Abstract
Apoptosis is a tightly regulated cell suicide program that plays an essential role in the maintenance of tissue homeostasis by eliminating unnecessary or harmful cells. Defects in this native defense mechanism promote malignant transformation and frequently confer chemoresistance to transformed cells. Indeed, the evasion of apoptosis has been recognized as a hallmark of cancer. Given that multiple mechanisms function at many levels to orchestrate the regulation of apoptosis, a multitude of opportunities for apoptotic dysregulation are present within the intricate signaling network of cell. Several of the molecular mechanisms by which cancer cells are protected from apoptosis have been elucidated. These advances have facilitated the development of novel apoptosis-inducing agents that have demonstrated single-agent activity against various types of cancers cells and/or sensitized resistant cancer cells to conventional cytotoxic therapies. Herein, we will highlight several of the central modes of apoptotic dysregulation found in cancer. We will also discuss several therapeutic strategies that aim to reestablish the apoptotic response, and thereby eradicate cancer cells, including those that demonstrate resistance to traditional therapies.
Keywords: evasion of apoptosis, oncogenic mutations, therapeutic targets
APOPTOSIS AND CANCER
Apoptosis is a tightly controlled cell suicide program that plays a fundamental role in development and tissue homeostasis by eliminating unnecessary and defective cells [Kerr et al., 1972; Raff, 1998]. Importantly, this evolutionary conserved form of programmed cell death eradicates potentially harmful cells, particularly genetically altered cells, and thereby serves to maintain the integrity of the organism [Ameisen, 2002]. The imperative function of appropriate apoptotic signaling in preserving the delicate balance between cell survival and cell death that is required to prevent disease is highlighted by the establishment of the evasion of apoptosis as a prominent hallmark of cancer [Hanahan and Weinberg, 2000].
Tumorigenesis requires defects in the cellular circuitry that promote uncontrolled proliferation, yet cellular proliferation mechanisms act within a complex, coordinated signaling network that serves to check aberrant proliferation by activating signaling pathways that induce cellular senescence or apoptosis [Lowe et al., 2004]. As a means to circumvent this protective apoptotic signaling response, cancer cells often harbor both proliferation-stimulating mutations and defects in the apoptotic circuitry, which act cooperatively to uncouple apoptosis from cellular proliferation programs [Evan and Vousden, 2001; Lowe et al., 2004]. Alternatively, cancer cells can evade apoptosis via the signaling of aberrantly active survival pathways [Kabore et al., 2004; Lowe et al., 2004]. Regardless of the mechanism, the suppression of apoptotic signaling confers an enhanced survival ability to cancer cells, which promotes their characteristic uncontrolled proliferation [Hanahan and Weinberg, 2000; Green and Evan, 2002; Lowe et al., 2004]. Furthermore, the dysregulation of apoptotic signaling is frequently implicated in drug resistance, as many anti-neoplastic agents exert their cytotoxic effects by inducing apoptosis [Lowe et al., 2004; Pommier et al., 2004; Blagosklonny, 2005; Fesik, 2005]. Given that apoptosis is regulated at several levels by multiple signaling pathways that are incorporated into an intricate cellular network, each mode of disrupted apoptotic signaling cannot be detailed in this review. Instead, we provide an overview of the key apoptotic mechanisms and highlight several of the means by which apoptosis is dysregulated in human cancers. Additionally, we briefly discuss the potential of targeting these specific apoptotic defects as novel therapeutic strategies for the treatment of cancer.
GENERAL FEATURES OF APOPTOSIS
Apoptosis is characterized by several morphological features, including blebbing of the plasma membrane, exposure of phosphatidylserine at the external surface of the cell membrane, cell shrinkage, chromatin condensation, and DNA fragmentation [Khosravi-Far and Esposti, 2004]. These distinctive alterations are triggered by the proteolytic activity of a family of cysteinyl aspartate-specific proteases, known as caspases, which dismantle the cell by cleaving critical cellular substrates, such as poly(ADP-ribose) polymerase (PARP). This cellular destruction concludes with the formation of apoptotic bodies that are subsequently eliminated by phagocytosis [Bucur et al., 2001; Khosravi-Far and Esposti, 2004].
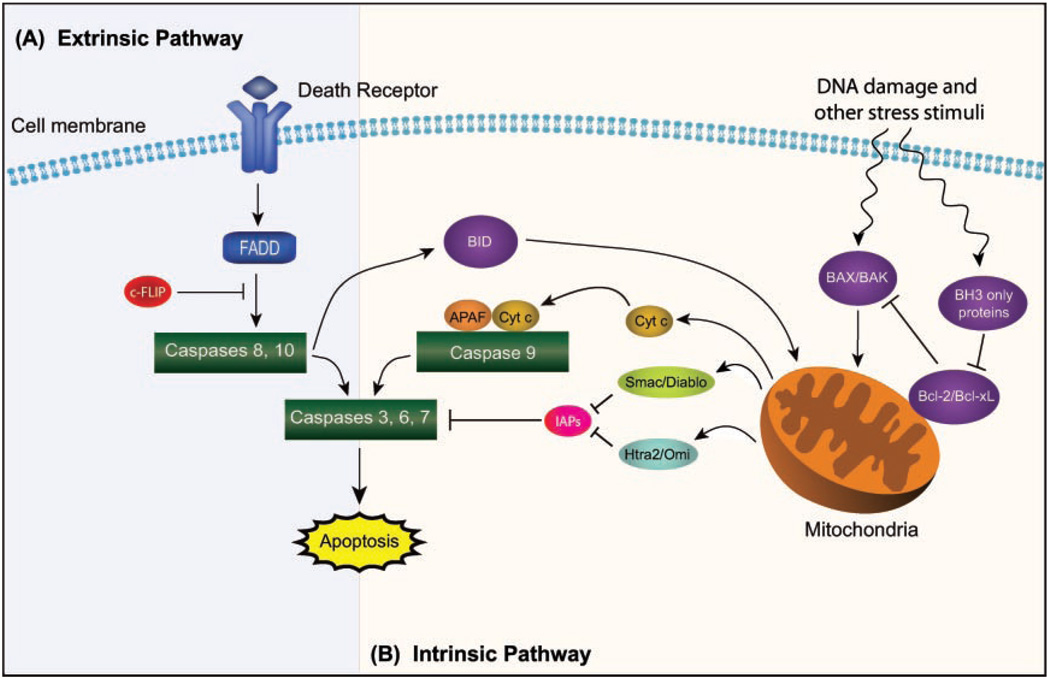
Caspases serve as one of the principal effectors of apoptosis. As such, the activity of these proteases is stringently controlled. One aspect of this regulation involves the synthesis of caspases as inactive zymogens, each of which requires the proteolytic removal of its N-terminal prodomain to generate the mature active caspsase [Nicholson, 1999]. A subset of caspases, termed initiator caspases, interact with specific adapter molecules that facilitate their autoprocessing. Upon activation, initiator caspases process a second class of caspases, known as executioner or effector caspases, which act on key cellular proteins, resulting in the demise of the cell [Nicholson, 1999; Stennicke and Salvesen, 2000]. As depicted in Figure 1, the induction of apoptosis can be mediated by death receptor-dependent or mitochondria-dependent apoptotic pathways, known as the extrinsic and intrinsic apoptotic pathways, respectively, both of which culminate in the activation of the executioner caspases and the consequent destruction of the cell [Jin and El-Deiry, 2005].
Fig. 1.
A schematic of the (A) extrinsic and (B) intrinsic apoptotic signaling pathways.
APOPTOTIC MACHINERY
The Extrinsic (Death Receptor-Dependent) Apoptotic Pathway
The extrinsic apoptotic pathway is activated by cell surface death receptors binding their respective cytokine ligands, such as FasL, tumor necrosis factor (TNF), and TNF-related apoptosis-inducing ligand (Apo2L, TRAIL) [Khosravi-Far and Esposti, 2004]. Death receptors are members of the TNF receptor superfamily and can play a role in mediating several distinct cellular functions, nevertheless most death receptors mainly act to initiate apoptosis, with the apoptosis-inducing ability of TNF receptor-1 (TNFR1), Fas (APO-1, CD95), DR4 (TRAIL receptor 1, TRAIL R1), and DR5 (TRAIL R2) being the most extensively characterized [Ashkenazi and Dixit, 1998; Baud and Karin, 2001; Jin and El-Deiry, 2005]. Ligand binding to the extracellular death receptor domain triggers the oligomerization of the death receptor, leading to the aggregation of a characteristic intracellular motif of death receptor family members, known as the death domain (DD). The complex of aggregated receptor domains recruits adaptor proteins containing DDs, such as FAS-associated death domain (FADD), via DD–DD interactions. These adaptor proteins function to sequester the inactive zymogen of initiator caspase-8 and/or caspase-10, resulting in the formation of the death-inducing signaling complex (DISC) [Jin and El-Deiry, 2005]. DISC formation facilitates a high local concentration of procaspase molecules and thereby promotes the auto-activation of caspase-8 [Boatright et al., 2003]. Activated initiator caspases in turn process and activate the downstream executioner caspases, including caspase-3, −6, and −7, which execute the destruction of the cell [Degterev et al., 2003].
Modification of the death receptor-dependent apoptotic signaling mechanism has been associated with several human cancers. Loss of the death-inducing activity of the Fas-FasL death receptor system [Muschen et al., 2000] and aberrant expression of cytosolic components of the death receptor-mediated apoptotic signaling pathways, including FADD [Tourneur et al., 2005], FLICE-inhibitory protein (c-FLIP) [Jin et al., 2004; Zhang et al., 2004; Kataoka, 2005], and caspases [Zhivotovsky and Orrenius, 2006], can contribute to cellular transformation. Fas-mediated apoptotic signaling has been found to be impaired in various cancer cells. Several defects that contribute to tumor cell resistance to Fas-mediated apoptosis have been observed, including the transcriptional silencing of Fas, a common oncogenic event in epithelial malignancies, and somatic mutations of Fas, which are often associated with germinal center (GC)-derived B-cell lymphomas [Muschen et al., 2002]. The loss of Fas function can promote the persistence of malignant cells by enabling these transformed cells to evade immunosurveillance and elimination mediated by FasL-expressing cytotoxic T cells [Muschen et al., 2002; Abramson and Shipp, 2005].
Deficiencies in downstream effector molecules of the death receptor signaling complexes can also play a role in carcinogenesis. In acute myelogenous leukemia (AML) cells, absent or low expression of FADD was frequently observed and predicted resistance to chemotherapy and a poor prognosis [Tourneur et al., 2004, 2005]. Furthermore, low or absent caspase-8 expression via hypermethylation of caspase-8 regulatory sequences has been reported in a number of tumor types, including neuroblastomas, medulloblastomas, and small cell lung cancer (SCLC) [Teitz et al., 2000; Shivapurkar et al., 2002; Zuzak et al., 2002]. In addition to gene silencing, the suppression of caspase-8 activity can be mediated by overexpression of c-FLIP, an anti-apoptotic protein that is recruited to the DISC and subsequently attenuates the auto-activation of caspase-8. Overexpression of c-FLIP has been reported in a variety of human cancers [Irmler et al., 1997]. Specifically, elevated levels of c-FLIP were observed in most of the colon cancer samples analyzed in a recent study [Korkolopoulou et al., 2007], and the levels c-FLIP were higher in the PC-3 and DU-145 prostate cancer cell lines compared with normal prostate stromal and epithelial cells [Voelkel-Johnson, 2003; Zhang et al., 2004]. Importantly, aberrant expression of c-FLIP has been shown to mediate resistance to cell death induced by stimulation of the TRAIL death receptors in prostate cancer cells [Zhang et al., 2004].
Considering that dysregulation of death receptor-dependent apoptotic signaling can contribute to the pathogenesis of numerous human malignancies and death receptor systems, particularly Fas-FasL, have been reported to play a role in the apoptotic response induced by anti-cancer therapy, restoring the functional activity of the death-receptor-mediated apoptotic program by targeting defects specific to certain cancer cells is a promising therapeutic approach [Fulda and Debatin, 2003]. In addition to its role in tumorigenesis, inhibition of caspase-8 activity by mechanisms involving c-FLIP [Bullani et al., 2001; Krueger et al., 2001; Kataoka, 2005] or transcriptional silencing [Fulda et al., 2001; Fulda and Debatin, 2002] has been associated with drug resistance. Consequently, therapeutic strategies that aim to induce the activation of caspase-8 can sensitize cancer cells to apoptosis-inducing therapies. The downregulation of c-FLIP by metabolic inhibitors has been shown to sensitize a variety of cancer cells to death-receptor-induced apoptosis [Fulda et al., 2000]. Moreover, the reestablishment of caspase-8 expression by interferon-mediated transcriptional activation, demethylation, or gene transfer has been found to sensitize previously resistant caspase-8-deficient tumor cells to death receptor- and drug-induced apoptosis [Fulda et al., 2001; Fulda and Debatin, 2002].
The therapeutic value of inducing apoptosis via the extrinsic pathway extends to cancer cells with defects other than those involving components of this pathway. While some cancer cells with certain oncogenic mutations are resistant to DNA-damaging therapeutic agents, inducing apoptosis by stimulating the extrinsic apoptotic machinery can overcome this resistance since the death receptor-mediated apoptotic pathways can function in a manner independent of the p53-mediated stress response induced by these agents. For example, the TRAIL ligand has been shown to induce apoptosis in a number of cancer cell lines, including those with aberrant p53 activity, but demonstrates little or no apoptotic activity in most normal cells [Almasan and Ashkenazi, 2003]. The preferential killing of cancer cells and apparent lack of systemic toxicity mediated by TRAIL-induced apoptosis has lead to the emergence of agonistic antibodies against the TRAIL death receptors or soluble versions of TRAIL as promising tumor-specific therapeutic agents that warrant further clinical investigation [Fesik, 2005; Bucur et al., 2006] (see Table I).
TABLE I.
Therapies Targeting the Extrinsic Pathway of Apoptosis
| Therapy | Target | Type of cancer (phase) | Company/reference |
|---|---|---|---|
| Agonist mAB | |||
| Mapatumumab (HGS-ETR1) | TRAIL-R1 | Non-Hodgkin’s lymphoma, NSCLC and multiple myeloma (II) |
Human Genome Sciencesa/ [Tolcher et al., 2007] |
| Lexatumumab (HGS-ETR2) | TRAIL-R2 | Various solid malignancies (I) |
Human Genome Sciencea/ [Plummer et al., 2007] |
| CS-1008 (humanized TRA-8) | TRAIL-R2 | Pancreatic cancer (II) | Daiichi Sankyo, Inc.a/ [DeRosier et al., 2007] |
| Soluble TRAIL | |||
| AMG 951 (rhApo2L/TRAIL) | TRAIL-R1 and TRAIL-R2 |
NSCLC (II) | Amgen and Genentecha/ [Daniel et al., 2007] |
| TRAIL-expressing adenovirus | TRAIL-R1 and TRAIL-R2 |
Preclinical | Introgen Therapeutics and VirRx/[Shashkova et al., 2008] |
| Recombinant TNF + chemotherapy | TNF receptors |
APPROVED in Europe in the isolated limb perfusion setting for treatment of irresectable soft tissue carcinomas and melanoma |
[van Horssen et al., 2006] |
NSCLC, non-small cell lug cancer.
