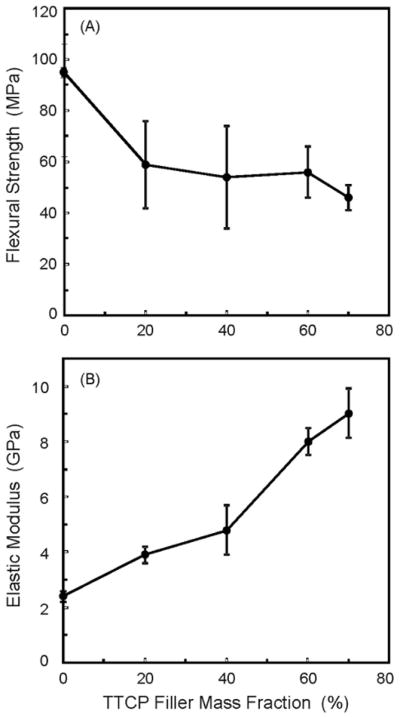
Effects of TTCP filler level on composite mechanical properties, from Group 1. Only TTCP was incorporated into the resin; no glass particles were used in the resin. The TTCP composites were photo-cured. Each value is the mean of six measurements with the error bar showing one standard deviation (mean ± sd; n = 6).
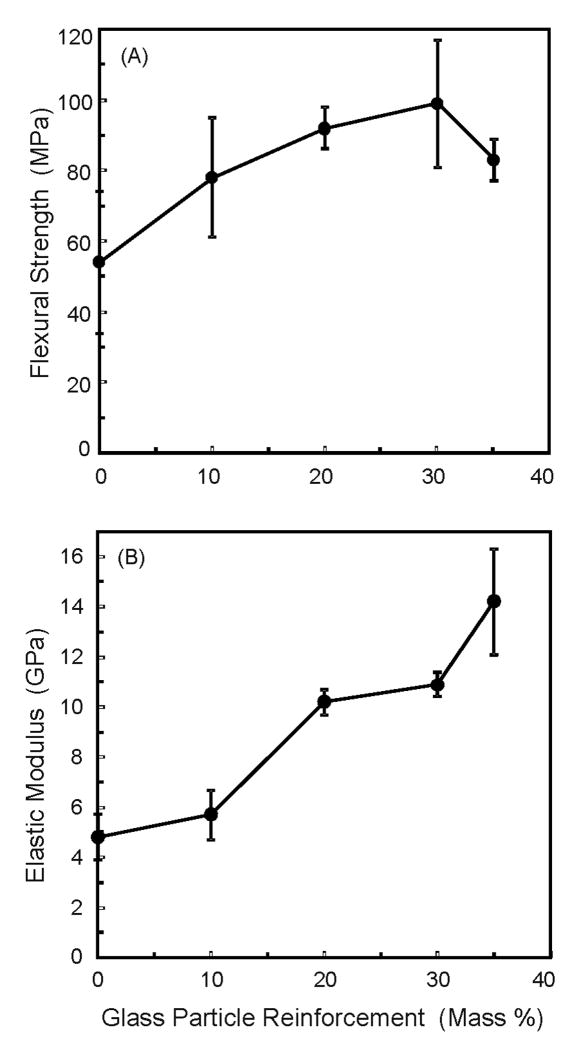
Figure 2 plots mechanical properties of Group 2 on the effects of glass filler reinforcement, while the TTCP mass fraction in the resin was fixed at 40%. Increasing the glass filler level increased the strength (p < 0.05). Flexural strength at 20% and 30% glass were (92 ± 6) MPa and (99 ± 18) MPa, respectively. Both values were higher than the (54 ± 20) MPa at 0% glass (p < 0.05). While the strengths were not significantly different from 10% glass to 35% glass (p > 0.1), the elastic modulus was monotonically increased, reaching (10.9 ± 0.5) GPa at 30% glass (Total filler mass fraction = 40% TTCP + 30% glass = 70%).
Figure 2.
