Abstract
The mammalian cytoskeletal proteins β- and γ-actin are highly homologous, but only β-actin is N-terminally arginylated, which regulates its function. Here we examined the metabolic fate of exogenously expressed arginylated and non-arginylated actin isoforms. Arginylated γ-actin, unlike β-, was highly unstable and was selectively ubiquitinated and degraded in vivo. This instability was regulated by the differences in the coding sequence between the two actin isoforms, which conferred different translation rates. γ-actin was translated more slowly than β-actin, and this slower processing resulted in the exposure of a normally hidden lysine residue for ubiquitination, leading to the preferential degradation of γ-actin upon arginylation. This degradation mechanism, coupled to nucleotide coding sequence, may regulate protein arginylation in vivo.
Actin is a major cytoskeletal component in eukaryotic cells. Mammalian β- and γ-actin (1-4) are encoded by different genes and differ significantly at the nucleotide level, but are nearly identical in their amino acid sequences, with only 4 homologous substitutions near their N-termini. N-terminal methionine (M) in the actin sequence is removed immediately after synthesis, exposing the aspartic acid (D) (in β-actin) or glutamic acid (E) (in γ- actin) that can undergo subsequent posttranslational modifications (5). Arginylation -- posttranslational addition of arginine (R) to proteins mediated by arginyltransferase (6) – affects cytoskeletal proteins (7) and regulates actin (8), however only β-actin is posttranslationally arginylated on the N-terminus (8). The mechanism for such a differentiation between these two highly similar actin isoforms is unclear.
While arginylated β-actin is metabolically stable (8), other proteins can be targeted for degradation via N-terminal arginylation (9). To test the possibility that such arginylation-dependent targeting may selectively affect arginylated γ- actin, we tested the metabolic stability of mammalian fusion constructs encoding wild type and N-terminally arginylated β- and γ- actins (termed M-and R-actin, respectively, see Fig. S3A and SOM Text 1-2 for further explanations and construct validation).
Arginylated γ-actin was indeed significantly less abundant than arginylated β-actin or non-arginylated actin isoforms (Fig. 1A, Fig. S3-4). Measurements of posttranslational degradation in the presence of cycloheximide showed that arginylated γ-actin was degraded significantly faster than other actin species (Fig. 1B and Fig. S5). Its levels specifically increased in the presence of proteasome inhibitor MG132 (Fig. 1C) suggesting that selective degradation of γ-actin was at least in part proteasome-dependent.
Figure 1. Arginylated γ-actin is selectively degraded.
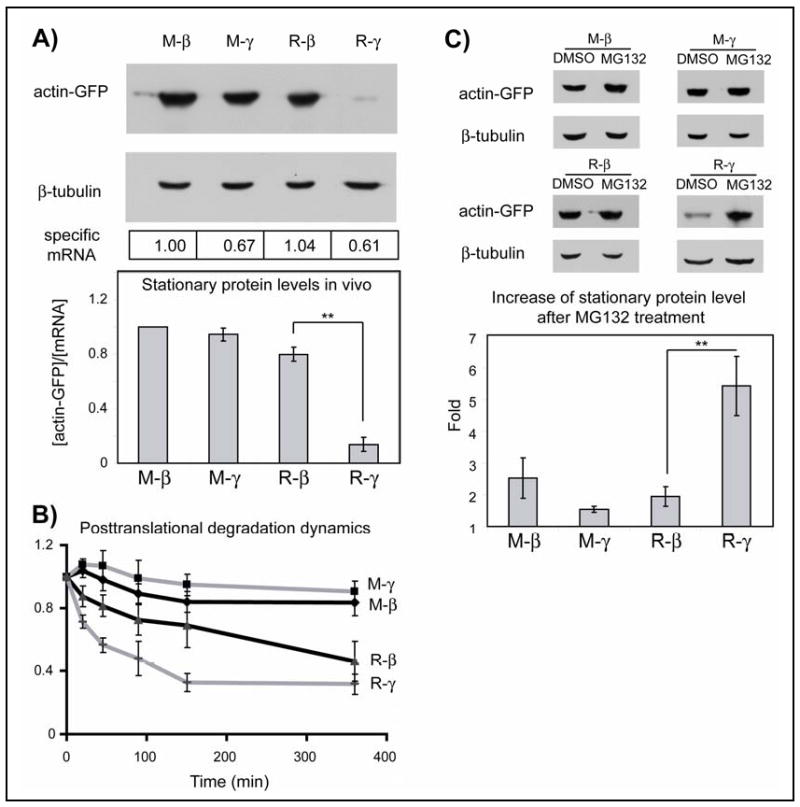
A. Top: Representative immunoblots of the lysates of HEK 293T cells transfected with actin-GFP constructs probed with anti-GFP and β-tubulin as a loading control. Boxed numbers at the bottom indicate the specific mRNA levels in each sample. Bottom: Quantifications of the stationary protein levels as the amounts of in vivo actin-GFP per mRNA unit, normalized to the level of M-β-actin (n= 4 for R-β, R-γ and 6 for M-β, M-γ). B. Changes in actin protein level over time in the presence of cycloheximide, normalized to zero time point (n= 3 for M-β, M-γ and 6 for R-β, R-γ). C. Top: representative immunoblots showing the stationary protein levels of the actin fusions and β-tubulin loading control in cells treated with DMSO or MG132. Bottom, quantification of the fold change of each fusion protein upon MG132 treatment compared to the DMSO-treated sample (n= 3 for M-β, M-γ, R-β and 6 for R-γ). Numbers in all panels represent mean +/-SEM; ** P<0.01, Student's t-test.