The Intrinsic (Mitochondria-Dependent) Apoptotic Pathway
The intrinsic apoptotic pathway is mediated by intrinsic signals that converge at the mitochondria in response to diverse cellular stressors, including UV radiation, gamma irradiation, heat, viral virulence factors, the majority of DNA-damaging agents, and the activation of some oncogenic factors [Kroemer, 2003; Green and Kroemer, 2004; Khosravi-Far and Esposti, 2004; Bouchier-Hayes et al., 2005]. The mitochondria acts as a central regulator of the intrinsic apoptotic pathway, as mitochondrial outer membrane permeabilization (MOMP) is regarded as the critical event in the mitochondria-mediated apoptotic pathway that commits the cell to apoptosis [Bouchier-Hayes et al., 2005]. MOMP prompts the cytosolic release of various proteins that are normally confined to the mitochondrial intermembrane space (IMS). Importantly, cytochrome c leaks into the cytosol and binds to apoptosis protease-activating factor 1 (Apaf-1) in a dATP-dependent manner to form a complex that recruits procaspase-9. The formation of this complex, known as the “apoptosome,” facilitates oligomerization and activation of caspase-9 [Li et al., 1997; Boatright et al., 2003; Jin and El-Deiry, 2005]. Activated caspase-9, in turn, processes and activates the executioner caspases-3, −6, and −7, which drive the execution of the cell [Slee et al., 1999].
Alterations in the expression of components of the intrinsic apoptotic machinery or its key regulators have been associated with various human cancers. Specifically, reduced expression of Apaf-1 has been observed in numerous human melanoma samples and correlates with disease progression [Baldi et al., 2004]. The frequent transcriptional silencing of Apaf-1 in metastatic melanomas is a result of the aberrant methylation of the promoter sequences in the gene [Soengas et al., 2001]. In addition to deficiencies in the components of the apoptosome, modulators of its formation have been implicated in the pathogenesis of cancer [Hajra and Liu, 2004]. As primary regulators of MOMP, the pivotal event in the intrinsic apoptotic pathway that enables apoptosome formation, members of the Bcl-2 family of proteins function as an apoptotic switch [Adams and Cory, 2007]. The Bcl-2 family members, each of which contains at least one of four Bcl-2 homology (BH) domains, termed BH1 to BH4, can be broadly classified into two groups: the anti-apoptotic Bcl-2 members, including Bcl-2, Bcl-xL, Mcl-1, Bcl-w, A1, and Bcl-B, and the pro-apoptotic members of the BH3-only and Bax-like subfamilies [Danial and Korsmeyer, 2004; Roset et al., 2007]. The relative levels of these antagonistic pro- and anti-apoptotic Bcl-2 family members, which counteract the activity of one another via direct interactions, mediate the induction of apoptosis, and the disruption of this protective balancing act can contribute to carcinogenesis.
Both overexpression of anti-apoptotic members and reduced expression of pro-apoptotic members have been linked to the aberrant apoptotic signaling involved in several cellular transformation mechanisms [Adams and Cory, 2007]. Bcl-2 or Bcl-xL promote survival by binding pro-apoptotic Bax-like subfamily members, namely Bax and Bak, and thereby inhibit the induction of MOMP mediated by the homooligomerization of these proteins in the mitochondrial membrane [Hinds and Day, 2005; Jin and El-Deiry, 2005]. Bcl-2 overexpression has been reported in a variety of human malignacies, including diffuse large B-cell lymphoma (DLBCL), AML, glioblastoma, melanoma, malignant pleural mesothelioma (MPM), prostate cancer, and lung cancer [Colombel et al., 1993; Ramsay et al., 1995; Kitagawa et al., 1996; Kaufmann et al., 1998; Deininger et al., 1999; Venditti et al., 2004; Abramson and Shipp, 2005; O’Kane et al., 2006]. The overexpression of Bcl-xL is another common oncogenic event that has been observed in several types of cancer, including colorectal adenocarcinomas, Kaposi’s sarcoma, and multiple myeloma (MM) [Foreman et al., 1996; Krajewska et al., 1996; Tu et al., 1998]. Additionally, in prostate cancer, Bcl-xL overexpression is associated with disease progression and the development of androgen resistance [Castilla et al., 2006]. In contrast, the BH3-only proteins, such as Bim, Bid, Puma, Bad, and Noxa, act as sensors of cellular damage and can promote apoptosis by binding the anti-apoptotic Bcl-2 family members, facilitating the release of the essential mediators of cell death, Bak and Bak, from inactive heterooligomeric complexes. The importance of the pro-apototic activity of Bax and Bak is highlighted by the high propensity of Bax/Bak-double-deficient mouse embryo fibroblasts (MEF) to undergo oncogenic transformation [Zong et al., 2001].
The BH3-only protein Bid can also serve as a link between the extrinsic and intrinsic apoptotic pathways. Upon activation of death receptor systems, the cleavage of Bid by caspase-8 generates the activated C-terminal Bid fragment, known as tBid, which translocates to the mitochondria and subsequently induces the release of cytochrome c [Khosravi-Far and Esposti, 2004]. The engagement of the mitochondria-dependent apoptotic pathway via the caspase-8-mediated cleavage of Bid is necessary to elicit a complete apoptotic response in response to the Fas/FasL system-initiated death signal in some types of cells [Wang, 2001]. Thus, the overexpression of anti-apoptotic Bcl-2 proteins can inhibit Fas-mediated apoptosis in several cell types, as demonstrated by the inhibition of anti-Fas-induced apoptosis in MCF7 breast cancer cells with elevated levels of Bcl-xL despite the activation of casapase-8 in these cells [Srinivasan et al., 1998]. Accordingly, Bcl-xL-overexpressing cells that rely on the induction of the mitochondria-dependent apoptotic pathway to amplify the extrinsic apoptotic response are resistant to certain drugs that activate Fas-mediated apoptosis, and the role of this dependence in mediating a cell type specific response to cytotoxic drugs can have important therapeutic implications [Fulda et al., 2001].
In addition to the upstream regulation of MOMP induction by the Bcl-2 proteins, the intrinsic apoptotic pathway is regulated downstream of apoptosome formation by several mediators of caspase activation [Wang, 2001; Hajra and Liu, 2004]. The inhibitor of apoptosis proteins (IAPs) are a family of caspase inhibitors that directly bind caspases-3, −7 and/or −9 and thereby impair the activity of these critical effectors of apoptosis [Schimmer, 2004]. Elevated levels of IAPs have been found in numerous types of malignant cells, and the overexpression of these anti-apoptotic proteins is associated with chemoresistance and serves as a poor prognosis marker in several types of cancer [Schimmer, 2004; Zhivotovsky and Orrenius, 2006]. The differential expression of survivin, an IAP that inhibits caspases-3 and −7, in malignant cells and normal adult cells has been demonstrated by the detection of survivin expression in each tumor cell line of the NCI 60 cell line panel, but not in untransformed cells, [Tamm et al., 1998] and by the prominent expression of survivin in lung, colon, pancreas, prostate, and breast cancer cells, in vivo, with undetectable expression in the corresponding non-neoplastic cell types [Ambrosini et al., 1997]. Frequent overexpression of another IAP, XIAP, has also been observed in the NCI 60 tumor cell line panel [Fong et al., 2000], and in AML patients, a high level of XIAP has been associated with poor prognosis [Tamm et al., 2000]. While survivin partially inhibited Bax or Fas-induced apoptosis in cotransfection experiments using 293 cells, cell death was almost entirely blocked by XIAP under the same conditions [Tamm et al., 1998]. The potent anti-apoptotic activity of XIAP is, in part, attributable to its ability to suppress both the death receptor- and mitochondria-dependent apoptotic pathways, as XIAP acts to inhibit caspases-3, −7, and −9 [Deveraux et al., 1998].
Given that aberrant levels of key mediators of the mitochondria-dependent apoptotic pathway have been implicated in tumorigenesis and chemoresistance, these regulatory proteins can serve as specific targets for apoptosis-inducing cancer therapeutics (see Table II). Efforts that implement this approach have involved designing inhibitors of the anti-apoptotic proteins that are frequently overexpressed in tumor cells, particularly Bcl-2, Bcl-xL, and IAPs [Fulda and Debatin, 2004; Fesik, 2005]. The association of the overexpression of anti-apoptotic Bcl-2 family members, especially Bcl-2 and Bcl-xL, with resistance to various anti-cancer therapies, as demonstrated by the strong negative correlation of Bcl-xL expression levels with sensitivity of cancer cells to a panel of 122 standard chemotherapy agents [Amundson et al., 2000], makes these proteins attractive therapeutic targets [Fesik, 2005]. A promising therapeutic approach to block the action of these anti-apoptotic proteins involves the development of both peptide mimetics and small molecule inhibitors that mimic the BH3 domain of the BH3-only subfamily members and thereby bind and neutralize anti-apoptotic Bcl-2 family members [Fesik, 2005; Zhang et al., 2007]. For instance, a potent small molecule inhibitor of Bcl-2, Bcl-xL, and Bcl-w, termed ABT-737, can enhance the cytotoxicity of chemotherapeutic agents and displays single-agent activity against some cancer cells, including SCLC cells, causing complete regression of SCLC tumor xenografts in mice [Oltersdorf et al., 2005].
TABLE II.
Therapies Targeting the Intrinsic Pathway of Apoptosis
| Therapy | Target | Type of cancer (phase) | Company/reference |
|---|---|---|---|
| Small molecule inhibitors of Bcl-2/Bcl-xL | |||
| Obatoclax (GX15-070) | Pan-Bcl-2 inhibitor | Hematological malignancies, NSCLC, and SCLC (I–II) |
Gemin Xa/[Perez-Galan et al., 2007] |
| ABT-263 | Bcl-2, Bcl-xL, and Bcl-w |
Lymphomas, CLL, and SCLC (I) | Abbott and Genentecha/[Tuma, 2007] |
| BH3Is | Bcl-xL | Preclinical | [Degterev et al., 2001; Ray et al., 2005] |
| ABT-737 | Bcl-2, Bcl-xL, and Bcl-w |
Preclinical | Abbott Laboratories/[Oltersdorf et al., 2005] |
| Bcl-2 ASOs | |||
| Genasense (G3139) | Bcl-2 | CLL, CML, melanoma, breast cancer, and colorectal cancer (I–III) |
Genta Incorporateda/[Anon, 2007b] |
| SPC2996 | Bcl-2 | CLL (I/II) | Santaris Pharmaa/[Tilly et al., 2007] |
| Peptide-based inhibitors of Bcl-2/Bcl-xL | |||
| BH3 domain of Bak fused to a PTD |
Bcl-xL | Preclinical | [Holinger et al., 1999; Brewis et al., 2003] |
| SAHBs | Bcl-xL | Preclinical | [Walensky et al., 2004] |
| MKT-077 (lipophilic cation) | Mitochondria | Solid tumors (II—discontinued trial due to toxicity) |
[Bouchier-Hayes et al., 2005; Deocaris et al., 2007] |
| IAP inhibitors | |||
| Gene delivery of SMAC (natural IAP inhibitor) |
XIAP | Preclinical | [McNeish et al., 2003] |
| SMAC mimetics | XIAP | Preclinical | [Sun et al., 2004] |
| Capped tripeptides with unnatural amino acids |
XIAP | Preclinical | [Oost et al., 2004] |
| Non-peptidic mimetic of SMAC |
XIAP, cIAP-1 and cIAP-2 |
Preclinical | [Li et al., 2004] |
| IAP ASOs | |||
| AEG35156 (XIAP ASO) | XIAP | Pancreatic cancer, breast cancer, NSCLC, and AML (I/II) |
Aegera Therapeuticsa/[LaCasse et al., 2006] |
| LY2181308 (survivin ASO) | Survivin | Hepatocellular carcinoma (I/II) | Lilly and ISIS Pharmaceuticalsa |
NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; CLL, chronic lymphocytic leukemia; ASO, anti-sense oligonucleotide; CML, chronic myelogenous leukemia; PTD, protein transduction domain; SAHBs, stabilized alpha-helix of BCL-2 domains; IAP, inhibitor of apoptosis protein; SMAC, second mitochondrial-derived activator of caspase; AML, acute myelogenous leukemia.
Similar to anti-apoptotic Bcl-2 proteins, elevated expression in most human malignancies and a role in the resistance of cancer cells to various pro-apoptotic stimuli, including chemotherapeutic agents, make IAPs promising molecular targets for the development of cancer therapeutics [Vucic and Fairbrother, 2007]. Several IAP-targeted therapies have been developed, including anti-sense oligonucleotides against XIAP and survivin and small molecule inhibitors of XIAP, and some have entered into clinical trials [Amantana et al., 2004; Schimmer, 2004; Schimmer and Dalili, 2005; Vucic and Fairbrother, 2007]. XIAP inhibitors have been shown to suppress tumor growth in xenograft mouse models and sensitize cancer cells to chemotherapeutic and radiation treatments, highlighting XIAP as a significant factor in the resistance of several types of cancer cells to apoptosis-inducing agents [Schimmer, 2004; Schimmer et al., 2004; Schimmer and Dalili, 2005]. Specifically, downregulation of XIAP by the adenoviral vector-mediated delivery of an anti-sense agent has been found to induce apoptosis in chemoresistant ovarian cancer cells [Sasaki et al., 2000] and sensitize lung cancer cells to radiation therapy [Holcik et al., 2000]. Furthermore, the inhibition of XIAP expression, using an anti-sense XIAP phosphorodiamidate morpholino oligomer (PMO), has been shown to induce apoptosis and increase caspase-3 activity as well as enhance the apoptotic effects of cisplatin and TRAIL in human androgen-insensitive DU145 prostate cancer cells [Amantana et al., 2004].
REGULATORY MECHANISMS OF THE APOPTOTIC PATHWAYS
Cellular Stress-Induced Apoptosis
The regulation of apoptosis at several levels is essential to maintain the fundamental equilibrium between cell survival and cell death that is characteristic of healthy tissues. Disruption of this balance by alterations in the expression or function of proteins that serve as mediators of survival or apoptotic signaling pathways can lead to enhanced cellular survival, thus promoting the development and progression of cancer [Kabore et al., 2004]. Consequently, the cell has several defense mechanisms that are activated upon the introduction of cellular stressors as a means to safeguard this balance. In particular, the tumor suppressor p53 plays a key role in the prevention of aberrant cellular proliferation and the preservation of genomic integrity by inducing either DNA repair or apoptosis in response to several stress stimuli, including DNA damage and oncogene overexpression [Vogelstein et al., 2000; Fuster et al., 2007]. The activation of p53 is mediated by several post-translational modification processes, including phosphorylation, acetylation, and ubiquitination [Fuster et al., 2007]. The oncoprotein murine double minute 2 (MDM2; known as HDM2 in humans) is a critical negative regulator of p53 that acts to block the binding of the transcription machinery to p53 and to promote the proteasomal degradation of p53 through its p53-specific E3 ubiquitin ligase activity [Garcia-Echeverria et al., 2000; Fuster et al., 2007].
