Abstract
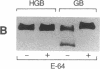
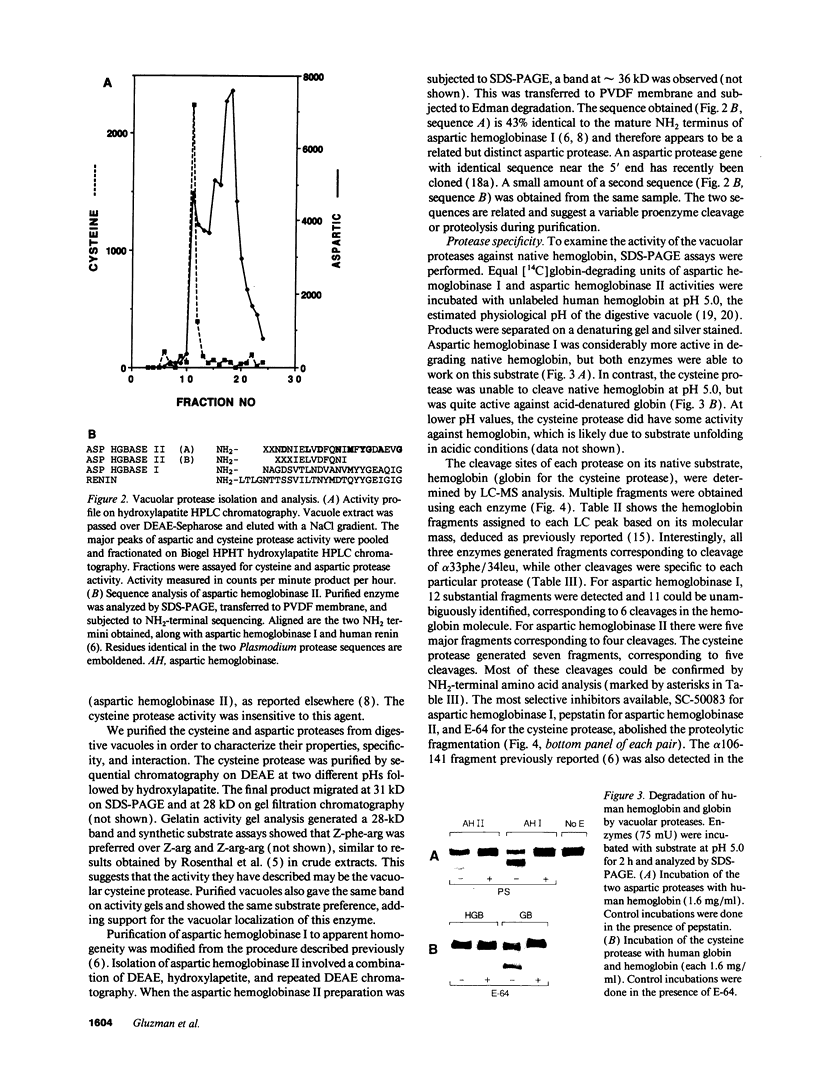
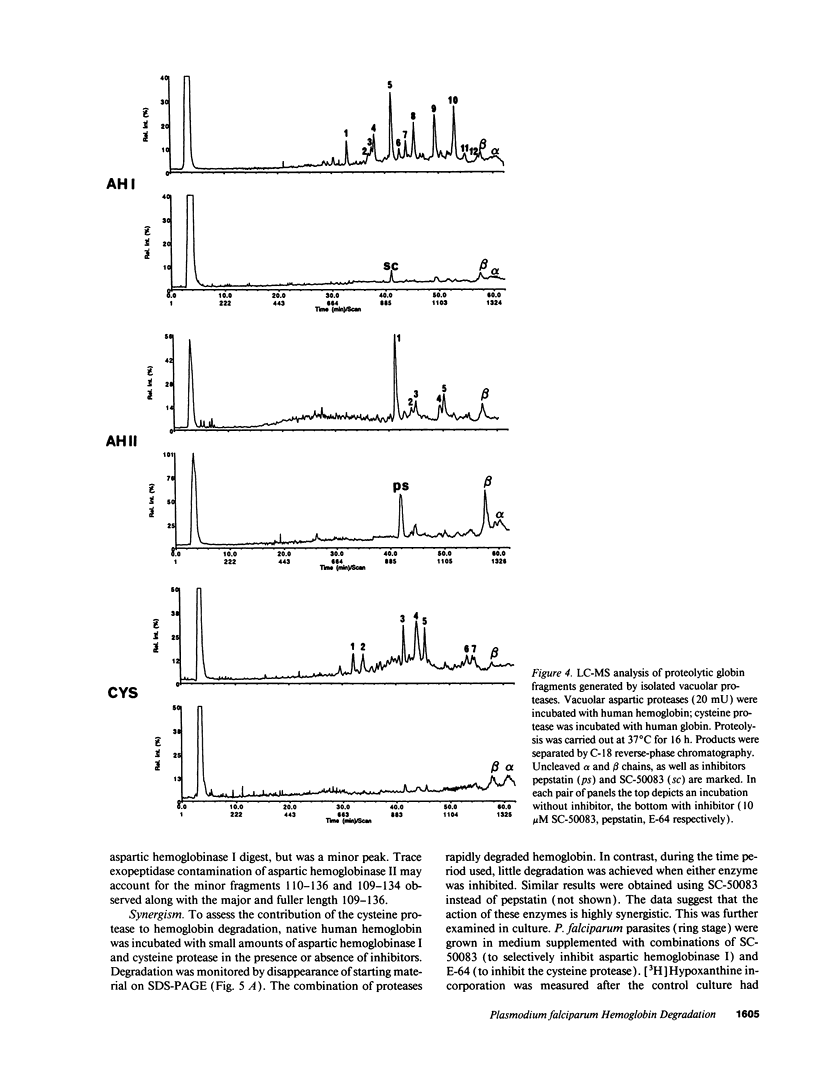
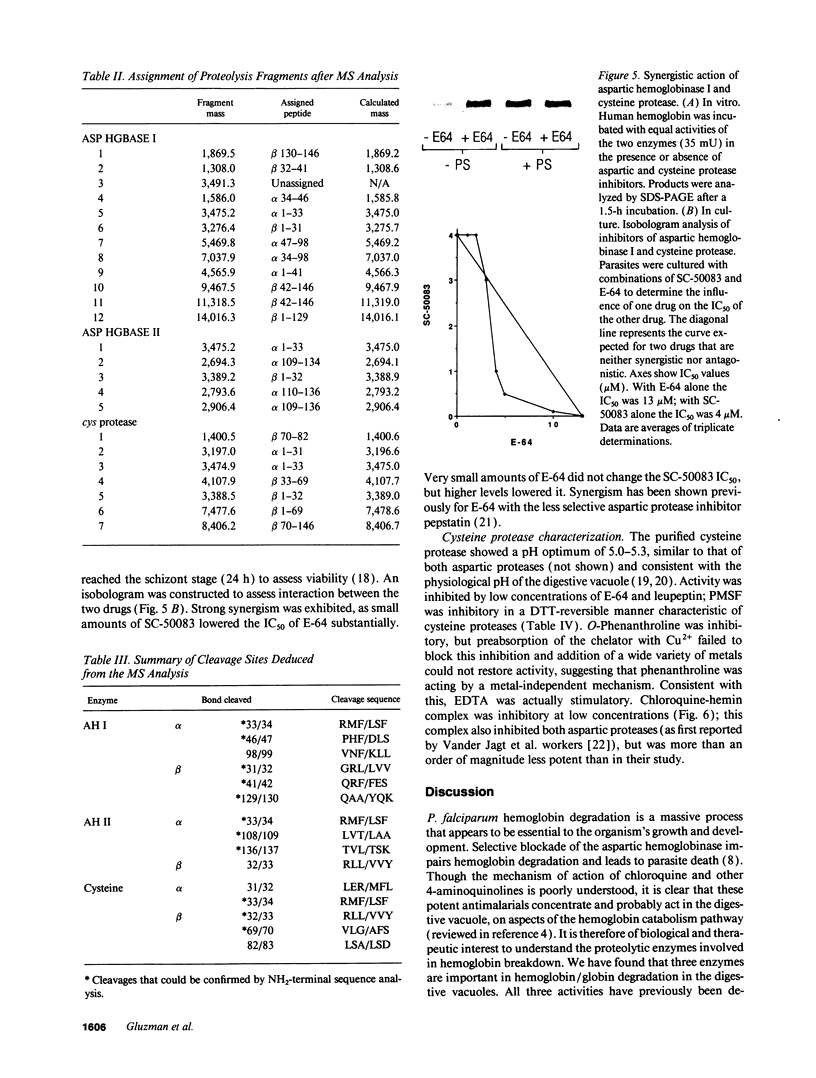
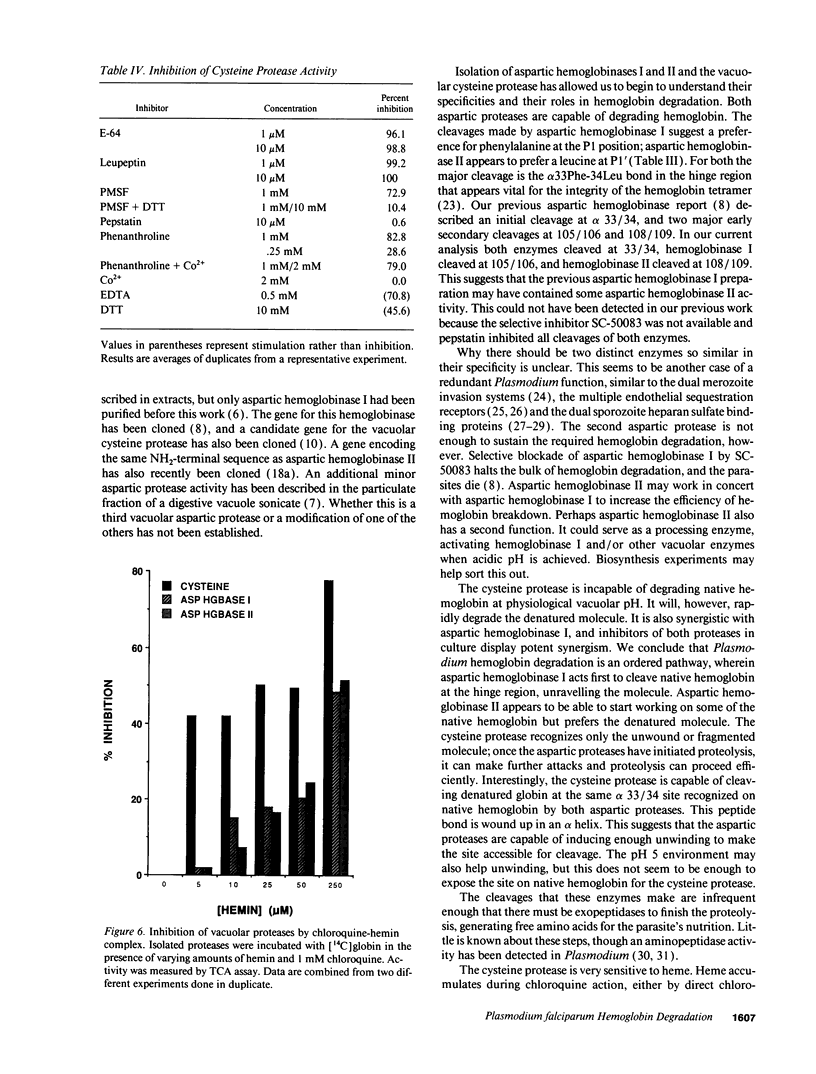
The human malaria parasite, Plasmodium falciparum, degrades nearly all its host cell hemoglobin during a short segment of its intraerythrocytic development. This massive catabolic process occurs in an acidic organelle, the digestive vacuole. Aspartic and cysteine proteases have been implicated in this pathway. We have isolated three vacuolar proteases that account for most of the globin-degrading activity of the digestive vacuole. One is the previously described aspartic hemoglobinase that initiates hemoglobin degradation. A second aspartic protease is capable of cleaving hemoglobin with an overlapping specificity, but seems to prefer acid-denatured globin. The third is a cysteine protease that does not recognize native hemoglobin but readily cleaves denatured globin. It is synergistic with the aspartic hemoglobinase, both by in vitro assay of hemoglobin degradation, and by isobologram analysis of protease inhibitor-treated parasites in culture. The cysteine protease is highly sensitive to chloroquine-heme complex, suggesting a possible mechanism of 4-aminoquinoline antimalarial action. The data suggest an ordered pathway of hemoglobin catabolism that presents an excellent target for chemotherapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Hepler P. K., Huff C. G., Sprinz H. The feeding mechanism of avian malarial parasites. J Cell Biol. 1966 Feb;28(2):355–373. doi: 10.1083/jcb.28.