Effects of glass filler reinforcement on composite mechanical properties, from Group 2. The TTCP level was the same at 40%, and the glass filler level was varied. Total fillers = TTCP + glass particles. The TTCP-glass composites were photo-cured. Increasing the glass filler level significantly increased the composite strength and elastic modulus (p < 0.05). Each value is mean ± sd; n = 6.
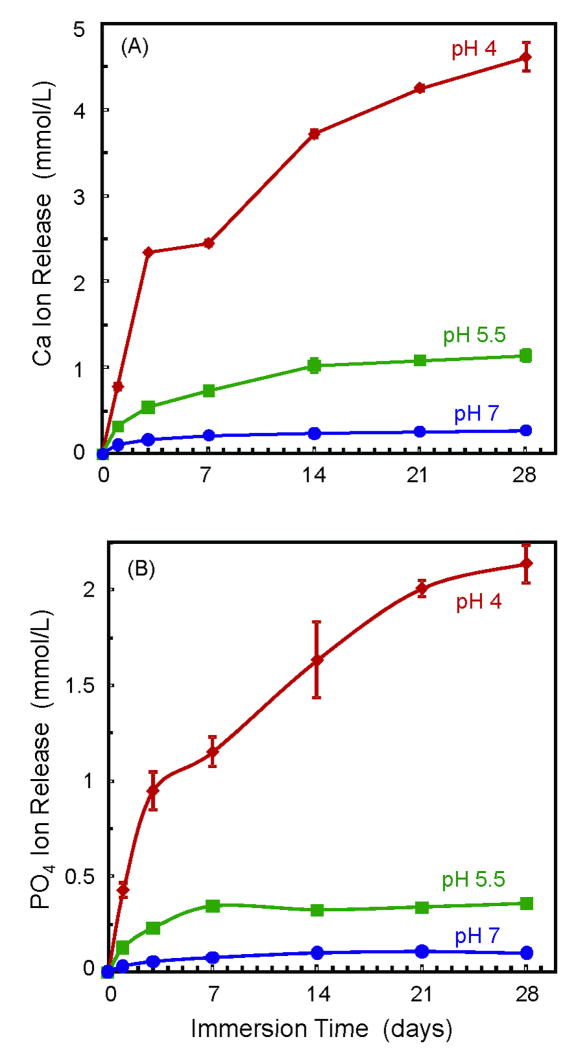
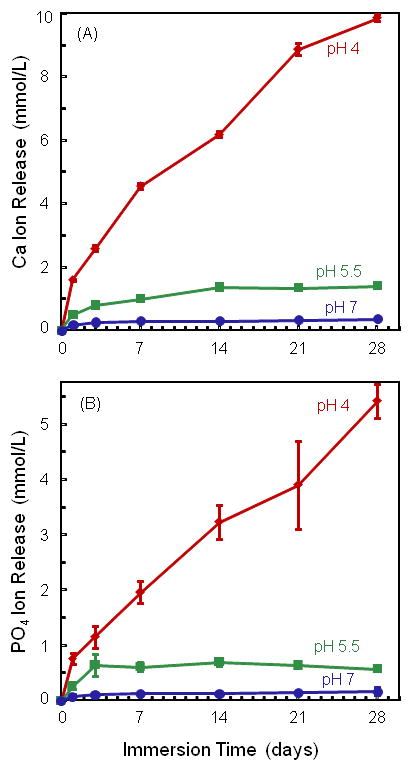
Figure 3 plots Ca and PO4 ion release for the composite containing 40% TTCP and 30% glass. It was selected because it had good strength and elastic modulus, and the composite paste was relatively easily mixed. Two-way ANOVA showed significant effects of solution pH and immersion time, with a significant interaction between the two variables (p < 0.05). Lowering the pH substantially increased the amount of ion release. At 28 d, the released Ca concentration (mean ± sd; n = 3) was (4.61 ± 0.18) mmol/L at pH of 4, much higher than (1.14 ± 0.07) mmol/L at pH of 5.5, and (0.27 ± 0.01) mmol/L at pH of 7 (p < 0.05).