Apoptosis induction by p53 is a critical aspect of its tumor suppressor function. p53 can induce apoptosis by binding to DNA in a sequence specific fashion to activate the transcription of its pro-apoptotic gene targets [Yu and Zhang, 2005]. Specifically, p53 can induce the expression of several pro-apoptotic Bcl-2 proteins, including Bax and the BH3-only subfamily members Puma, Noxa, and Bid, and thereby promote the activation of the mitochondria-dependent apoptotic pathway [Schuler and Green, 2005; Yu and Zhang, 2005]. While p53-mediated apoptosis is primarily associated with the intrinsic apoptotic pathway, the transcriptional activity of p53 can also promote apoptosis by activating components of the extrinsic apoptotic pathway [Fridman and Lowe, 2003]. For example, p53 has been shown to regulate the expression of death receptor-encoding genes, including DR4, DR5, and Fas [Schuler and Green, 2005; Yu and Zhang, 2005]. In addition to the undeniable importance of the transcriptional regulation function of p53 in apoptosis induction, accumulating evidence supports transcriptional-independent mechanisms of p53-mediated apoptosis, whereby p53 is linked to the intrinsic apoptotic pathway through direct interactions with Bcl-2 family proteins [Schuler and Green, 2005; Yee and Vousden, 2005]. Upon initiation of p53-dependent apoptosis, but not during p53-independent apoptosis, a fraction of p53 has been found to translocate to the mitrochondria [Marchenko et al., 2000; Sansome et al., 2001]. Mitochondrial p53 has been reported to interact with both anti-apoptotic Bcl-2 family members and the pro-apoptotic Bcl-2 protein Bak, which disrupts inactive heterodimeric complexes of Bax and the anti-apoptotic BAK–MCL-1 complex, respectively, to promote the homooligomerization of Bax-like proteins, thereby triggering MOMP and apoptosis [Fuster et al., 2007].
In view of the central role of p53 in mediating several anti-proliferative processes to prevent aberrant cell proliferation, thereby earning the title “guardian of the genome,” loss of functional p53 facilitates cellular transformation by promoting the inappropriate survival of cells and the persistence and evolution of genetic defects [Fridman and Lowe, 2003]. In addition to the remarkably high incidence of p53 inactivation in human tumors, with most cancers exhibiting mutations or aberrant regulation of p53 [Hainaut et al., 1998; Momand et al., 1998], evidence of the critical function of p53 as a tumor suppressor has been provided by the generation of genetically altered mice lacking p53, as these p53 knockout mice rapidly develop tumors at a high frequency [Attardi and Jacks, 1999]. The enhanced tumor development observed in several p53-deficient mice models has been associated with defective apoptosis, underscoring the significant role of the apoptosis-inducing activity of p53 in tumor suppression [Vousden and Lu, 2002; Fridman and Lowe, 2003].
Inactivating p53 gene mutations have been found in an extensive number of cancers, with a high prevalence in malignancies of the lung, colon, stomach, and esophagus [Soussi, 2000]. As these mutations are most commonly point missense mutations within the region encoding the conserved DNA binding domain (exons 5–8), mutation of the p53 gene generally results in the generation of p53 mutants that lack the transactivation function of wild-type p53 (wt-p53) [Soussi, 2000]. Alterations in the key regulators of p53 stability and function have also been identified as a mechanism for p53 inactivation in human cancers [Fuster et al., 2007]. In particular, upregulation of MDM-2 [Momand et al., 1998], the main inhibitor of p53, or downregulation of p14ARF [Sato et al., 2002], a direct inhibitor of MDM-2, is fairly common in certain cancers. For example, hypermethylation of the p14ARF gene, resulting in the suppression of p14ARF expression, has been reported in sporadic [Esteller et al., 2000] and ulcerative colitis-associated colorectal carcinomas [Sato et al., 2002].
In recognition of the fact that p53 inactivation is a feature of the majority of human cancers, great efforts have been made toward developing therapeutic agents that can restore wt-p53 transcriptional activity [Fuster et al., 2007] (see Table III). The importance of this therapeutic approach is underscored by the association of loss of p53 function with increased cancer aggressiveness and resistance to anti-cancer therapies [Bossi and Sacchi, 2007]. Several strategies that aim to reinstate wt-p53 function, particularly gene transfer of wt-p53, inhibition of the MDM2-p53 interaction, and chemical restoration of wt-p53 activity, have been pursued. Despite the need for further improvements that reduce toxicity or increase anti-tumor efficiency, some p53-activating agents have shown encouraging results, supporting the continued investigation of wt-p53 reactivation as a means to develop a potent tumor-specific therapy [Bossi and Sacchi, 2007; Selivanova and Wiman, 2007].
TABLE III.
Therapies Targeting Regulatory Mechanisms of Apoptosis
| Therapy | Target | Type of cancer (phase) | Company/reference |
|---|---|---|---|
| Restoration of the p53 pathway | |||
| INGN 201 (Ad5CMV-p53) | Adenovirus-mediated delivery of wt-p53 |
NSCLC (I), breast cancer (II), and head and neck cancer (III) |
Introgen Therapeuticsa/[Anon, 2007a; Tolcher et al., 2006] |
| MDM2 inhibitors | |||
| MI-63 and MI-147 | Disruption of the MDM2-p53 interaction |
Preclinical | Ascenta/http://www.ascenta.com |
| Nutlin-3a | Disruption of the MDM2-p53 interaction |
Preclinical | [Kojima et al., 2006; Drakos et al., 2007] |
| p53 reactivaton | |||
| RITA | wt-p53 | Preclinical | [Issaeva et al., 2004; Krajewski et al., 2005] |
| CP-31398 | wt-p53 and mutant p53 | Preclinical | [Wischhusen et al., 2003] |
| PRIMA-1 | Various p53 mutants | Preclinical | [Bykov et al., 2002; Rehman et al., 2005] |
| Inhibition of the PI3K–Akt | |||
| PI3K inhibitors | |||
| XL 147 | PI3K | Solid tumors (I) | Exelixisa |
| XL 765 | PI3K and mTOR | Solid tumors (I) | Exelixisa |
| PX-866 | PI3K | Preclinical | [Howes et al., 2007] |
| Akt inhibitors | |||
| GSK690693 | Akt | Lymphomas and solid tumors (I) |
Glaxo Smith Klinea |
| TCN-PM (VD-0002) | Akt | Metastatic cancers with activated Akt (I) |
VioQuest Pharmaceuticalsa/ [Ravandi et al., 2007] |
| Perifosine (KRX-0401) | Akt | Leukemias (II) and solid cancers, including NSCLC, gliomas, GIST, and renal cancer (I-II) |
AOI Pharmaceuticals, NCIa/ [Elrod et al., 2007] |
| KP372-1 | Akt | Preclinical | [Mandal et al., 2005] |
| A-443654 | Akt | Preclinical | [Luo et al., 2005] |
| mTOR inhibitors | |||
| Rapamycin (Sirolimus) | mTOR |
FDA APPROVED as an immunosuppressant |
Wyeth, NCIa [Seeliger et al., 2007] |
| Clinical—many cancers (mainly I or II) |
|||
| CCI-779 (Temsirolimus, Torisel) |
mTOR |
FDA APPROVED for advanced renal cell carcinoma |
Wyetha/[Rini et al., 2007] |
| Clinical—gynecologic malignancies (I), multiple myeloma (I/II), breast cancer (II), and MCL (III) |
|||
| RAD001 (Everolimus) | mTOR | Many cancers, including kidney cancer (I–II), breast cancer (I–II), and mCRC (II–III) |
Novaritisa/[Lane and Lebwohl, 2006] |
| AP23573 (Deforolimus) | mTOR | Hematological malignancies (II), sarcomas (II–III), and other malignancies (I) |
Ariad Pharmaceuticalsa/ [Wan and Helman, 2007] |
| Inhibition of the Ras-Raf-MEK-ERK pathway | |||
| FTIs | |||
| SCH66336 (Lonafarnib) | Ras and other targets | Many cancers (II-III) | Schering-Plougha/[Morgillo and Lee, 2006] |
| Tipifarnib (R115777) | Ras and other targets | Leukemias and solid tumors (I), including breast cancer (II) |
NCIa/[Armand et al., 2007] |
| ISIS 2503 (H-Ras ASO) | H-Ras | Pancreatic cancer and colorectal cancer (II) |
NCIa/[Adjei et al., 2003] |
| Raf inhibitors | |||
| Sorafenib (BAY 43–9006, Nexavar) |
B-Raf, Raf-1, VEGFR-2, VEGFR-3, PDGFR and KIT |
FDA APPROVED for advanced renal cancer and unresectable hepatocelluar carcinoma |
Bayera/http://www.nexavar.com [Hahn and Stadler, 2006; Gridelli et al., 2007] |
| Clinical—many cancers, including breast cancer, melanoma, and NSCLC (II–III) |
|||
| XL281 | B-Raf, Raf-1, and mutant B-Raf(V600E) |
Solid tumors (I) | Elexisa |
| PLX4032 | Mutant B-Raf(V600E) | Melanoma with oncogenic B-Raf(V600E) (I) |
Plexxikona |
| LErafAON (Raf-1 ASO) | Raf-1 | Advanced cancers (I) | Neopharma/[Dritschilo et al., 2006] |
| MEK inhibitors | |||
| PD325901 | MEK | Colon cancer, breast cancer, and melanoma (I) |
Pfizera/[LoRusso et al., 2005] |
| AZD6244 (ARRY-142886) | MEK | NSCLC, melanoma, hepato- cellular carcinoma, mCRC, and pancreatic cancer (II) |
AstraZenecaa/[Yeh et al., 2007] |
| XL518 | MEK | Solid tumors (I) | Exelixisa |
| Inhibition of RTKs | |||
| Monoclonal antibodies | |||
| Cetuximab (Erbitux) | EGFR |
FDA APPROVED for mCRC and advanced head and neck cancer |
Bristol-Myers Squibb, ImClone Systemsa/[Blick and Scott, 2007], |
| Clinical—various solid tumors (II–III) |
|||
| Panitumumab (Vectibix, ABX-EGF) |
EGFR |
FDA APPROVED for mCRC |
Amgena/[Messersmith and Hidalgo, 2007] |
| Clinical—NSCLC (II) and head and neck cancer (III) |
|||
| Matuzumab (EMD 72000) |
EGFR | NSCLC and gastric cancer (II) | EMD Pharmaceuticalsa/ [Yoshida et al., 2008] |
| Trastuzumab | HER2 |
FDA APPROVED for metastatic breast cancers with HER2 overexpression |
Genentecha/[Hudis, 2007] |
| Clinical—breast cancer (I–III) | |||
| Tyrosine kinase inhibitors | |||
| Gefitinib (Iressa, ZD1839) |
EGFR |
RESTRICTED FDA APPROVAL for NSCLC patients that have already received and benefited from this therapy |
AstraZenecaa/[Blackhall et al., 2006] |
| Clinical—various solid tumors (II–III) |
|||
| Erlotinib (Tarceva, OSI-774) |
EGFR |
FDA APPROVED for locally advanced or metastatic NSCLC and unresectable, locally advanced, or metastatic pancreatic cancer (in combination with gemcitabine) |
Genentech, OSI Pharmaceuticals, Rochea/[Moore et al., 2007] |
| Clinical—various solid tumors (II–III) |
|||
| EKB-569 (irreversible inhibitor) |
EGFR | Colorectal cancer (II) and NSCLC (II) |
Wyetha/[Erlichman et al., 2006; Yoshimura et al., 2006] |
| HKI-272 (irreversible inhibitor) |
EGFR and HER2 | Preclinical studies indicated that irreversible inhibitors may be effective in patients with EGFR mutations that confer gefitinib or erlotinib resistance |
Wyetha/[Rabindran et al., 2004; Kwak et al., 2005] |
| Clinical—breast cancer (I–II) and NSCLC (II) |
|||
| AEE788 | EGFR, HER2, and VEGFR2 |
Glioblastoma (I/II) | Novartis, NCIa/[Traxler et al., 2004; Goudar et al., 2005] |
| Inhibition of BCR-ABL | |||
| Imatinib Mesylate (IM, Gleevec, Glivec, STI571) |
BCR-ABL (ABL kinases), PDGFR, and KIT |
FDA APPROVED for CML and BCR-ABL-positive ALL |
Novartisa/[Cohen et al., 2005; Deininger, 2007] |
| Clinical—many cancers, including GIST (II–III) |
|||
| Dasatinib (Sprycel, BMS354825) |
BCR-ABL, IM-resistant BCR-ABL mutants (except T315I), PDGFR, KIT, and SRC kinases |
FDA APPROVED for CML and BCR-ABL-positive ALL in adult patients with resistance or intolerance to prior therapy, including IM |
Bristol-Myers Squibba/[Olivieri and Manzione, 2007] |
| Clinical—solid tumors (I) | |||
| Nilotinib (Tasigna, AMN107) |
BCR-ABL, IM-resistant BCR-ABL mutants (except T315I), PDGFR, and KIT |
FDA APPROVED for CML in adult patients resistant/ intolerant to prior therapy, including IM |
Novartisa/[Kujawski and Talpaz, 2007] |
| Clinical—GIST patients with resistance to both IM and sunitinib (III) |
|||
| SKI-606 | BCR-ABL and SRC kinases |
CML and BCR-ABL-positive ALL (I/II) and breast cancer (II) |
Wyetha/[Konig et al., 2008] |
| MK-0457 (aurora kinase inhibitor; VX-680) |
BCR-ABL and IM-resistant BCR-ABL mutants, including BCR- ABL(T3151) |
CML and BCR-ABL-positive ALL with T315I mutation (I/II) |
Mercka/[Giles et al., 2007] |
NSCLC, non-small cell lung cancer; TCN-PM, Triciribine Phosphate Monohydrate (TCN-PM); GIST, gastrointestinal stromal tumors; NCI, National Cancer Institute; MCL, mantle cell lymphoma; mCRC, metastatic colorectal cancer; FTIs, farnesyl transferase inhibitors; ASO, anti-sense oligonucleotide; VEGFR, vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor; RTKs, receptor tyrosine kinases; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor type 2; CML, chronic myelogenous leukemia; ALL, acute lymphocytic leukemia.
In addition to p53, the Jun N-terminal kinases (JNKs) are activated in response to some cellular stressors and can act to mediate apoptosis [Herr and Debatin, 2001]. The JNKs are considered key modulators of diverse cellular processes, including cell proliferation, cell survival, DNA repair, and cell death [Karin and Gallagher, 2005]. While the JNK pathway has been implicated in mediating both pro-and anti-apoptotic effects that are dependent upon cellular context, JNK signaling can target several apoptotic molecules, using both transcription-dependent and transcription-independent mechanisms, to promote apoptosis [Herr and Debatin, 2001]. JNK activation results in the phosphorylation and subsequent activation of certain members of the AP-1 family of transcription factors, including c-Jun and ATF-2, thereby promoting apoptosis via triggering the expression of the pro-apoptotic c-Jun target genes, such as the death ligand-encoding genes FasL and TNF [Herr and Debatin, 2001]. JNK signaling may also play a role in the mitochondria-dependent apoptotic pathway by phosphorylating Bcl-2 and Bcl-xL, which could serve to inactivate these key anti-apoptotic proteins [Herr and Debatin, 2001].