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly E., Jambou R., Savel J., Jaureguiberry G. Plasmodium falciparum: differential sensitivity in vitro to E-64 (cysteine protease inhibitor) and Pepstatin A (aspartyl protease inhibitor). J Protozool. 1992 Sep-Oct;39(5):593–599. doi: 10.1111/j.1550-7408.1992.tb04856.x. [DOI] [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Cerami C., Frevert U., Sinnis P., Takacs B., Clavijo P., Santos M. J., Nussenzweig V. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992 Sep 18;70(6):1021–1033. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Chevli R., Fitch C. D. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980 Apr 15;19(8):1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottavio-Martin D., Ravel J. M. Radiolabeling of proteins by reductive alkylation with [14C]formaldehyde and sodium cyanoborohydride. Anal Biochem. 1978 Jul 1;87(2):562–565. doi: 10.1016/0003-2697(78)90706-6. [DOI] [PubMed] [Google Scholar]
- Francis S. E., Gluzman I. Y., Oksman A., Knickerbocker A., Mueller R., Bryant M. L., Sherman D. R., Russell D. G., Goldberg D. E. Molecular characterization and inhibition of a Plasmodium falciparum aspartic hemoglobinase. EMBO J. 1994 Jan 15;13(2):306–317. doi: 10.1002/j.1460-2075.1994.tb06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U., Sinnis P., Cerami C., Shreffler W., Takacs B., Nussenzweig V. Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. J Exp Med. 1993 May 1;177(5):1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary T. G., Jensen J. B., Ginsburg H. Uptake of [3H]chloroquine by drug-sensitive and -resistant strains of the human malaria parasite Plasmodium falciparum. Biochem Pharmacol. 1986 Nov 1;35(21):3805–3812. doi: 10.1016/0006-2952(86)90668-4. [DOI] [PubMed] [Google Scholar]