Figure 3.
Ca and PO4 ion release from composite containing 40% TTCP and 30% glass. Lowering the pH substantially increased the amount of ion release. Each value is mean ± sd; n = 3.
At 28 d, the PO4 concentration was (2.14 ± 0.14) mmol/L at pH of 4, higher than (0.36 ± 0.01) mmol/L at pH of 5.5, and (0.10 ± 0.01) mmol/L at pH of 7 (p < 0.05).
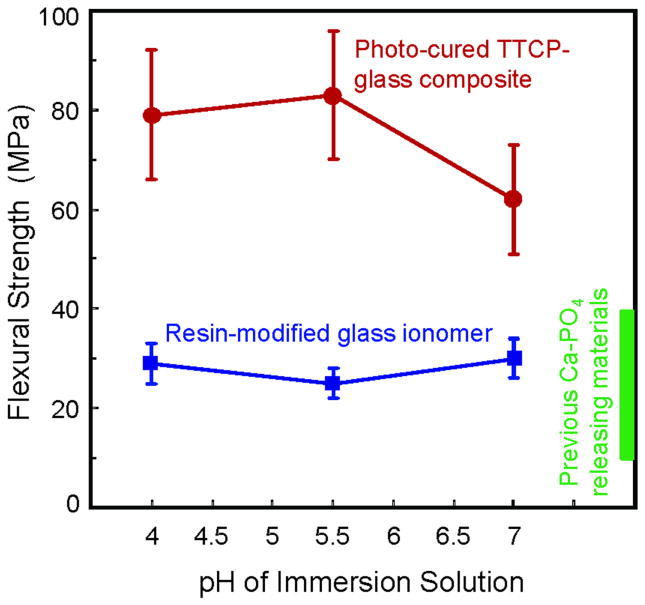
Figure 4 plots the results from Group 3 on the effect of solution pH on mechanical properties of the immersed specimens. The TTCP composite contained 40% TTCP and 30% glass, which was the same as that in Figure 3. Its strength was (79 ± 13) MPa after immersion for 28 days at pH 4, (83 ± 13) MPa at pH 5.5, and (62 ± 11) MPa at pH 7. These results were not significantly different from each other (p > 0.1). However, these strength values were lower than the (99 ± 18) MPa for specimens without immersion (Fig. 2) (p < 0.05).
Figure 4.
Effect of solution pH on mechanical properties of immersed specimens, from Group 3. Specimens were immersed for 28 days, in solutions with pH of 7, 5.5, and 4, respectively. The box at the right axis indicates the reported strength range of previous Ca-PO4 materials immersed at neutral pH.26-29 Varying the solution pH from 4 to 7 had only minor effects on the strength and elastic modulus of TTCP-glass composite and Vitremer. The composite with 40% TTCP and 30% glass had much higher strength than Vitremer and previously-reported Ca-PO4 materials. Each value is mean ± sd; n = 6.
For Vitremer, the strengths were (29 ± 4) MPa, (25 ± 3) MPa, and (30 ± 4) MPa, at pH of 4, 5.5, and 7, respectively. They were not significantly different from each other (p > 0.1). However, the strength of the TTCP-glass composite was significantly higher than that of Vitremer at each pH (p < 0.05). The rectangular box at the right axis of Figure 4 indicates the reported strengths of previous CaP materials. Previous studies reported a biaxial flexural strength of 40-50 MPa for CaP materials before immersion; the strength decreased to 10-20 MPa after 90 days of immersion at neutral pH.28,29 Other studies reported that the strength of the ACP composite decreased to about 40 MPa after 11 days of immersion at neutral pH.26,27 The elastic modulus of the TTCP-glass composite ranged from 8 to 9.6 GPa, and that of Vitremer ranged from 7.9 to 9.5 GPa, which were not significantly different from each other (p > 0.1).