Modification of the JNK pathway has been implicated in the pathogenesis of certain cancers. The aberrant expression of Bcl-w in gastric cancer cells has been reported to exert its pro-survival effect by suppressing the activation of JNK signaling [Lee et al., 2003]. In addition, reduced levels of the JNK effector ATF-2 have been associated with the development of mammary tumors, as Atf-2−/− mouse embryonic fibroblasts (MEFs) have been shown to exhibit reduced levels of apoptosis in response to some stresses and Atf-2+/− mice have been shown to have a high propensity for mammary tumor development [Maekawa et al., 2007, 2008]. Still, exploiting the JNK pathway as a therapeutic target for the development of apoptosis-inducing anti-cancer agents requires consideration of both its pro- and anti-apoptotic effects. While the apoptotic response of cancer cells to certain stress-inducing agents can depend on JNK activation [Singh et al., 2007; Xia et al., 2007], inhibition of JNK signaling is also being explored as a therapeutic strategy in cancer since JNK inhibitors can impair the DNA repair response in cancer cells and thereby enhance the cytotoxic effects of DNA-damaging agents [Potapova et al., 2001; Karin and Gallagher, 2005].
Survival Factor-Mediated Inhibition of Apoptosis
In addition to the induction of stress-induced apoptotic signaling, control of the promotion of cellular proliferation by survival signaling pathways is crucial for maintaining the homeostasis of healthy tissues. Because survival mechanisms, including the phosphatidylinositol 3-kinase (PI3K)-Akt, NF-κB, and Ras-Raf-MEK-ERK pathways, are dynamically linked to the apoptotic machinery in a complex cellular signaling network, activation of survival signaling can serve to block apoptotic signaling [Kabore et al., 2004]. This integration of cell survival and cell death programs is often exploited by cancer cells, using constitutive activation of survival signaling as a means of protection against the apoptotic response that would normally be elicited by various cues, including the removal of growth factors and/or genomic defects [Kabore et al., 2004].
The PI3K-Akt pathway, a downstream mediator of several cell surface receptors, particularly growth factor-stimulated receptor tyrosine kinases (RTKs), can use multiple mechanisms to exert an anti-apoptotic effect, as the activity of several key regulators of apoptosis are mediated by Akt phosphorylation [Vivanco and Sawyers, 2002]. One such class of Akt targets is the forkhead box O (FoxO) family of transcription factors, which can play an important role in controlling cellular death, proliferation, and survival through modulation of the expression of cell-cycle inhibitory genes and pro-apoptotic genes [Greer and Brunet, 2005; Lam et al., 2006; Jagani et al., 2008]. Direct phosphorylation of FoxO1 and FoxO3a by Akt serves as a key inhibitory mechanism of their transcriptional activity by promoting the nuclear exclusion and subsequent ubiquitination-mediated degradation of these transcription factors. In this manner, Akt promotes cell survival by blocking the FoxO-induced expression of pro-apoptotic gene targets such as FasL, TRAIL, and Bim [Greer and Brunet, 2005; Lam et al., 2006; Jagani et al., 2008]. Akt also regulates the activity of mammalian target of rapamycin (mTOR) complex 1 (mTORC1), a key mediator of cell growth, proliferation, and survival that can function to inhibit apoptosis in a cellular context-dependent manner [Castedo et al., 2002; Corradetti and Guan, 2006; Wullschleger et al., 2006]. The mTORC1 effector S6K1 may mediate the anti-apoptotic effects of mTOR by phosphorylating the BH3-only protein Bad on serine 136, resulting in the inactivation of the pro-apoptotic activity of Bad [Harada et al., 2001]. In addition, Akt itself has been shown to phosphorylate serine 136 of Bad, and this phosphorylation has been found to effectively suppress Bad-induced cell death [Datta et al., 1997].
Since Akt activation triggers multiple signaling mechanisms that inhibit both the intrinsic and extrinsic apoptotic pathways, abnormalities in the PI3K-Akt pathway can play a major role in mediating the evasion of apoptosis in cancer cells [Kabore et al., 2004]. One such aberration involves activating mutations of PIK3CA, the gene encoding the p110α catalytic subunit of PI3K. Somatic missense mutations of PIK3CA are common in several cancers, including ovarian and breast cancers [Levine et al., 2005]. The two most common types of PI3K mutants, arising from mutations of PIK3CA within the regions encoding the helical and kinase domains, are potent inducers of Akt activation and oncogenic transformation [Kang et al., 2005; Liu and Roberts, 2006]. In addition to PI3K mutations, several other genetic aberrations that result in the constitutive activation of Akt have been reported. The widespread prevalence of aberrant Akt activity in human cancers strongly implies that Akt activation is a key mediator of carcinogenesis [Testa and Bellacosa, 2001; Hay, 2005; Hennessy et al., 2005]. Each of the highly conserved Akt family members, Akt1, Akt2, and Akt3, has been associated with specific types of cancer. For instance, gene amplification and/or mRNA overexpression of Akt2 has been found in a significant fraction of ovarian and pancreatic cancers, and upregulation of Akt3 mRNA expression has been observed in hormone-insensitive breast tumors [Testa and Bellacosa, 2001]. Furthermore, a point mutation in the plekstrin homology domain (PHD) of Akt1 that leads to persistent plasma membrane localization and consequent constitutive activation of the mutant has been found in breast, ovarian, and colorectal cancers [Carpten et al., 2008]. Moreover, the loss or downregulation of the tumor suppressor PTEN, an inhibitor of PI3K-dependent activation of Akt, is a common mechanism for constitutive Akt activation in many human cancers, especially in prostate and endometrial cancers, melanoma, and glioblastoma [Sansal and Sellers, 2004].
In light of the prominent role of aberrant Akt activation in neoplastic transformation, the PI3K-Akt pathway components, including both Akt itself [West et al., 2002] and its downstream effectors [Arden, 2006; Petroulakis et al., 2006], have emerged as potential therapeutic targets in the treatment of a variety of cancers [Hennessy et al., 2005] (see Table III). For instance, reinstating FoxO activity represents a promising therapeutic approach for a wide range of cancers, as a deficiency of functional FoxO can lead to uncontrolled cellular proliferation and accumulation of DNA damage [Arden, 2006; Lam et al., 2006]. Furthermore, inactivation of the transcriptional function of FoxO1 has been observed in PTEN-null tumor cells and restoration of FoxO activity has been found to induce cell death in a manner which parallels reconstitution with PTEN, thereby indicating that FoxOs play a key role in mediating the tumor suppressor function of PTEN [Nakamura et al., 2000]. Consequently, the preclinical development of therapeutic agents that restore the transcriptional activity of FoxO1 by inhibiting its nuclear export has been initiated [Kau et al., 2003; Schroeder et al., 2005].
In addition to the potential therapeutic effects of targeting the PI3K-Akt pathway components with monotherapeutic approaches, agents directed against elements of the PI3K-Akt pathway in combination with standard therapies have great promise to enhance the effectiveness of current therapies. In several types of cancer cells, inhibition of PI3K-Akt signaling has been shown to reverse resistance to a variety of treatments, including chemotherapy, hormone therapy, and targeted therapy [Beeram et al., 2007; Lu et al., 2007; Yu et al., 2008]. Considering that the PI3K-Akt pathway is frequently implicated in conferring therapy resistance to cancer cells, combination therapies of conventional agents with drugs aimed at re-establishing the apoptotic response by suppressing PI3K-Akt signaling may serve as a means to overcome therapy resistance in a clinical setting, particularly in cancers with genetic aberrations that activate the PI3K-Akt pathway [Hennessy et al., 2005].
The therapeutic effect of attenuating PI3K-Akt signaling is underscored by the essential role of PI3K-Akt pathway inhibition in mediating the therapeutic efficacy of a number of the drugs targeted against deregulated RTKs that aberrantly activate multiple downstream signal transduction mechanisms, including the PI3K-Akt pathway [Hennessy et al., 2005]. Given that constitutive activation of RTKs, often due to overexpression or mutational activation, plays a critical role in tumorigenesis, many agents that selectively target these cell surface receptors have been developed. Importantly, several anti-RTK therapies have demonstrated efficacy in clinical trials, both as a monotherapy and in combinational therapies [Gschwind et al., 2004; Guillemard and Saragovi, 2004]. In particular, Trastuzumab (Herceptin) is a monoclonal antibody against the extracellular domain of human epidermal growth factor receptor type 2 (HER2), an upstream RTK of PI3K that is frequently overexpressed in invasive breast cancers. Trastuzumab acts to neutralize HER2 signaling and thus prevents the pathological effects of HER2 overexpression, which includes the inhibition of apoptosis through constitutive activation of the PI3K-Akt-mTOR pathway [Yarden and Sliwkowski, 2001; Hudis, 2007]. Following the validation of its efficacy and safety, both alone and in combination with chemotherapy, by multiple clinical trials, Trastuzumab was approved by the FDA for the treatment of breast cancers with HER-2 overexpression and has since revolutionized the treatment and prognosis of women with HER2-positive breast cancer [Hudis, 2007].
Another important mediator of cell survival is NF-κB, as this transcription factor has emerged as a major player in apoptosis regulation [Dutta et al., 2006]. Activation of NF-κB by certain TNF family members, particularly TNF, promotes the expression of anti-apoptotic proteins and consequently blocks apoptosis. Targets of NF-κB-mediated transcriptional activation include the genes that encode the IAPs, the anti-apoptotic Bcl-2 family members Bcl-xL and A1, c-FLIP, and TNF-associated factor 1 (TRAF1) and 2 (TRAF2) [Karin and Lin, 2002; Karin et al., 2002]. The anti-apoptotic activity of NF-κB can also be induced in response to DNA-damaging agents, including chemotherapeutics and radiation, thereby protecting cancer cells from death by these treatments [Dutta et al., 2006].
While NF-κB is best known as a central regulator of the inflammatory and immune responses, the importance of its more recently discovered role in controlling cell proliferation, apoptosis, and cell migration is highlighted by compelling evidence that links dysregulation of NF-κB with oncogenesis [Baldwin, 2001]. Indeed, NF-κB has been found to be constitutively activated in various malignancies and to play a role in several aspects of carcinogenesis, acting to enhance cellular proliferation, block apoptosis, and promote the angiogenic and metastatic potential of cancer cells [Basseres and Baldwin, 2006; Escarcega et al., 2007]. The significance of NF-κB activity in tumor growth and progression has been supported by studies that have demonstrated that inhibition of NF-κB activity results in decreased tumorigenicity and suppressed angiogenesis and metastasis of human melanoma and ovarian cancer cells in mice [Huang et al., 2000a,b]. In addition, reports of a mutual transcriptional antagonism between NF-κB and p53, which involves the downregulation of p53-mediated transcriptional activation by TNF-activated NF-κB, suggest that NF-κB dysregulation can also contribute to tumorogenesis by promoting the persistence and evolution of genomic defects through inhibition of the tumor suppressor function of p53 [Webster and Perkins, 1999; Ikeda et al., 2000].
The activation of NF-κB signaling not only promotes neoplastic transformation and cancer progression, but also serves as a protective mechanism used by cancer cells to escape death induced by several cancer therapies. Chemotherapy and radiation treatments have been shown to activate NF-κB in some tumor cells, and inhibition of NF-κB activity has been reported to strongly enhance the apoptosis-inducing activity of TNF, radiation, and the chemotherapeutic agents daunorubicin and CPT-11 [Wang et al., 1996, 1999]. The significant role of NF-κB activation in suppressing the apoptotic potential of chemotherapeutic agents has been further evidenced by numerous studies using various chemotherapies and a variety of strategies to block NF-κB activity [Nakanishi and Toi, 2005]. Given that NF-κB acts as a key player in carcinogenesis and chemoresistance, the NF-κB signaling pathway represents an appealing target for the development of anti-cancer agents [Nakanishi and Toi, 2005; Olivier et al., 2006]. The intense interest in targeting the NF-κB signaling pathway for pharmaceutical intervention is highlighted by the fact that over 750 inhibitors of NF-κB activity have been identified [Gilmore and Herscovitch, 2006].
While the significant progress that has been made in the development of agents that act as specific inhibitors of NF-κB provides much hope for the advancement of one such agent into clinical trials, the attenuation of the anti-apoptotic activity of NF-κB by therapeutics that target general cellular components, thereby exerting both NF-κB-dependent and -independent biological effects, has already been shown to elicit a clinical response in cancer patients [Karin et al., 2004; Olivier et al., 2006]. Notably, the anti-neoplastic activity of the proteasome inhibitor bortezomib (Velcade), an FDA-approved drug for second line treatment of MM, has been largely associated with inhibition of the NF-κB survival pathway [Adams, 2004; Olivier et al., 2006]. In addition to MM cells, bortezomib has demonstrated single-agent activity against a range of cancer cells, particularly non-Hodgkin’s lymphoma (NHL) and non-small cell lung cancer (NSCLC) cells, and bortezomib has been shown to sensitize MM, NHL, and NSCLC cells to various anti-cancer agents [Leonard et al., 2006]. Based on strong preclinical evidence, a number of clinical trials investigating the efficacy of bortezomib, both as a single agent and in combination therapy, in several subtypes of NHL have been initiated, and the clinical results thus far indicate that bortezomib-based combination therapy is a promising treatment strategy for lymphoma patients [Leonard et al., 2006].Considering that NF-κB-mediated resistance to apoptosis is a common feature of many tumor cells, this approach of using agents that block NF-κB activity to enhance the clinical activity of standard apoptosis-inducing therapies may extend to a wide range of cancers. Yet, in certain cell type- and/or stimulus-dependent contexts, NF-κB activation has also been shown to sensitize cells to apoptosis. Further investigation into the mechanisms that direct NF-κB to exert a tumor suppressor activity, as opposed to its generally anti-apoptotic activity, will provide insight into the scenarios in which NF-κB-targeted therapeutic agents, both alone and in combination with standard therapies, will offer therapeutic benefits [Dutta et al., 2006].
The small GTPase Ras functions as yet another central regulator of cellular proliferation and survival by coupling the activation of cell surface receptors, including RTKs, to downstream cytoplasmic effectors [Roberts and Der, 2007]. The stimulation of various cell surface receptors by extracellular stimuli induces the conversion of human Ras proteins, termed H-Ras, N-Ras, and K-Ras, from an inactive GDP-bound form to an active GTP-bound form. Activated Ras subsequently triggers several signaling mechanisms, including mitogen-activated protein kinase (MAPK) cascades, which are comprised of three proteins that function as a signaling relay to mediate a variety of cellular responses [Kolch, 2000; Mitin et al., 2005; Roberts and Der, 2007]. In particular, the Raf-MEK-ERK pathway is a well characterized Ras effector pathway that plays an important role in both cellular proliferation and programmed cell death [Kolch, 2000].
Once activated, ERK, the terminal serine/ threonine kinase of the Raf-MEK-ERK cascade, phosphorylates and thereby regulates the activities of a number of substrates, including 90 kDa ribosomal protein S6 kinase (RSK) and multiple transcription factors. In this manner, ERK signaling facilitates the transduction of an extracellular signal from cell surface receptors to DNA transcription factors and thus induces alterations in gene expression [Roberts and Der, 2007].