- Goldberg D. E. Hemoglobin degradation in Plasmodium-infected red blood cells. Semin Cell Biol. 1993 Oct;4(5):355–361. doi: 10.1006/scel.1993.1042. [DOI] [PubMed] [Google Scholar]
- Goldberg D. E., Slater A. F., Beavis R., Chait B., Cerami A., Henderson G. B. Hemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. J Exp Med. 1991 Apr 1;173(4):961–969. doi: 10.1084/jem.173.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. E., Slater A. F., Cerami A., Henderson G. B. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: an ordered process in a unique organelle. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2931–2935. doi: 10.1073/pnas.87.8.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyang F. N., Poole B., Trager W. Peptidases from Plasmodium falciparum cultured in vitro. Mol Biochem Parasitol. 1982 Apr;5(4):263–273. doi: 10.1016/0166-6851(82)90034-2. [DOI] [PubMed] [Google Scholar]
- Hadley T. J., Klotz F. W., Pasvol G., Haynes J. D., McGinniss M. H., Okubo Y., Miller L. H. Falciparum malaria parasites invade erythrocytes that lack glycophorin A and B (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J Clin Invest. 1987 Oct;80(4):1190–1193. doi: 10.1172/JCI113178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui G. S., Palmer K. L., Siddiqui W. A. Use of human plasma for continuous in vitro cultivation of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1984;78(5):625–626. doi: 10.1016/0035-9203(84)90222-0. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. A perspective on antimalarial action: effects of weak bases on Plasmodium falciparum. Biochem Pharmacol. 1986 Feb 15;35(4):547–552. doi: 10.1016/0006-2952(86)90345-x. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H., Gluzman I. Y. Antimalarials increase vesicle pH in Plasmodium falciparum. J Cell Biol. 1985 Dec;101(6):2302–2309. doi: 10.1083/jcb.101.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Müller H. M., Reckmann I., Hollingdale M. R., Bujard H., Robson K. J., Crisanti A. Thrombospondin related anonymous protein (TRAP) of Plasmodium falciparum binds specifically to sulfated glycoconjugates and to HepG2 hepatoma cells suggesting a role for this molecule in sporozoite invasion of hepatocytes. EMBO J. 1993 Jul;12(7):2881–2889. doi: 10.1002/j.1460-2075.1993.tb05950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tandon N. N., Magowan C., Jamieson G. A., Chulay J. D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989 Mar 17;243(4897):1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- Orjih A. U., Banyal H. S., Chevli R., Fitch C. D. Hemin lyses malaria parasites. Science. 1981 Nov 6;214(4521):667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. J., Lee G. K., Smith R. E. Inhibition of a Plasmodium vinckei cysteine proteinase cures murine malaria. J Clin Invest. 1993 Mar;91(3):1052–1056. doi: 10.1172/JCI116262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P. J., McKerrow J. H., Aikawa M., Nagasawa H., Leech J. H. A malarial cysteine proteinase is necessary for hemoglobin degradation by Plasmodium falciparum. J Clin Invest. 1988 Nov;82(5):1560–1566. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P. J., Nelson R. G. Isolation and characterization of a cysteine proteinase gene of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Mar;51(1):143–152. doi: 10.1016/0166-6851(92)90209-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. J., Wollish W. S., Palmer J. T., Rasnick D. Antimalarial effects of peptide inhibitors of a Plasmodium falciparum cysteine proteinase. J Clin Invest. 