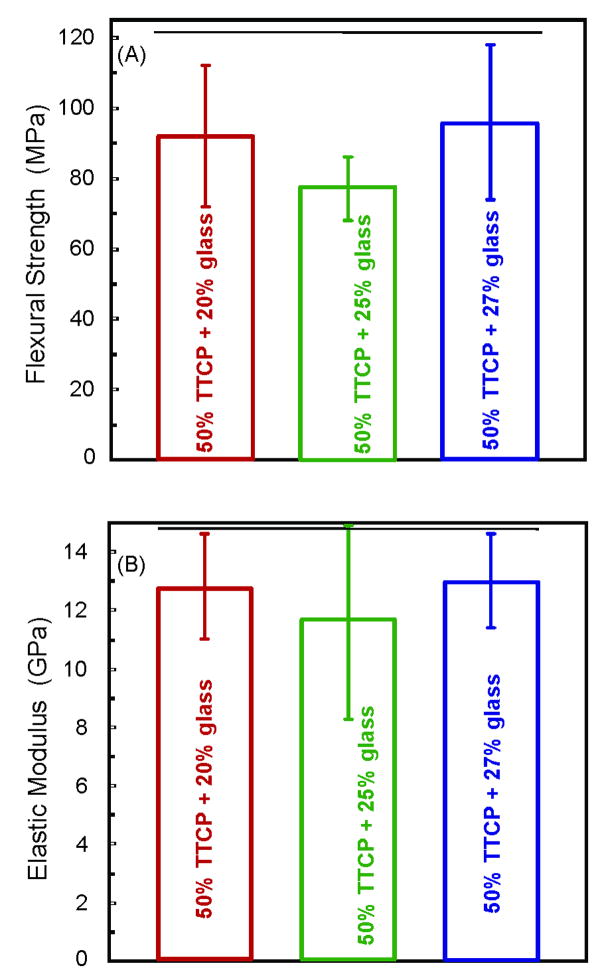
Figure 5 plots the results from Group 4, in which the TTCP filler level was increased to 50% (higher than the 40% TTCP in Fig. 2). The purpose was to increase the TTCP filler level for higher Ca and PO4 release, without compromising the composite mechanical properties. Indeed, the strengths of the experimental composites represented in Figure 5 matched the strengths of those shown in Figure 2A (p > 0.1). In Figure 5A, the flexural strength (mean ± sd; n = 6) was (92 ± 20) MPa, (77 ± 9) MPa, and (95 ± 22) MPa, at 20%, 25% and 27% glass, respectively. They were not significantly different from each other, and neither was the elastic modulus (Figure 5B) (p > 0.1). However, the resin paste was drier and more difficult to mix at 50% TTCP plus 25% or 27% glass. The paste at 50% TTCP and 20% glass was relatively easier to mix. Therefore, the composite with 50% TTCP and 20% glass was selected for Ca and PO4 release measurement in Figure 6.
Figure 5.
Group 4 with a TTCP filler level of 50% (higher than the 40% TTCP in Figure 2), reinforced with various glass filler amounts. The purpose was to increasing the TTCP filler level for increased Ca and PO4 release, without compromising the mechanical properties. Horizontal line indicates values that are not significantly different from each other (p > 0.1). The strengths of Figure 5 were similar to those in Figure 2 (p > 0.1). Each value is mean ± sd; n = 6.
Figure 6.
Ca and PO4 release from photo-cured composite containing 50% TTCP and 20% glass (mean ± sd; n = 3). Increasing the TTCP fillers to 50% significantly increased the Ca and PO4 release (as compared to the release at 40% TTCP in Figure 3).