Much evidence indicates that the Raf-MEF-ERK cascade plays an important role in blocking apoptosis [Shelton et al., 2003]. Constitutively activated MEK has been shown to suppress the apoptotic response, in contrast to the promotion of apoptosis induced by a dominant negative MEK mutant, and constitutively activated ERK, as well as activated MEK, has been found to protect NIH3T3 fibroblasts against death induced by doxorubicin [von Gise et al., 2001]. Furthermore, while the Raf-MEF-ERK signaling pathway has been shown to impair apoptosis without concomitant Akt activation, this MAPK cascade has also been reported to interact with the PI3K-Akt pathway in a cooperative manner to mediate a more extensive anti-apoptotic effect [Shelton et al., 2003].
The vital role of Ras in the control of cellular growth processes is underscored by the potent transforming ability of constitutively activated Ras mutants together with the remarkable prevalence of mutationally activated Ras in human malignancies, with 30% of all human cancers harboring activating Ras mutations, including 90% of pancreatic tumors [Roberts and Der, 2007]. Mutational activation of Ras generally involves a point mutation within particular codons of one of the three Ras-encoding genes, HRAS, NRAS, and KRAS, resulting in the generation of Ras protein products with a single amino acid substitution at position 12, 13, or 61 [Bos, 1989]. These Ras mutants are locked in an active GTP-bound state and thus trigger the persistent activation of downstream effector pathways in a stimulus-independent manner [Roberts and Der, 2007]. Several lines of evidence indicate that the Raf-MEK-ERK cascade is a critical downstream mediator of Ras-induced oncogenesis, yet Raf-independent signaling pathways have also been shown to significantly contribute to Ras-mediated transformation in some cell types [Khosravi-Far et al., 1996; Plattner et al., 1999].
While both Raf-dependent and Raf-independent pathways appear to act as key players in Ras-induced cellular transformation, in either an independent or synergistic manner [Khosravi-Far et al., 1996], aberrant activation of Raf-MEF-ERK signaling has been directly associated with the pathogenesis of several cancers [Mercer and Pritchard, 2003]. Specifically, activating point mutations of BRAF, one of the three genes encoding Raf proteins, have been found in a variety of cancers, both with and without the co-occurrence of RAS mutations [Mercer and Pritchard, 2003; Sieben et al., 2004]. Nearly all of the known somatic mutations of BRAF give rise to a protein product with an enhanced kinase activity, the most common of which harbors a V600E mutation within its kinase domain [Davies et al., 2002; Sridhar et al., 2005]. The transforming activity of these activated B-Raf mutants in NIH3T3 cells and their presence in a wide range of cancers, with a particularly high incidence in malignant melanoma, highlight the significance role of aberrant Raf-MEK-ERK signaling in oncogenesis [Davies et al., 2002; Sridhar et al., 2005]. While the BRAF V600E mutation generally occurs independently of RAS muations, both types of oncogenic mutations result in similar cancer types and induce constitutive ERK signaling, suggesting that the deregulation of ERK signaling serves as a common mechanism by which Ras and B-Raf drive tumor development [Mercer and Pritchard, 2003].
In addition to activating mutations of Ras or Raf, genetically or epigenetically modified RTK signaling can trigger enhanced Raf-MEK-ERK signaling. As with the PI3K-Akt pathway, aberrant activation of upstream RTKs can result in hyperactivation of the Raf-MEK-ERK pathway and thereby promote malignant transformation and cancer progression [Paul and Mukhopadhyay, 2004]. As critical mediators of key signaling pathways that control cellular proliferation, apoptosis, and angiogenesis, RTKs are tightly regulated in normal cells and deregulation of their activity, predominantly by mutational activation or overexpression, has been found to contribute to the pathogenesis of numerous cancers [Bennasroune et al., 2004]. In particular, epidermal growth factor receptor (EGFR) is overexpressed and/or constitutively activated in many cancers, and upregulation of EGFR has been implicated in the early stages of tumor development and associated with poor prognosis [Grandis and Sok, 2004]. An elevated level of EGFR, often attributable to aberrant transcriptional activation, is common in a wide array of malignancies, including head and neck, colon, lung, breast, renal, ovarian, and prostate cancers. Mutations of EGFR that render the receptor constitutively active and consequently enable ligand-independent signaling have also been reported in a variety of cancers, with an especially high frequency in gliomas [Grandis and Sok, 2004]. For example, an EGFR mutant with a truncated extracellular domain (EGFR-vIII) is commonly found in glioblastomas, and EGFRvIII-mediated tumorogenesis has been associated with an enhanced activation of Ras and the downstream Raf-MEK-ERK cascade [Montgomery et al., 1995; Prigent et al., 1996; Feldkamp et al., 1999].
The upregulation of ERK signaling, by any of the aforementioned activating mechanisms, has been implicated in several tumor-promoting processes, including the evasion of apoptosis [Sridhar et al., 2005]. Constitutive activation of the Raf-MEK-ERK signaling pathway has been associated with resistance to apoptosis in melanoma cells, as hyperactive signaling of the ERK effector RSK has been reported to mediate the persistent phosphorylation and consequent inactivation of pro-apoptotic Bad [Eisenmann et al., 2003]. Additionally, ERK signaling can play an important role in promoting cell survival by phosphorylating several transcription factors, leading to alterations in gene transcription that result in increased levels of several proteins that inhibit apoptosis, including anti-apoptotic Bcl-2 family members and IAPs [Henson and Gibson, 2006]. Moreover, phosphorylation of Bcl-2 and Bim by ERK has been shown to play a protective role against apoptosis by blocking Bcl-2 degradation and accelerating Bim degradation [Breitschopf et al., 2000; Luciano et al., 2003].
Considering the emergence of the Raf-MEK-ERK pathway as a mechanism for apoptosis suppression in certain contexts along with the high incidence of hyperactive Raf-MEK-ERK signaling in cancer, inhibition of Raf-MEK-ERK signaling is an attractive anti-cancer therapeutic approach [Koo et al., 2002; Sridhar et al., 2005]. Significant efforts have been made toward the development of effective therapeutic agents that specifically target components of the Raf-MEK-ERK pathway and its upstream regulators (see Table III). A number of inhibitors of Raf and MEK have been designed, and several of these agents have demonstrated antineoplastic activity against a wide range of cancers with only mild toxicity in preclinical and early clinical analysis, supporting the continued development and evaluation of Raf and MEK inhibitors [Roberts and Der, 2007]. Furthermore, since a series of post-translational modifications, including farsenylation, serves as a key mechanism that directs Ras signaling, farnesyl transferase inhibitors (FTIs) have been developed as a means to block the post-translational processing of Ras and thereby prevent its activation. While these agents fail to inactivate K- and N-Ras, FTIs inhibit the activity of H-Ras, as well as other proteins that are mediated by farsenylation, and thus have exhibited significant anti-tumor activity in preclinical and clinical studies [Basso et al., 2005, 2006]. Moreover, two strategies aimed at inhibiting EGFR signaling have been successfully developed: monoclonal antibodies against the extracellular domain of EGFR and small molecule inhibitors targeting the EGFR intracellular tyrosine kinase domain. Numerous anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors have demonstrated clinical activity, as single agents and/or in combination with conventional cytotoxic therapies, with a handful of these agents already receiving FDA approval for use in various cancers, including colorectal cancer and NSCLC, and many others progressing into late-stage clinical evaluation [Dassonville et al., 2007; Overman and Hoff, 2007; Roberts and Der, 2007].
Despite the clinical success of several EGFR-targeted therapies, limitations in utilizing these agents have emerged and their optimal use has not yet been elucidated [Dassonville et al., 2007; Ho et al., 2007; Overman and Hoff, 2007]. Various mechanisms of resistance to drugs directed against EGFR have been reported [Pao et al., 2005; Dassonville et al., 2007], yet using a combination of agents to simultaneously target EGFR and the downstream Raf-MEK-ERK signaling pathway may overcome some forms of resistance [Benvenuti et al., 2007]. In addition, as acquired resistance to EGFR-targeted therapies has been associated with overproduction of vascular endothelial growth factor (VEGF), dual inhibition of EGFR and VEGF receptor (VEGFR), either by using a combination of drugs that target these RTKs separately or a single agent that acts to block both EGFR and VEGFR signaling, is a promising therapeutic strategy for several cancers, particularly NSCLC [Byers and Heymach, 2007]. The therapeutic potential of targeting multiple signaling molecules is highlighted by the significant clinical benefits provided by sorafenib [Hahn and Stadler, 2006; Rini, 2006] and sunitinib [Christensen, 2007], two targeted therapies with potent inhibitory activity against multiple tyrosine kinases, including the angiogenic RTKs platelet-derived growth factor receptor (PDGFR) and VEGFR. In addition to the demonstrated efficacy and safety of these agents in the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors, encouraging clinical data suggest that sorafenib and sunitinib may prove to be effective therapies for NSCLC [Gridelli et al., 2007]. As strategies for the development of anticancer therapies continue to progress and evolve, the emerging role of multi-kinase inhibitors in cancer therapy has prompted a discussion regarding the advantages and disadvantages of highly selective monotherapies verses multi-targeted kinase inhibitors [Sebolt-Leopold and English, 2006].
Evasion of Apoptosis by Cancer-Specific Fusion Proteins
Distinct chromosomal translocations in leukemias and in solid tumors often result in gene fusion and consequent generation of tumor-specific chimeric oncoproteins [Rabbitts, 1994], such as NPM-ALK, the product of a fusion gene that is commonly observed in anaplastic large cell lymphoma (ALCL) [Shiota et al., 1995], and BCR-ABL, a fusion gene product that is the causative agent of chronic myelogenous leukemia (CML) and a subset of acute lymphocytic leukemia (ALL) cases [Rowley, 1973; Melo, 1996; Deininger, 2007]. As products of genetic defects, the functional activities of various fusion proteins are inherently dysregulated, and their aberrant activities are associated with several oncogenic processes, including constitutive activation of survival pathways and suppression of apoptotic signaling [Deininger et al., 2000; Slupianek et al., 2001; Coluccia et al., 2004; Melo and Deininger, 2004; Steelman et al., 2004; Hosokawa, 2005]. In human ALCL-derived cells, the constitutive activity of NPM-ALK imparts a robust survival signal through the aberrant activation of multiple signal transduction pathways, including the Jak-Stat and PI3K-Akt pathways. In this manner, NPM-ALK exerts an anti-apoptotic effect, in part, by promoting the expression of Bcl-xL [Slupianek et al., 2001; Coluccia et al., 2004]. Similarly, the oncogenic potential of BCR-ABL, resulting from its constitutive tyrosine kinase activity, involves the activation of several survival mechanisms. In particular, the PI3K-Akt pathway has been reported to play a key role in BCR-ABL-induced leukemogenesis [Skorski et al., 1997; Van Etten, 2004; Ren, 2005]. BCR-ABL mediates the evasion of apoptosis, at least in part, by inducing the hyperactivation of Akt, thereby suppressing the FoxO-regulated expression of the pro-apoptotic factors TRAIL and Bim [Jagani et al., 2008].
The identification of the molecular pathogenesis of CML has revolutionized the core strategies employed in the development of cancer treatments by enabling the introduction of rationally designed therapies [Hehlmann et al., 2007] (see Table III). Imatinib mesylate (Gleevec, Glivec), a small molecule inhibitor of BCR-ABL, has been shown to exert an anti-proliferative effect in BCR-ABL-positive leukemia cells [Deininger and Druker, 2003] by inducing apoptosis in a caspase-dependent manner [Dan et al., 1998]. While this targeted therapy has shown a remarkable clinical response and is the standard first line treatment for all phases of CML, the emergence of imatinib resistance as a significant limitation in the treatment of CML warrants the investigation of alternative therapeutic strategies, including targeting the downstream signaling pathways of BCR-ABL that are important in the pathogenesis of CML [Ren, 2005; Deininger, 2007].
CONCLUSION
The essential role of apoptosis in preventing tumor-promoting processes, particularly aberrant cellular proliferation and the accumulation of genetic defects, has clearly been confirmed since the prospect that apoptosis serves as a barrier to cancer was initially suggested by Kerr et al. [1972]. The evasion of apoptosis is now well recognized as a prominent hallmark of cancer, and tremendous progress has been made in defining the molecular mechanisms by which cancer cells acquire resistance to apoptosis. In view of the significance of apoptosis suppression in mediating the development and progression of cancer, targeting the molecular defects responsible for the abrogation of apoptosis has emerged as a promising therapeutic approach for treating an array of cancers. While classical cancer therapies act on normal cells as well as cancer cells, thus causing adverse side effects, and fail to elicit a therapeutic response in apoptotic-deficient cells, therapeutic agents that restore apoptotic signaling are expected to selectively target and eradicate cancer cells dependent on apoptosis-suppressing oncogenic mutations for survival and to sensitize cancer cells to other anti-neoplastic agents. Indeed, the therapeutic potential of this approach has been realized, as highlighted by the clinical success of several agents directed against various identified mechanisms that act, at least in part, to protect cancer cells from apoptosis. Despite the achievements thus far, the therapeutic strategy of reinstating the apoptotic response remains an enormous challenge. This approach must be tailored to specific subsets of cancers, as various cancers cells bear distinct sets of defects. Thus, considering the high grade of heterogeneity between tumors, even of the same type, exploiting the induction of apoptosis as a means to eliminate cancer cells requires extensive efforts toward the further investigation of the specific modes of apoptotic dysregulation. Nevertheless, the therapeutic limitations of existing drugs, often attributable to apoptosis resistance, and the encouraging results of preclinical and clinical studies clearly validate the continued search for agents aimed at reestablishing the apoptotic response.
ACKNOWLEDGMENTS
We thank Jane Hayward, media specialist, Beth Israel Deaconess Medical Center, for assistance with figure preparation. O.B. is supported by a fellowship from the Lady TATA Memorial Trust, London, U.K. R.K. is an American Cancer Society Scholar. This work was also funded by National Institutes of Health grants (CA105306 and HL080192) awarded to R.K.
Grant sponsor: NIH; Grant numbers: CA105306, HL080192; Grant sponsor: American Cancer Society; Grant number: RSG 03-012-01-CCG; Grant sponsor: The Lady Tata Memorial Trust, London, UK.