1991 Nov;88(5):1467–1472. doi: 10.1172/JCI115456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzinska M. A., Trager W., Bray R. S. Pinocytotic uptake and the digestion of hemoglobin in malaria parasites. J Protozool. 1965 Nov;12(4):563–576. doi: 10.1111/j.1550-7408.1965.tb03256.x. [DOI] [PubMed] [Google Scholar]
- Slater A. F., Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992 Jan 9;355(6356):167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Baack B. R., Hunsaker L. A. Purification and characterization of an aminopeptidase from Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jan;10(1):45–54. doi: 10.1016/0166-6851(84)90017-3. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Campos N. M. Characterization of a hemoglobin-degrading, low molecular weight protease from Plasmodium falciparum. Mol Biochem Parasitol. 1986 Mar;18(3):389–400. doi: 10.1016/0166-6851(86)90095-2. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yayon A., Cabantchik Z. I., Ginsburg H. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 1984 Nov;3(11):2695–2700. doi: 10.1002/j.1460-2075.1984.tb02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vander Jagt D. L., Hunsaker L. A., Campos N. M., Scaletti J. V. Localization and characterization of hemoglobin-degrading aspartic proteinases from the malarial parasite Plasmodium falciparum. Biochim Biophys Acta. 1992 Aug 21;1122(3):256–264. doi: 10.1016/0167-4838(92)90401-x. [DOI] [PubMed] [Google Scholar]