Figure 6 plots Ca and PO4 release from the composite with 50% TTCP and 20% glass. Compared with Figure 3 for the composite with 40% TTCP, increasing the TTCP filler mass fraction to 50% significantly increased the Ca and PO4 release (p < 0.05). pH also had a significant effect. At 28 d, the Ca concentration was (9.85 ± 0.13) mmol/L at pH of 4, much higher than (1.39 ± 0.03) at pH of 5.5, and (0.37 ± 0.02) at pH of 7 (p < 0.05). At 28 d, the PO4 concentration was (5.40 ± 0.34) mmol/L at pH of 4, higher than (0.57 ± 0.03) at pH of 5.5, and (0.16 ± 0.07) at pH of 7 (p < 0.05).
Discussion
The methodology of incorporating a reinforcing filler and a Ca and PO4 ion-releasing filler into the same resin matrix showed promise in the development of load-bearing, caries-inhibiting dental composites. Previous studies used whiskers for reinforcement and yielded a whitish but relatively opaque paste, which may be suitable for load-bearing posterior restorations.30-32 In the present study, a novel TTCP-glass composite was developed using ball-milled fine TTCP particles, esthetic dental glass particles, and a photo-cured resin. The TTCP provided Ca and PO4 ion release from the composite to inhibit caries; the glass fillers provided the needed mechanical reinforcement. The flexural strength of the TTCP composite without glass was about 50-60 MPa, about 1/2 that of the unfilled resin (Figure 1), which was similar to previous CaP composites.26,28 CaP fillers did not reinforce the resin, likely because these fillers were not strong mechanically, and they were not silanized hence did not bond chemically to the resin. A recent study showed that silanization of CaP fillers did not increase the strength of the composite,32 likely because the silane did not bond to CaP fillers. Furthermore, silanization of CaP fillers was not desirable because it reduced the Ca and PO4 ion release.32 One approach to overcoming this drawback was to incorporate glass co-fillers for reinforcement. The present study demonstrated that this dual-filler method increased the flexural strength of the TTCP composite from about 50 MPa to 90 MPa (Figure 2). In addition, the elastic modulus was increased by 2-3 fold.
In comparison with previous studies, the amorphous calcium phosphate (ACP) composite had a flexural strength of (56 ± 16) MPa for specimens without immersion.43 Another CaP composite had strength of 40-60 MPa for specimens without immersion.29 Therefore, the flexural strength of approximately 90 MPa of the new photo-cured TTCP-glass composite was higher than the strengths of previous Ca and PO4 releasing restoratives.
Immersion for a month in solutions at pH of 7, 5.5, and 4 reduced the composite flexural strength, while the variation in solution pH did not have a significant effect on the composite strength. The photo-cured TTCP-glass composite had an average strength (for specimens immersed at all three pH) of 75 MPa, about 25% lower than the strength of specimens before immersion. The strength of the photo-cured TTCP-glass composite was 2-fold higher than the commercial fluoride releasing control (Vitremer). In comparison, a previously-reported CaP material had a flexural strength of 10-20 MPa after 90-d of immersion at neutral pH.29 The strength of the ACP-composite decreased to 40 MPa after 11-d of immersion at neutral pH.26 These strengths were similar to that of Vitremer, hence these CaP materials have the potential to be useful in low-load-bearing restorations. In comparison, the new photo-cured TTCP-glass composite's average strength of 75 MPa after immersion was much higher. This may enable the ion-releasing composite to be used in higher load-bearing restorations. The higher strength was likely because the new TTCP-glass composite relied on dental glass fillers for reinforcement, while previous CaP materials relied solely on the CaP fillers for reinforcement, without a glass co-filler.26-29 Therefore, the photo-cured TTCP-glass composite is expected to have a better long-term durability than previous CaP composites that do not have a stable reinforcement phase. Future study will investigate the long-term water-aging and wear behavior of the new TTCP-glass composite. Further study should also examine the cyclic fatigue properties and fracture toughness using notched specimens for these Ca and PO4 ion-releasing composites.4,15,17,39
As shown in Figure 3, the photo-cured TTCP-glass composite was “smart” and dramatically increased the Ca and PO4 release when the pH was reduced from neutral to a cariogenic pH of 4. It is generally accepted that the local plaque pH of > 6 is the safe area, pH of 6.0 - 5.5 is the potentially cariogenic area, and pH of 5.5 - 4 is the cariogenic area for cavity formation.33,34 It is advantageous that the TTCP-glass composite could substantially increase Ca and PO4 release at lower pH, when these ions are most needed to combat caries. In contrast, the ion release was minimal at neutral pH, so that these ions would not be wasted at neutral pH, and the release capability of the smart composite would not be quickly exhausted. In several previous studies, the release of fluoride and chlorhexidine from restoratives at neutral and acidic pH was measured, which showed more release at lower pH.44-46 Other studies on CaP composites reported Ca and PO4 release at neutral pH only, without reporting release at cariogenic pH.26-32 A recent study from our laboratory reported Ca and PO4 release at different pH, but that composite was relatively opaque and could not be photo-cured. Based on our literature search, the present study is the first report on a photo-cured “smart” composite with Ca and PO4 release, showing a needed, substantial increase in ion release at cariogenic pH.