REFERENCES
- Abramson JS, Shipp MA. Advances in the biology and therapy of diffuse large B-cell lymphoma: Moving toward a molecularly targeted approach. Blood. 2005;106:1164–1174. doi: 10.1182/blood-2005-02-0687. [DOI] [PubMed] [Google Scholar]
- Adams J. The proteasome: A suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjei AA, Dy GK, Erlichman C, Reid JM, Sloan JA, Pitot HC, Alberts SR, Goldberg RM, Hanson LJ, Atherton PJ, Watanabe T, Geary RS, Holmlund J, Dorr FA. A phase I trial of ISIS 2503, an antisense inhibitor of H-ras, in combination with gemcitabine in patients with advanced cancer. Clin Cancer Res. 2003;9:115–123. [PubMed] [Google Scholar]
- Almasan A, Ashkenazi A. Apo2L/TRAIL: Apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Amantana A, London CA, Iversen PL, Devi GR. X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol Cancer Ther. 2004;3:699–707. [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Ameisen JC. On the origin, evolution, and nature of programmed cell death: A timeline of four billion years. Cell Death Differ. 2002;9:367–393. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- Anon INGN 201:Ad-p53, Ad5CMV-p53, adenoviral p53, p53 gene therapy–introgen, RPR/INGN 201. Drugs R D. 2007a;8:176–187. doi: 10.2165/00126839-200708030-00005. [DOI] [PubMed] [Google Scholar]
- Anon Oblimersen: Augmerosen, BCL-2 antisense oligonucleotide—Genta, G 3139,GC 3139, oblimersen sodium. Drugs R D. 2007b;8:321–334. doi: 10.2165/00126839-200708050-00006. [DOI] [PubMed] [Google Scholar]
- Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41:709–717. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Armand JP, Burnett AK, Drach J, Harousseau JL, Low-enberg B, San Miguel J. The emerging role of targeted therapy for hematologic malignancies: Update on bortezomib and tipifarnib. Oncologist. 2007;12:281–290. doi: 10.1634/theoncologist.12-3-281. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Attardi LD, Jacks T. The role of p53 in tumour suppression: Lessons from mouse models. Cell Mol Life Sci. 1999;55:48–63. doi: 10.1007/s000180050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi A, Santini D, Russo P, Catricala C, Amantea A, Picardo M, Tatangelo F, Botti G, Dragonetti E, Murace R, Tonini G, Natali PG, Baldi F, Paggi MG. Analysis of APAF-1 expression in human cutaneous melanoma progression. Exp Dermatol. 2004;13:93–97. doi: 10.1111/j.0906-6705.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- Basso AD, Mirza A, Liu G, Long BJ, Bishop WR, Kirschmeier P. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem. 2005;280:31101–31108. doi: 10.1074/jbc.M503763200. [DOI] [PubMed] [Google Scholar]
- Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res. 2006;47:15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Beeram M, Tan QT, Tekmal RR, Russell D, Middleton A, DeGraffenried LA. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–1328. doi: 10.1093/annonc/mdm170. [DOI] [PubMed] [Google Scholar]
- Bennasroune A, Gardin A, Aunis D, Cremel G, Hubert P. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit Rev Oncol Hematol. 2004;50:23–38. doi: 10.1016/j.critrevonc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, Siena S, Bardelli A. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–2648. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- Blackhall F, Ranson M, Thatcher N. Where next for gefitinib in patients with lung cancer? Lancet Oncol. 2006;7:499–507. doi: 10.1016/S1470-2045(06)70725-2. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Carcinogenesis, cancer therapy and chemoprevention. Cell Death Differ. 2005;12:592–602. doi: 10.1038/sj.cdd.4401610. [DOI] [PubMed] [Google Scholar]
- Blick SK, Scott LJ. Cetuximab: A review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs. 2007;67:2585–2607. doi: 10.2165/00003495-200767170-00008. [DOI] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Bos JL. Ras oncogenes in human cancer: A review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Bossi G, Sacchi A. Restoration of wild-type p53 function in human cancer: Relevance for tumor therapy. Head Neck. 2007;29:272–284. doi: 10.1002/hed.20529. [DOI] [PubMed] [Google Scholar]
- Bouchier-Hayes L, Lartigue L, Newmeyer DD. Mitochondria: Pharmacological manipulation of cell death. J Clin Invest. 2005;115:2640–2647. doi: 10.1172/JCI26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschopf K, Haendeler J, Malchow P, Zeiher AM, Dimmeler S. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: Molecular characterization of the involved signaling pathway. Mol Cell Biol. 2000;20:1886–1896. doi: 10.1128/mcb.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewis ND, Phelan A, Normand N, Choolun E, O’Hare P. Particle assembly incorporating a VP22-BH3 fusion protein, facilitating intracellular delivery, regulated release, and apoptosis. Mol Ther. 2003;7:262–270. doi: 10.1016/s1525-0016(02)00054-0. [DOI] [PubMed] [Google Scholar]
- Bucur O, Nat R, Cretoiu D, Popescu LM. Phagocytosis of apoptotic cells by microglia in vitro. J Cell Mol Med. 2001;5:438–441. doi: 10.1111/j.1582-4934.2001.tb00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur O, Ray S, Bucur MC, Almasan A. APO2 ligand/ tumor necrosis factor-related apoptosis-inducing ligand in prostate cancer therapy. Front Biosci. 2006;11:1549–1568. doi: 10.2741/1903. [DOI] [PubMed] [Google Scholar]
- Bullani RR, Huard B, Viard-Leveugle I, Byers HR, Irmler M, Saurat JH, Tschopp J, French LE. Selective expression of FLIP in malignant melanocytic skin lesions. J Invest Dermatol. 2001;117:360–364. doi: 10.1046/j.0022-202x.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- Byers LA, Heymach JV. Dual targeting of the vascular endothelial growth factor and epidermal growth factor receptor pathways: Rationale and clinical applications for non-small-cell lung cancer. Clin Lung Cancer. 2007;8 Suppl 2:S79–S85. doi: 10.3816/clc.2007.s.006. [DOI] [PubMed] [Google Scholar]
- Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8:282–288. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, Thomas JE. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2008;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- Castedo M, Ferri KF, Kroemer G. Mammalian target of rapamycin (mTOR): Pro- and anti-apoptotic. Cell Death Differ. 2002;9:99–100. doi: 10.1038/sj.cdd.4400978. [DOI] [PubMed] [Google Scholar]
- Castilla C, Congregado B, Chinchon D, Torrubia FJ, Japon MA, Saez C. Bcl-xL is overexpressed in hormone-resistant prostate cancer and promotes survival of LNCaP cells via interaction with proapoptotic Bak. Endocrinology. 2006;147:4960–4967. doi: 10.1210/en.2006-0502. [DOI] [PubMed] [Google Scholar]
- Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18 Suppl 10:x3–x10. doi: 10.1093/annonc/mdm408. [DOI] [PubMed] [Google Scholar]
- Cohen MH, Johnson JR, Pazdur R. U. S. Food and Drug Administration Drug Approval Summary: Conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin Cancer Res. 2005;11:12–19. [PubMed] [Google Scholar]
- Colombel M, Symmans F, Gil S, O’Toole KM, Chopin D, Benson M, Olsson CA, Korsmeyer S, Buttyan R. Detection of the apoptosis-suppressing oncoprotein bc1–2 in hormone-refractory human prostate cancers. Am J Pathol. 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- Coluccia AM, Perego S, Cleris L, Gunby RH, Passoni L, Marchesi E, Formelli F, Gambacorti-Passerini C. Bcl-XL down-regulation suppresses the tumorigenic potential of NPM/ALK in vitro and in vivo. Blood. 2004;103:2787–2794. doi: 10.1182/blood-2003-09-3144. [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: Do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- Dan S, Naito M, Tsuruo T. Selective induction of apoptosis in Philadelphia chromosome-positive chronic myelogenous leukemia cells by an inhibitor of BCR - ABL tyrosine kinase, CGP 57148. Cell Death Differ. 1998;5:710–715. doi: 10.1038/sj.cdd.4400400. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Daniel D, Yang B, Lawrence DA, Totpal K, Balter I, Lee WP, Gogineni A, Cole MJ, Yee SF, Ross S, Ashkenazi A. Cooperation of the proapoptotic receptor agonist rhApo2L/TRAIL with the CD20 antibody rituximab against non-Hodgkin lymphoma xenografts. Blood. 2007;110:4037–4046. doi: 10.1182/blood-2007-02-076075. [DOI] [PubMed] [Google Scholar]
- Dassonville O, Bozec A, Fischel JL, Milano G. EGFR targeting therapies: Monoclonal antibodies versus tyrosine kinase inhibitors. Similarities and differences. Crit Rev Oncol Hematol. 2007;62:53–61. doi: 10.1016/j.critrevonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, Yuan J. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- Deininger MW. Optimizing therapy of chronic myeloid leukemia. Exp Hematol. 2007;35:144–154. doi: 10.1016/j.exphem.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Deininger MW, Druker BJ. Specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol Rev. 2003;55:401–423. doi: 10.1124/pr.55.3.4. [DOI] [PubMed] [Google Scholar]
- Deininger MH, Weller M, Streffer J, Meyermann R. Antiapoptotic Bcl-2 family protein expression increases with progression of oligodendroglioma. Cancer. 1999;86:1832–1839. doi: 10.1002/(sici)1097-0142(19991101)86:9<1832::aid-cncr27>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- Deocaris CC, Widodo N, Shrestha BG, Kaur K, Ohtaka M, Yamasaki K, Kaul SC, Wadhwa R. Mortalin sensitizes human cancer cells to MKT-077-induced senescence. Cancer Lett. 2007;252:259–269. doi: 10.1016/j.canlet.2006.12.038. [DOI] [PubMed] [Google Scholar]
- DeRosier LC, Buchsbaum DJ, Oliver PG, Huang ZQ, Sellers JC, Grizzle WE, Wang W, Zhou T, Zinn KR, Long JW, Vickers SM. Combination treatment with TRA-8 anti death receptor 5 antibody and CPT-11 induces tumor regression in an orthotopic model of pancreatic cancer. Clin Cancer Res. 2007;13:5535s–5543s. doi: 10.1158/1078-0432.CCR-07-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakos E, Thomaides A, Medeiros LJ, Li J, Leventaki V, Konopleva M, Andreeff M, Rassidakis GZ. Inhibition of p53-murine double minute 2 interaction by nutlin-3A stabilizes p53 and induces cell cycle arrest and apoptosis in Hodgkin lymphoma. Clin Cancer Res. 2007;13:3380–3387. doi: 10.1158/1078-0432.CCR-06-2581. [DOI] [PubMed] [Google Scholar]
- Dritschilo A, Huang CH, Rudin CM, Marshall J, Collins B, Dul JL, Zhang C, Kumar D, Gokhale PC, Ahmad A, Ahmad I, Sherman JW, Kasid UN. Phase I study of liposome-encapsulated c-raf antisense oligodeoxyribonucleotide infusion in combination with radiation therapy in patients with advanced malignancies. Clin Cancer Res. 2006;12:1251–1259. doi: 10.1158/1078-0432.CCR-05-1260. [DOI] [PubMed] [Google Scholar]
- Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo HM. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res. 2003;63:8330–8337. [PubMed] [Google Scholar]
- Elrod HA, Lin YD, Yue P, Wang X, Lonial S, Khuri FR, Sun SY. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther. 2007;6:2029–2038. doi: 10.1158/1535-7163.MCT-07-0004. [DOI] [PubMed] [Google Scholar]
- Erlichman C, Hidalgo M, Boni JP, Martins P, Quinn SE, Zacharchuk C, Amorusi P, Adjei AA, Rowinsky EK. Phase I study of EKB-569, an irreversible inhibitor of the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol. 2006;24:2252–2260. doi: 10.1200/JCO.2005.01.8960. [DOI] [PubMed] [Google Scholar]
- Escarcega RO, Fuentes-Alexandro S, Garcia-Carrasco M, Gatica A, Zamora A. The transcription factor nuclear factor-kappa B and cancer. Clin Oncol (R Coll Radiol) 2007;19:154–161. doi: 10.1016/j.clon.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Esteller M, Tortola S, Toyota M, Capella G, Peinado MA, Baylin SB, Herman JG. Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res. 2000;60:129–133. [PubMed] [Google Scholar]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- Feldkamp MM, Lala P, Lau N, Roncari L, Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–1453. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- Fong WG, Liston P, Rajcan-Separovic E, St Jean M, Craig C, Korneluk RG. Expression and genetic analysis of XIAP-associated factor 1 (XAF1) in cancer cell lines. Genomics. 2000;70:113–122. doi: 10.1006/geno.2000.6364. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Wrone-Smith T, Boise LH, Thompson CB, Polverini PJ, Simonian PL, Nunez G, Nickoloff BJ. Kaposi’s sarcoma tumor cells preferentially express Bcl-xL. Am J Pathol. 1996;149:795–803. [PMC free article] [PubMed] [Google Scholar]
- Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. IFNgamma sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–2308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Death receptor signaling in cancer therapy. Curr Med Chem Anticancer Agents. 2003;3:253–262. doi: 10.2174/1568011033482404. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Targeting apoptosis pathways in cancer therapy. Curr Cancer Drug Targets. 2004;4:569–576. doi: 10.2174/1568009043332763. [DOI] [PubMed] [Google Scholar]
- Fulda S, Meyer E, Debatin KM. Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res. 2000;60:3947–3956. [PubMed] [Google Scholar]
- Fulda S, Meyer E, Friesen C, Susin SA, Kroemer G, Debatin KM. Cell type specific involvement of death receptor and mitochondrial pathways in drug-induced apoptosis. Oncogene. 2001;20:1063–1075. doi: 10.1038/sj.onc.1204141. [DOI] [PubMed] [Google Scholar]
- Fuster JJ, Sanz-Gonzalez SM, Moll UM, Andres V. Classic and novel roles of p53: Prospects for anticancer therapy. Trends Mol Med. 2007;13:192–199. doi: 10.1016/j.molmed.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Garcia-Echeverria C, Chene P, Blommers MJ, Furet P. Discovery of potent antagonists of the interaction between human double minute 2 and tumor suppressor p53. J Med Chem. 2000;43:3205–3208. doi: 10.1021/jm990966p. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Cortes J, Jones D, Bergstrom D, Kantarjian H, Freedman SJ. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007;109:500–502. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- Goudar RK, Shi Q, Hjelmeland MD, Keir ST, McLendon RE, Wikstrand CJ, Reese ED, Conrad CA, Traxler P, Lane HA, Reardon DA, Cavenee WK, Wang XF, Bigner DD, Friedman HS, Rich JN. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101–112. [PubMed] [Google Scholar]
- Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Gridelli C, Maione P, Del Gaizo F, Colantuoni G, Guerriero C, Ferrara C, Nicolella D, Comunale D, De Vita A, Rossi A. Sorafenib and sunitinib in the treatment of advanced non-small cell lung cancer. Oncologist. 2007;12:191–200. doi: 10.1634/theoncologist.12-2-191. [DOI] [PubMed] [Google Scholar]
- Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: Targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- Guillemard V, Saragovi HU. Novel approaches for targeted cancer therapy. Curr Cancer Drug Targets. 2004;4:313–326. doi: 10.2174/1568009043332989. [DOI] [PubMed] [Google Scholar]