Comparing Figure 3 with Figure 6 indicates that when the TTCP mass fraction in the composite was increased from 40% to 50%, the Ca and PO4 release was significantly increased. A previous study investigated the effect of CaP filler level on ion release, using another CaP compound.31 It was shown that the ion release increased with increasing filler level at a rate faster than being linear.31 For example, when the CaP filler level was doubled, the ion release was more than doubled. This was because increasing the CaP filler level not only increased the source of ion release, it also increased the interfaces in the resin which served as relatively easier passageways for diffusion of water and ions.31 This is likely why in the present study, increasing the TTCP by a mere 10% substantially increased the ion release.
In previous studies, the ACP-composites released Ca and PO4 ions to yield a Ca concentration of 0.3-1.0 mmol/L, and a PO4 concentrations of 0.2-0.7 mmol/L.26 Another study on remineralizing CaP cement reported a Ca concentration of 0.5 mmol/L, and a PO4 concentration of 0.1 mmol/L, measured in a similar manner.28 It was shown that when Ca and PO4 were released, they reprecipitated to form hydroxyapatite outside the composite and inside the tooth lesions, significantly increasing the mineral content of the lesion, thereby remineralizing the lesion.25,28 The Ca and PO4 concentrations released from the new TTCP-glass composite were relatively high. This suggests that the mechanically-stronger, photo-cured TTCP-glass composite might also effectively remineralize tooth lesions. Further studies should investigate the in situ caries inhibition capability of the smart TTCP-glass composite.
Regarding potential clinical applications, the composite with 40% TTCP and 30% glass could be used in load-bearing restorations, for which the current CaP composites and resin-modified glass ionomer restoratives are mechanically too weak. The composite with 50% TTCP and 20% glass, with a higher Ca and PO4 releasing capability, could be used in treatments where complete removal of caries tissue is contra-indicated, in teeth where carious lesions are beginning to occur, and for patients at high risk for dental caries including those receiving radiation treatments or with dry mouth. Further studies are needed to explore these applications and the efficacy of the photo-cured smart TTCP-glass composite.
Summary
Currently-available posterior composites and hybrid composites can be used as stress-bearing restorations, but they generally do not release Ca, PO4 or F ions. On the other hand, restoratives that do release Ca, PO4 or F ions are relatively weak and cannot be used in large stress-bearing restorations. Therefore, there is a need to develop new composites that are mechanically strong while at the same time have sustained release of Ca, PO4 and F ions to inhibit caries. In the present study, a new calcium phosphate composite was developed using a photo-cured Bis-GMA/TEGDMA resin, esthetic dental glass particles, and ball-milled fine TTCP particles. The flexural strength of the TTCP-glass composite was 2-fold higher than previously-known CaP composites and a resin-modified glass ionomer. The photo-cured TTCP-glass composite was “smart” and increased the Ca and PO4 ion release by an order of magnitude, when the pH was reduced from neutral to a cariogenic pH of 4, when these ions are most needed to inhibit tooth caries. Decreasing the solution pH did not significantly decrease the mechanical properties of the composite. The photo-cured, smart CaP composite, with mechanical properties higher than those of previous Ca, PO4 and fluoride releasing restoratives, may have potential to provide the necessary combination of load-bearing and caries-inhibiting capabilities.
Acknowledgments
We thank Dr. James H. Yen and William F. Guthrie of the Statistical Engineering Division of the National Institute of Standards and Technology (NIST) for help with statistical analysis. We thank Dr. Laurence C. Chow, Dr. Shozo Takagi and Dr. Limin Sun of the Paffenbarger Research Center of the American Dental Association, and Dr. Joseph M. Antonucci of NIST for discussions. We are also grateful to Esstech (Essington, PA) for kindly providing the resin monomers. This study was supported by NIH R01 DE14190 and DE17974 (HX), Maryland Nano-Biotechnology Initiative Award (HX), and the University of Maryland Dental School.
References
- 1.American Dental Association (ADA) The 1999 survey of dental services rendered. Chicago: ADA Survey Center; 2002. [Google Scholar]
- 2.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 3.Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 4.Drummond JL, Bapna MS. Static and cyclic loading of fiber-reinforced dental resin. Dent Mater. 2003;19:226–231. doi: 10.1016/s0109-5641(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 5.Imazato S. Review: Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 6.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. J Dent Res. 2005;84:822–826. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 7.Wan Q, Sheffield J, McCool J, Baran GR. Light curable dental composites designed with colloidal crystal reinforcement. Dent Mater. 2008;24:1694–1701. doi: 10.1016/j.dental.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stansbury JW. Cyclopolymerizable monomers for use in dental resin composites. J Dent Res. 1990;69:844–848. doi: 10.1177/00220345900690030201. [DOI] [PubMed] [Google Scholar]
- 9.Eick JD, Byerley TJ, Chappell RP, Chen GR, Bowles CQ, Chappelow CC. Properties of expanding SOC/epoxy copolymers for dental use in dental composites. Dent Mater. 1993;9:123–127. doi: 10.1016/0109-5641(93)90088-8. [DOI] [PubMed] [Google Scholar]
- 10.Ferracane JL, Mitchem JC. Properties of posterior composites: Results of round robin testing for a specification. Dent Mater. 1994;10:92–99. doi: 10.1016/0109-5641(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 11.Loza-Herrero MA, Rueggeberg FA, Caughman WF, Schuster GS, Lefebvre CA, Gardner FM. Effect of heating delay on conversion and strength of a post-cured resin composite. J Dent Res. 1998;77:426–431. doi: 10.1177/00220345980770021201. [DOI] [PubMed] [Google Scholar]
- 12.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 13.Krämer N, García-Godoy F, Reinelt C, Frankenberger R. Clinical performance of posterior compomer restorations over 4 years. Am J Dent. 2006;19:61–66. [PubMed] [Google Scholar]
- 14.Tyas M. Correlation between fracture properties and clinical performance of composite resins in posterior teeth. Aust Dent J. 1990;35:46–49. doi: 10.1111/j.1834-7819.1990.tb03027.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferracane JL, Berge HX. Fracture toughness of experimental dental composites aged in ethanol. J Dent Res. 1995;74:1418–1423. doi: 10.1177/00220345950740071501. [DOI] [PubMed] [Google Scholar]
- 16.Ruddell DE, Maloney MM, Thompson JY. Effect of novel filler particles on the mechanical and wear properties of dental composites. Dent Mater. 2002;18:72–80. doi: 10.1016/s0109-5641(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 17.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watts DC, Issa M, Ibrahim A, Wakiaga J, Al-Samadani K, Al-Azraqi M, Silikas N. Edge strength of resin-composite margins. Dent Mater. 2008;24:129–133. doi: 10.1016/j.dental.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. International Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 22.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Prim Dent Care. 2002;9:31–36. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 23.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 24.Jokstad A, Bayne S, Blunck U, Tyas M, Wilson N. Quality of dental restorations. FDI Commision Projects 2-95. International Dent J. 2001;51:117–158. doi: 10.1002/j.1875-595x.2001.