- Hahn O, Stadler W. Sorafenib. Curr Opin Oncol. 2006;18:615–621. doi: 10.1097/01.cco.0000245316.82391.52. [DOI] [PubMed] [Google Scholar]
- Hainaut P, Hernandez T, Robinson A, Rodriguez-Tome P, Flores T, Hollstein M, Harris CC, Montesano R. IARC Database of p53 gene mutations in human tumors and cell lines: Updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998;26:205–213. doi: 10.1093/nar/26.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra KM, Liu JR. Apoptosome dysfunction in human cancer. Apoptosis. 2004;9:691–704. doi: 10.1023/B:APPT.0000045786.98031.1d. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA. 2001;98:9666–9670. doi: 10.1073/pnas.171301998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370:342–350. doi: 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Henson ES, Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: Implications for cancer therapy. Cell Signal. 2006;18:2089–2097. doi: 10.1016/j.cellsig.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603–2614. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Day CL. Regulation of apoptosis: Uncovering the binding determinants. Curr Opin Struct Biol. 2005;15:690–699. doi: 10.1016/j.sbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Ho C, Davies A, Mack P, Gandara D. Dual inhibition: Combining epidermal growth factor-targeted therapies in non-small-cell lung cancer. Clin Lung Cancer. 2007;8:420–424. doi: 10.3816/CLC.2007.n.025. [DOI] [PubMed] [Google Scholar]
- Holcik M, Yeh C, Korneluk RG, Chow T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene. 2000;19:4174–4177. doi: 10.1038/sj.onc.1203765. [DOI] [PubMed] [Google Scholar]
- Holinger EP, Chittenden T, Lutz RJ. Bak BH3 peptides antagonize Bcl-xL function and induce apoptosis through cytochrome c-independent activation of caspases. J Biol Chem. 1999;274:13298–132304. doi: 10.1074/jbc.274.19.13298. [DOI] [PubMed] [Google Scholar]
- Hosokawa Y. Anti-apoptotic action of API2-MALT1 fusion protein involved in t(11;18)(q21;q21) MALT lymphoma. Apoptosis. 2005;10:25–34. doi: 10.1007/s10495-005-6059-6. [DOI] [PubMed] [Google Scholar]
- Howes AL, Chiang GG, Lang ES, Ho CB, Powis G, Vuori K, Abraham RT. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther. 2007;6:2505–2514. doi: 10.1158/1535-7163.MCT-06-0698. [DOI] [PubMed] [Google Scholar]
- Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000a;6:2573–2581. [PubMed] [Google Scholar]
- Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000b;60:5334–5339. [PubMed] [Google Scholar]
- Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Sun X, Li Y, Zhang Y, Eckner R, Doi TS, Takahashi T, Obata Y, Yoshioka K, Yamamoto K. p300/CBP-dependent and -independent transcriptional interference between NF-kappaB RelA and p53. Biochem Biophys Res Commun. 2000;272:375–379. doi: 10.1006/bbrc.2000.2786. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, Pramanik A, Selivanova G. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10:1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: A matter of evasion of apoptosis. Biochim Biophys Acta. 2008;1785:63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- Jin TG, Kurakin A, Benhaga N, Abe K, Mohseni M, Sandra F, Song K, Kay BK, Khosravi-Far R. Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5. J Biol Chem. 2004;279:55594–55601. doi: 10.1074/jbc.M401056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabore AF, Johnston JB, Gibson SB. Changes in the apoptotic and survival signaling in cancer cells and their potential therapeutic implications. Curr Cancer Drug Targets. 2004;4:147–163. doi: 10.2174/1568009043481551. [DOI] [PubMed] [Google Scholar]
- Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Gallagher E. From JNK to pay dirt: Jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: From innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: A treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005;25:31–58. doi: 10.1615/critrevimmunol.v25.i1.30. [DOI] [PubMed] [Google Scholar]
- Kau TR, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, Clardy J, Sellers WR, Silver PA. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, Reed JC. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood. 1998;91:991–1000. [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R, Esposti MD. Death receptor signals to mitochondria. Cancer Biol Ther. 2004;3:1051–1057. doi: 10.4161/cbt.3.11.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R, White MA, Westwick JK, Solski PA, Chrzanowska-Wodnicka M, Van Aelst L, Wigler MH, Der CJ. Oncogenic Ras activation of Raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Y, Wong F, Lo P, Elliott M, Verburgt LM, Hogg JC, Daya M. Overexpression of Bcl-2 and mutations in p53 and K-ras in resected human non-small cell lung cancers. Am J Respir Cell Mol Biol. 1996;15:45–54. doi: 10.1165/ajrcmb.15.1.8679221. [DOI] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, McQueen T, O’Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108:993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- Konig H, Holyoake TL, Bhatia R. Effective and selective inhibition of chronic myeloid leukemia primitive hematopoietic progenitors by the dual Src/Abl kinase inhibitor SKI-606. Blood. 2008;111:2329–2338. doi: 10.1182/blood-2007-05-092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HM, VanBrocklin M, McWilliams MJ, Leppla SH, Duesbery NS, Woude GF. Apoptosis and melanogenesis in human melanoma cells induced by anthrax lethal factor inactivation of mitogen-activated protein kinase kinase. Proc Natl Acad Sci USA. 2002;99:3052–3057. doi: 10.1073/pnas.052707699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkolopoulou P, Saetta AA, Levidou G, Gigelou F, Lazaris A, Thymara I, Scliri M, Bousboukea K, Michalopoulos NV, Apostolikas N, Konstantinidou A, Tzivras M, Patsouris E. c-FLIP expression in colorectal carcinomas: Association with Fas/FasL expression and prognostic implications. Histopathology. 2007;51:150–156. doi: 10.1111/j.1365-2559.2007.02723.x. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422–2427. [PubMed] [Google Scholar]
- Krajewski M, Ozdowy P, D’Silva L, Rothweiler U, Holak TA. NMR indicates that the small molecule RITA does not block p53-MDM2 binding in vitro. Nat Med. 2005;11:1135–1136. doi: 10.1038/nm1105-1135. author reply 1136–1137. [DOI] [PubMed] [Google Scholar]
- Kroemer G. Mitochondrial control of apoptosis: An introduction. Biochem Biophys Res Commun. 2003;304:433–435. doi: 10.1016/s0006-291x(03)00614-4. [DOI] [PubMed] [Google Scholar]
- Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: Regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski L, Talpaz M. Strategies for overcoming imatinib resistance in chronic myeloid leukemia. Leuk Lymphoma. 2007;48:2310–2322. doi: 10.1080/10428190701665988. [DOI] [PubMed] [Google Scholar]
- Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, Harris PL, Driscoll DR, Fidias P, Lynch TJ, Rabindran SK, McGinnis JP, Wissner A, Sharma SV, Isselbacher KJ, Settleman J, Haber DA. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci USA. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCasse EC, Cherton-Horvat GG, Hewitt KE, Jerome LJ, Morris SJ, Kandimalla ER, Yu D, Wang H, Wang W, Zhang R, Agrawal S, Gillard JW, Durkin JP. Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res. 2006;12:5231–5241. doi: 10.1158/1078-0432.CCR-06-0608. [DOI] [PubMed] [Google Scholar]
- Lam EW, Francis RE, Petkovic M. FOXO transcription factors: Key regulators of cell fate. Biochem Soc Trans. 2006;34:722–726. doi: 10.1042/BST0340722. [DOI] [PubMed] [Google Scholar]
- Lane HA, Lebwohl D. Future directions in the treatment of hormone-sensitive advanced breast cancer: The RAD001 (Everolimus)-letrozole clinical program. Semin Oncol. 2006;33:S18–S25. doi: 10.1053/j.seminoncol.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Lee HW, Lee SS, Lee SJ, Um HD. Bcl-w is expressed in a majority of infiltrative gastric adenocarcinomas and suppresses the cancer cell death by blocking stress-activated protein kinase/c-Jun NH2-terminal kinase activation. Cancer Res. 2003;63:1093–1100. [PubMed] [Google Scholar]
- Leonard JP, Furman RR, Coleman M. Proteasome inhibition with bortezomib: A new therapeutic strategy for non-Hodgkin’s lymphoma. Int J Cancer. 2006;119:971–979. doi: 10.1002/ijc.21805. [DOI] [PubMed] [Google Scholar]
- Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, Boyd J. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11:2875–2878. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- Liu Z, Roberts TM. Human tumor mutants in the p110alpha subunit of PI3K. Cell Cycle. 2006;5:675–677. doi: 10.4161/cc.5.7.2605. [DOI] [PubMed] [Google Scholar]
- LoRusso P, Krishnamurthi S, Rinehart J. A phase I-II clinical study of a second generation oral MEK inhibitor, PD 0325901, in patients with advanced cancer. ASCO Meeting Abstracts. 2005;23:3011. [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, Mills GB, Hortobagyi GN, Esteva FJ, Yu D. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shoemaker AR, Liu X, Woods KW, Thomas SA, de Jong R, Han EK, Li T, Stoll VS, Powlas JA, Oleksijew A, Mitten MJ, Shi Y, Guan R, McGonigal TP, Klinghofer V, Johnson EF, Leverson JD, Bouska JJ, Mamo M, Smith RA, Gramling-Evans EE, Zinker BA, Mika AK, Nguyen PT, Oltersdorf T, Rosenberg SH, Li Q, Giranda VL. Potent and selective inhibitors of Akt kinases slow the progress of tumors in vivo. Mol Cancer Ther. 2005;4:977–986. doi: 10.1158/1535-7163.MCT-05-0005. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Shinagawa T, Sano Y, Sakuma T, Nomura S, Nagasaki K, Miki Y, Saito-Ohara F, Inazawa J, Kohno T, Yokota J, Ishii S. Reduced levels of ATF-2 predispose mice to mammary tumors. Mol Cell Biol. 2007;27:1730–1744. doi: 10.1128/MCB.01579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Sano Y, Shinagawa T, Rahman Z, Sakuma T, Nomura S, Licht JD, Ishii S. ATF-2 controls transcription of Maspin and GADD45 alpha genes independently from p53 to suppress mammary tumors. Oncogene. 2008;27:1045–1054. doi: 10.1038/sj.onc.1210727. [DOI] [PubMed] [Google Scholar]
- Mandal M, Kim S, Younes MN, Jasser SA, El-Naggar AK, Mills GB, Myers JN. The Akt inhibitor KP 372-1suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Br J Cancer. 2005;92:1899–1905. doi: 10.1038/sj.bjc.6602595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- McNeish IA, Bell S, McKay T, Tenev T, Marani M, Lemoine NR. Expression of Smac/DIABLO in ovarian carcinoma cells induces apoptosis via a caspase-9-mediated pathway. Exp Cell Res. 2003;286:186–198. doi: 10.1016/s0014-4827(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88:2375–2384. [PubMed] [Google Scholar]
- Melo JV, Deininger MW. Biology of chronic myelogenous leukemia–signaling pathways of initiation and transformation. Hematol Oncol Clin North Am. 2004;18:545–568. doi: 10.1016/j.hoc.2004.03.008. vii–viii. [DOI] [PubMed] [Google Scholar]
- Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta. 2003;1653:25–40. doi: 10.1016/s0304-419x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- Messersmith WA, Hidalgo M. Panitumumab, a monoclonal anti epidermal growth factor receptor antibody in colorectal cancer: Another one or the one? Clin Cancer Res. 2007;13:4664–4666. doi: 10.1158/1078-0432.CCR-07-0065. [DOI] [PubMed] [Google Scholar]
- Mitin N, Rossman KL, Der CJ. Signaling interplay in Ras superfamily function. Curr Biol. 2005;15:R563–R574. doi: 10.1016/j.cub.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453–3459. doi: 10.1093/nar/26.15.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RB, Moscatello DK, Wong AJ, Cooper JA, Stahl WL. Differential modulation of mitogen-activated protein (MAP) kinase/extracellular signal-related kinase kinase and MAP kinase activities by a mutant epidermal growth factor receptor. J Biol Chem. 1995;270:30562–30566. doi: 10.1074/jbc.270.51.30562. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Morgillo F, Lee HY. Lonafarnib in cancer therapy. Expert Opin Investig Drugs. 2006;15:709–719. doi: 10.1517/13543784.15.6.709. [DOI] [PubMed] [Google Scholar]
- Muschen M, Warskulat U, Beckmann MW. Defining CD95 as a tumor suppressor gene. J Mol Med. 2000;78:312–325. doi: 10.1007/s001090000112. [DOI] [PubMed] [Google Scholar]
- Muschen M, Rajewsky K, Kronke M, Kuppers R. The origin of CD95-gene mutations in B-cell lymphoma. Trends Immunol. 2002;23:75–80. doi: 10.1016/s1471-4906(01)02115-9. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–8982. doi: 10.1128/mcb.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- O’Kane SL, Pound RJ, Campbell A, Chaudhuri N, Lind MJ, Cawkwell L. Expression of bcl-2 family members in malignant pleural mesothelioma. Acta Oncol. 2006;45:449–453. doi: 10.1080/02841860500468927. [DOI] [PubMed] [Google Scholar]
- Olivier S, Robe P, Bours V. Can NF-kappaB be a target for novel and efficient anti-cancer agents? Biochem Pharmacol. 2006;72:1054–1068. doi: 10.1016/j.bcp.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Olivieri A, Manzione L. Dasatinib: A new step in molecular target therapy. Ann Oncol. 2007;18 Suppl 6:vi42–vi46. doi: 10.1093/annonc/mdm223. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Oost TK, Sun C, Armstrong RC, Al-Assaad AS, Betz SF, Deckwerth TL, Ding H, Elmore SW, Meadows RP, Olejniczak ET, Oleksijew A, Oltersdorf T, Rosenberg SH, Shoemaker AR, Tomaselli KJ, Zou H, Fesik SW. Discovery of potent antagonists of the antiapoptotic protein XIAP for the treatment of cancer. J Med Chem. 2004;47:4417–4426. doi: 10.1021/jm040037k. [DOI] [PubMed] [Google Scholar]
- Overman MJ, Hoff PM. EGFR-targeted therapies in colorectal cancer. Dis Colon Rectum. 2007;50:1259–1270. doi: 10.1007/s10350-007-0228-3. [DOI] [PubMed] [Google Scholar]
- Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, Zakowski MF, Heelan RT, Kris MG, Varmus HE. KRAS mutations and primary resistance of lung adeno-carcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MK, Mukhopadhyay AK. Tyrosine kinase—Role and significance in cancer. Int J Med Sci. 2004;1:101–115. doi: 10.7150/ijms.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX 15-070synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: Implications for cancer and anticancer therapy. Br J Cancer. 2006;94:195–199. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner R, Gupta S, Khosravi-Far R, Sato KY, Perucho M, Der CJ, Stanbridge EJ. Differential contribution of the ERK and JNK mitogen-activated protein kinase cascades to Ras transformation of HT1080 fibrosarcoma and DLD-1 colon carcinoma cells. Oncogene. 1999;18:1807–1817. doi: 10.1038/sj.onc.1202482. [DOI] [PubMed] [Google Scholar]
- Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, de Bono J. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: Molecular interaction maps and networks. Oncogene. 2004;23:2934–2949. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- Potapova O, Basu S, Mercola D, Holbrook NJ. Protective role for c-Jun in the cellular response to DNA damage. J Biol Chem. 2001;276:28546–28553. doi: 10.1074/jbc.M102075200. [DOI] [PubMed] [Google Scholar]
- Prigent SA, Nagane M, Lin H, Huvar I, Boss GR, Feramisco JR, Cavenee WK, Huang HS. Enhanced tumorigenic behavior of glioblastoma cells expressing a truncated epidermal growth factor receptor is mediated through the Ras-Shc-Grb2 pathway. J Biol Chem. 1996;271:25639–25645. doi: 10.1074/jbc.271.41.25639. [DOI] [PubMed] [Google Scholar]
- Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994;372:143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, Hallett WA, Johnson BD, Nilakantan R, Overbeek E, Reich MF, Shen R, Shi X, Tsou HR, Wang YF, Wissner A. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- Raff M. Cell suicide for beginners. Nature. 1998;396:119–122. doi: 10.1038/24055. [DOI] [PubMed] [Google Scholar]
- Ramsay JA, From L, Kahn HJ. bcl-2 protein expression in melanocytic neoplasms of the skin. Mod Pathol. 1995;8:150–154. [PubMed] [Google Scholar]
- Ravandi F, Lancet J, Giles F, Plunkett W, Williams B, Burton M, Faderl S, Estrov Z, Borthakur G, Akinsanmi L. Phase I study of triciribine phosphate monohydrate, a specific inhibitor of AKt phosphorylation, in adult patients with advanced hematologic malignancies. ASH Annual Meeting Abstracts. 2007;110:913. [Google Scholar]
- Ray S, Bucur O, Almasan A. Sensitization of prostate carcinoma cells to Apo2L/TRAIL by a Bcl-2 family protein inhibitor. Apoptosis. 2005;10:1411–1418. doi: 10.1007/s10495-005-2490-y. [DOI] [PubMed] [Google Scholar]
- Rehman A, Chahal MS, Tang X, Bruce JE, Pommier Y, Daoud SS. Proteomic identification of heat shock protein 90 as a candidate target for p53 mutation reactivation by PRIMA-1 in breast cancer cells. Breast Cancer Res. 2005;7:R765–R774. doi: 10.1186/bcr1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- Rini BI. Sorafenib. Expert Opin Pharmacother. 2006;7:453–461. doi: 10.1517/14656566.7.4.453. [DOI] [PubMed] [Google Scholar]
- Rini B, Kar S, Kirkpatrick P. Temsirolimus. Nat Rev Drug Discov. 2007;6:599–600. [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Roset R, Ortet L, Gil-Gomez G. Role of Bcl-2 family members on apoptosis: What we have learned from knock-out mice. Front Biosci. 2007;12:4722–4730. doi: 10.2741/2421. [DOI] [PubMed] [Google Scholar]
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- Sansome C, Zaika A, Marchenko ND, Moll UM. Hypoxia death stimulus induces translocation of p53 protein to mitochondria. Detection by immunofluorescence on whole cells. FEBS Lett. 2001;488:110–115. doi: 10.1016/s0014-5793(00)02368-1. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Sheng Y, Kotsuji F, Tsang BK. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659–5666. [PubMed] [Google Scholar]
- Sato F, Harpaz N, Shibata D, Xu Y, Yin J, Mori Y, Zou TT, Wang S, Desai K, Leytin A, Selaru FM, Abraham JM, Meltzer SJ. Hypermethylation of the p14(ARF) gene in ulcerative colitis-associated colorectal carcinogenesis. Cancer Res. 2002;62:1148–1151. [PubMed] [Google Scholar]
- Schimmer AD. Inhibitor of apoptosis proteins: Translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- Schimmer AD, Dalili S. Targeting the IAP family of caspase inhibitors as an emerging therapeutic strategy. Hematology Am Soc Hematol Educ Program. 2005:215–219. doi: 10.1182/asheducation-2005.1.215. [DOI] [PubMed] [Google Scholar]
- Schimmer AD, Welsh K, Pinilla C, Wang Z, Krajewska M, Bonneau MJ, Pedersen IM, Kitada S, Scott FL, Bailly-Maitre B, Glinsky G, Scudiero D, Sausville E, Salvesen G, Nefzi A, Ostresh JM, Houghten RA, Reed JC. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- Schroeder FC, Kau TR, Silver PA, Clardy J. The psammaplysenes, specific inhibitors of FOXO1a nuclear export. J Nat Prod. 2005;68:574–576. doi: 10.1021/np049624z. [DOI] [PubMed] [Google Scholar]
- Schuler M, Green DR. Transcription, apoptosis and p53: Catch-22. Trends Genet. 2005;21:182–187. doi: 10.1016/j.tig.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006;441:457–462. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]
- Seeliger H, Guba M, Kleespies A, Jauch KW, Bruns CJ. Role of mTOR in solid tumor systems: A therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev. 2007;26:611–621. doi: 10.1007/s10555-007-9077-8. [DOI] [PubMed] [Google Scholar]
- Selivanova G, Wiman KG. Reactivation of mutant p53: Molecular mechanisms and therapeutic potential. Oncogene. 2007;26:2243–2254. doi: 10.1038/sj.onc.1210295. [DOI] [PubMed] [Google Scholar]
- Shashkova EV, Kuppuswamy MN, Wold WS, Doronin K. Anticancer activity of oncolytic adenovirus vector armed with IFN-alpha and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 2008;15:61–72. doi: 10.1038/sj.cgt.7701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JG, Steelman LS, Lee JT, Knapp SL, Blalock WL, Moye PW, Franklin RA, Pohnert SC, Mirza AM, McMahon M, McCubrey JA. Effects of the RAF/ MEK/ERK and PI3K/AKT signal transduction pathways on the abrogation of cytokine-dependence and prevention of apoptosis in hematopoietic cells. Oncogene. 2003;22:2478–2492. doi: 10.1038/sj.onc.1206321. [DOI] [PubMed] [Google Scholar]
- Shiota M, Nakamura S, Ichinohasama R, Abe M, Akagi T, Takeshita M, Mori N, Fujimoto J, Miyauchi J, Mikata A, Nanba K, Takami T, Yamabe H, Takano Y, Izumo T, Nagatani T, Mohri N, Nasu K, Satoh H, Katano H, Fujimoto J, Yamamoto T, Mori S. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: A distinct clinicopathologic entity. Blood. 1995;86:1954–1960. [PubMed] [Google Scholar]
- Shivapurkar N, Reddy J, Matta H, Sathyanarayana UG, Huang CX, Toyooka S, Minna JD, Chaudhary PM, Gazdar AF. Loss of expression of death-inducing signaling complex (DISC) components in lung cancer cell lines and the influence of MYC amplification. Oncogene. 2002;21:8510–8514. doi: 10.1038/sj.onc.1205941. [DOI] [PubMed] [Google Scholar]
- Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, Diss T, Warren B, Al Adnani M, De Goeij AP, Krausz T, Flanagan AM. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- Singh SV, Choi S, Zeng Y, Hahm ER, Xiao D. Guggulsterone-induced apoptosis in human prostate cancer cells is caused by reactive oxygen intermediate dependent activation of c-Jun NH2-terminal kinase. Cancer Res. 2007;67:7439–7449. doi: 10.1158/0008-5472.CAN-07-0120. [DOI] [PubMed] [Google Scholar]
- Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, Choi JK, Trotta R, Wlodarski P, Perrotti D, Chan TO, Wasik MA, Tsichlis PN, Calabretta B. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. Embo J. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2, −3, −6, −7, −8, and −10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slupianek A, Nieborowska-Skorska M, Hoser G, Morrione A, Majewski M, Xue L, Morris SW, Wasik MA, Skorski T. Role of phosphatidylinositol 3-kinase-Akt pathway in nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res. 2001;61:2194–2199. [PubMed] [Google Scholar]
- Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C, Lowe SW. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- Soussi T. The p53 tumor suppressor gene: From molecular biology to clinical investigation. Ann N Y Acad Sci. 2000;910:121–137. doi: 10.1111/j.1749-6632.2000.tb06705.x. discussion 137–139. [DOI] [PubMed] [Google Scholar]
- Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. 2005;4:677–685. doi: 10.1158/1535-7163.MCT-04-0297. [DOI] [PubMed] [Google Scholar]
- Srinivasan A, Li F, Wong A, Kodandapani L, Smidt R, Jr, Krebs JF, Fritz LC, Wu JC, Tomaselli KJ. Bcl-xL functions downstream of caspase-8 to inhibit Fas- and tumor necrosis factor receptor 1-induced apoptosis of MCF7 breast carcinoma cells. J Biol Chem. 1998;273:4523–4529. doi: 10.1074/jbc.273.8.4523. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/ MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Caspases—controlling intracellular signals by protease zymogen activation. Biochim Biophys Acta. 2000;1477:299–306. doi: 10.1016/s0167-4838(99)00281-2. [DOI] [PubMed] [Google Scholar]
- Sun H, Nikolovska-Coleska Z, Yang CY, Xu L, Tomita Y, Krajewski K, Roller PP, Wang S. Structure-based design, synthesis, and evaluation of conformationally constrained mimetics of the second mitochondria-derived activator of caspase that target the X-linked inhibitor of apoptosis protein/caspase-9 interaction site. J Med Chem. 2004;47:4147–4150. doi: 10.1021/jm0499108. [DOI] [PubMed] [Google Scholar]
- Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, Andreeff M, Reed JC. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–1803. [PubMed] [Google Scholar]
- Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly H, Coiffier B, Michallet AS, Radford JA, Geisler CH, Gadeberg O, Dalseg A, Steenken EJ, Worsaae Dalby L. Phase I/II study of SPC2996, an RNA antagonist of Bcl-2, in patients with advanced chronic lymphocytic leukemia (CLL) ASCO Meeting Abstracts. 2007;25:7036. [Google Scholar]
- Tolcher AW, Hao D, de Bono J, Miller A, Patnaik A, Hammond LA, Smetzer L, Van Wart Hood J, Merritt J, Rowinsky EK, Takimoto C, Von Hoff D, Eckhardt SG. Phase I, pharmacokinetic, and pharmacodynamic study of intravenously administered Ad5CMV-p53, an adenoviral vector containing the wild-type p53 gene, in patients with advanced cancer. J Clin Oncol. 2006;24:2052–2058. doi: 10.1200/JCO.2005.03.6756. [DOI] [PubMed] [Google Scholar]
- Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern W, Corey A, Cohen RB. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- Tourneur L, Delluc S, Levy V, Valensi F, Radford-Weiss I, Legrand O, Vargaftig J, Boix C, Macintyre EA, Varet B, Chiocchia G, Buzyn A. Absence or low expression of fas-associated protein with death domain in acute myeloid leukemia cells predicts resistance to chemotherapy and poor outcome. Cancer Res. 2004;64:8101–8108. doi: 10.1158/0008-5472.CAN-04-2361. [DOI] [PubMed] [Google Scholar]
- Tourneur L, Buzyn A, Chiocchia G. FADD adaptor in cancer. Med Immunol. 2005;4:1. doi: 10.1186/1476-9433-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler P, Allegrini PR, Brandt R, Brueggen J, Cozens R, Fabbro D, Grosios K, Lane HA, McSheehy P, Mestan J, Meyer T, Tang C, Wartmann M, Wood J, Caravatti G. AE E788: A dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- Tu Y, Renner S, Xu F, Fleishman A, Taylor J, Weisz J, Vescio R, Rettig M, Berenson J, Krajewski S, Reed JC, Lichtenstein A. BCL-X expression in multiple myeloma: Possible indicator of chemoresistance. Cancer Res. 1998;58:256–2262. [PubMed] [Google Scholar]
- Tuma RS. Novel small molecule promotes programmed cell death. Oncol Times. 2007;29:38–40. [Google Scholar]
- Van Etten RA. Mechanisms of transformation by the BCR-ABL oncogene: New perspectives in the post-imatinib era. Leuk Res. 2004;28 (Suppl 1):S21–S28. doi: 10.1016/j.leukres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- Venditti A, Del Poeta G, Maurillo L, Buccisano F, Del Principe MI, Mazzone C, Tamburini A, Cox C, Panetta P, Neri B, Ottaviani L, Amadori S. Combined analysis of bcl-2 and MDR1 proteins in 256 cases of acute myeloid leukemia. Haematologica. 2004;89:934–939. [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Voelkel-Johnson C. An antibody against DR4 (TRAIL-R1) in combination with doxorubicin selectively kills malignant but not normal prostate cells. Cancer Biol Ther. 2003;2:283–290. doi: 10.4161/cbt.2.3.398. [DOI] [PubMed] [Google Scholar]
- von Gise A, Lorenz P, Wellbrock C, Hemmings B, Berberich-Siebelt F, Rapp UR, Troppmair J. Apoptosis suppression by Raf-1 and MEK1 requires MEK- and phosphatidylinositol 3-kinase-dependent signals. Mol Cell Biol. 2001;21:2324–2336. doi: 10.1128/MCB.21.7.2324-2336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: The cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Vucic D, Fairbrother WJ. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res. 2007;13:5995–6000. doi: 10.1158/1078-0432.CCR-07-0729. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Helman LJ. The biology behind mTOR inhibition in sarcoma. Oncologist. 2007;12:1007–1018. doi: 10.1634/theoncologist.12-8-1007. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: Enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- Webster GA, Perkins ND. Transcriptional cross talk between NF-kappaB and p53. Mol Cell Biol. 1999;19:3485–3495. doi: 10.1128/mcb.19.5.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M. CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene. 2003;22:8233–8245. doi: 10.1038/sj.onc.1207198. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xia S, Li Y, Rosen EM, Laterra J. Ribotoxic stress sensitizes glioblastoma cells to death receptor induced apoptosis: Requirements for c-Jun NH2-terminal kinase and Bim. Mol Cancer Res. 2007;5:783–792. doi: 10.1158/1541-7786.MCR-06-0433. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yee KS, Vousden KH. Complicating the complexity of p53. Carcinogenesis. 2005;26:1317–1322. doi: 10.1093/carcin/bgi122. [DOI] [PubMed] [Google Scholar]
- Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, Parry J, Smith D, Brandhuber BJ, Gross S, Marlow A, Hurley B, Lyssikatos J, Lee PA, Winkler JD, Koch K, Wallace E. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Okamoto I, Okabe T, Iwasa T, Satoh T, Nishio K, Fukuoka M, Nakagawa K. Matuzumab and cetuximab activate the epidermal growth factor receptor but fail to trigger downstream signaling by Akt or Erk. Int J Cancer. 2008;122:1530–1538. doi: 10.1002/ijc.23253. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Kudoh S, Kimura T, Mitsuoka S, Matsuura K, Hirata K, Matsui K, Negoro S, Nakagawa K, Fukuoka M. EKB-569, a new irreversible epidermal growth factor receptor tyrosine kinase inhibitor, with clinical activity in patients with non-small cell lung cancer with acquired resistance to gefitinib. Lung Cancer. 2006;51:363–368. doi: 10.1016/j.lungcan.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–858. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li JH, Xu XM, Liu S, Chen J, Liu F, Qi YL, Deng Q, Cao J, Liu SQ, Luo HS, Yu JP. Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int J Cancer. 2008;122:433–443. doi: 10.1002/ijc.23049. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jin TG, Yang H, DeWolf WC, Khosravi-Far R, Olumi AF. Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res. 2004;64:7086–7091. doi: 10.1158/0008-5472.CAN-04-1498. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivotovsky B, Orrenius S. Carcinogenesis and apoptosis: Paradigms and paradoxes. Carcinogenesis. 2006;27:1939–1945. doi: 10.1093/carcin/bgl035. [DOI] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuzak TJ, Steinhoff DF, Sutton LN, Phillips PC, Eggert A, Grotzer MA. Loss of caspase-8 mRNA expression is common in childhood primitive neuroectodermal brain tumour/medulloblastoma. Eur J Cancer. 2002;38:83–91. doi: 10.1016/s0959-8049(01)00355-0. [DOI] [PubMed] [Google Scholar]