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 25.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75:1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 26.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 27.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53B:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 29.Dickens SH, Flaim GM, Floyd CJE. Effect of resin composition on mechanical and physical properties of calcium phosphate filled bonding systems. Polymer Preprints. 2004;45:329–330. [Google Scholar]
- 30.Xu HHK, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC. Nano dicalcium phosphate anhydrous-whisker composites with high strength and Ca and PO4 release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu HHK, Weir MD, Sun L, Takagi S, Chow LC. Effect of calcium phosphate nanoparticles on Ca-PO4 composites. J Dent Res. 2007;86:378–383. doi: 10.1177/154405910708600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu HHK, Weir MD, Sun L. Dental nanocomposites with Ca-PO4 release: Effects of reinforcement, dicalcium phosphate particle size and silanization. Dent Mater. 2007;23:1482–1491. doi: 10.1016/j.dental.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hefferren JJ, Koehler HM. Foods, nutrition and dental health. Pathotox Publishers; Park Forest South, IL: 1981. p. 138. [Google Scholar]
- 34.Thylstrup A, Fejerskov O. Textbook of cariology. Copenhagen, Denmark: Munksgaard; 1986. pp. 145–146. [Google Scholar]
- 35.Xu HHK, Weir MD, Sun L. Calcium and phosphate ion releasing composite: Effect of pH on release and mechanical properties. Dent Mater. 2009;25:535–542. doi: 10.1016/j.dental.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow LC. Calcium phosphate cements: Chemistry, properties, and applications. Mater Res Symp Proc. 2000;599:27–37. [Google Scholar]
- 37.Xu HHK, Weir MD, Simon CG. Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dent Mater. 2008;24:1212–1222. doi: 10.1016/j.dental.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu HHK. Long-term water aging of whisker-reinforced polymer-matrix composites. J Dent Res. 2003;82:48–52. doi: 10.1177/154405910308200111. [DOI] [PubMed] [Google Scholar]
- 39.Xu HHK, Quinn JB, Giuseppetti AA. Wear and mechanical properties of nano-silica-fused whisker composites. J Dent Res. 2004;83:930–935. doi: 10.1177/154405910408301208. [DOI] [PubMed] [Google Scholar]
- 40.ISO/FDIS 4049: Dentistry – polymer-based fillings, restorative and luting materials. Third. Geneva, Switzerland: International Organization for Standardization; 2000. [Google Scholar]
- 41.ASTM D 790-03. Standard test methods for flexural properties of unreinforced and reinforced plastic and electrical insulating materials. West Conshohocken, PA: ASTM International; 2004. [Google Scholar]
- 42.Vogel GL, Chow LC, Brown WE. A microanalytical procedure for the determination of calcium, phosphate and fluoride in enamel biopsy samples. Caries Res. 1983;17:23–31. doi: 10.1159/000260645. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell JNR, Antonucci JM, Skrtic D. Mechanical properties of amorphous calcium phosphate composites. J Dent Res. 2005;84 doi: 10.1177/0883911506064476. IADR Abstract No. 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carey CM, Spencer M, Gove RJ, Eichmiller FC. Fluoride release from a resin-modified glass-ionomer cement in a continuous-flow system: effect of pH. J Dent Res. 2003;82:829–832. doi: 10.1177/154405910308201013. [DOI] [PubMed] [Google Scholar]
- 45.Anusavice KJ, Zhang NZ, Shen C. Effect of CaF2 content on rate of fluoride release from filled resins. J Dent Res. 2005;84:440–444. doi: 10.1177/154405910508400508. [DOI] [PubMed] [Google Scholar]
- 46.Anusavice KJ, Zhang NZ, Shen C. Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res. 2006;85:950–954. doi: 10.1177/154405910608501016. [DOI] [PMC free article] [PubMed] [Google Scholar]