To examine whether, in addition to the amino acid sequences, differences in β- and γ-actin coding sequences (Fig. S6A) could be involved in regulating their differential stability, we produced ‘converted’ actin isoforms, termed β-coded-γ-actin (βc-γ-actin) and γ-coded-β-actin (γc-β-actin), that used the coding sequence of β-actin to produce the amino acid sequence of γ-actin, and vice versa (Fig. S6B). Such conversion resulted in a reversal of the intracellular levels of arginylated β- and γ-actin (Fig. 2A, Fig. S7-8). Despite identical amino acid sequences, arginylated γc-β-actin was much less abundant than arginylated β-actin (>2 fold), while R-βc-γ-actin was >4 fold more abundant than R-γ-actin. Such codon switching did not significantly affect posttranslational degradation dynamics (Fig. 2C, Fig. S9), or intracellular levels of non-arginylated β- and γ-actin (Fig. 2B), indicating that the effect of codon switching was co-translational and specifically mediated by arginylation. Thus, changes in intracellular level of arginylated actin isoforms after the codon switching resulted from changes in their co-translational and not posttranslational degradation. This difference was mainly defined by the first 100 nucleotides of the actin coding sequence (Fig. S10).
Figure 2. Coding sequence affects stationary protein levels of arginylated actin isoforms without affecting their posttranslational degradation dynamics.
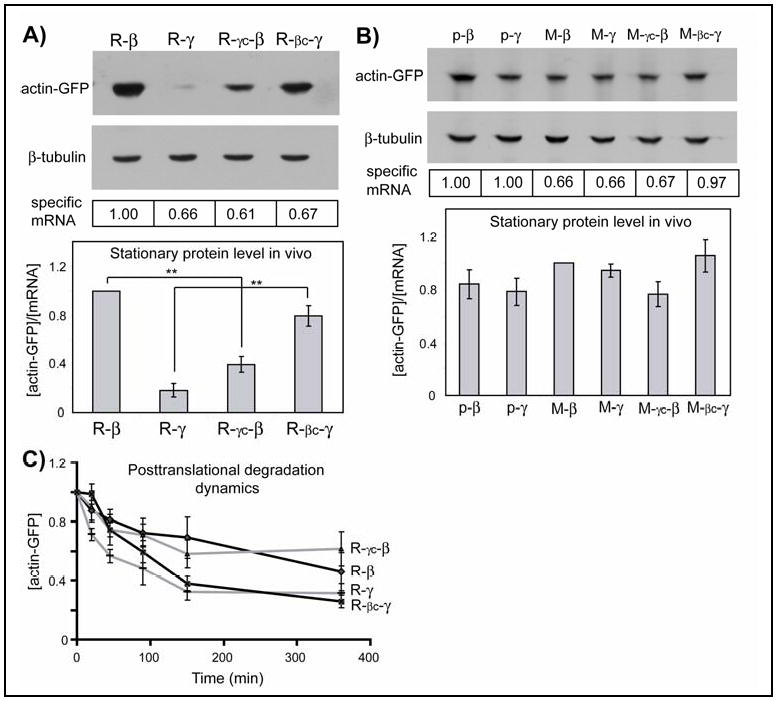
A, B. Representative immunoblots of the lysates of HEK 293T cells transfected with actin-GFP constructs probed with anti-GFP and β-tubulin as a loading control. Boxed numbers at the bottom indicate corresponding mRNA levels. Histogram: stationary protein levels of each actin species quantified per mRNA unit and normalized to the level of R-β- (A) or M-β-actin (B). In A, n=5; in B, n=3 for p-β and p-γ-, the constructs that contain no N-terminal Ub fusion (Fig. S2, SOM Text 2), M- γc-β, and M- βc-γ and 6 for M- β, M-γ. C. Changes in actin protein levels over time in the presence of cycloheximide, normalized to the protein level at zero time point (n= 5 for R- γc-β, R-βc- γ- and 6 for R-β, R- γ). Numbers in all panels represent mean +/- SEM; ** P<0.01, Student's t-test.
The cotranslational events that contribute to the overall protein stability include translation speed and/or co-translational degradation(10). To test whether β- and γ-actin have different translation speeds, we compared the dynamics of accumulation of β and γ-actin constructs (that contain identical translation initiation and termination sites) in the presence of proteasome inhibitor MG132 in vivo, and in vitro in rabbit reticulocyte lysates. In both tests, γ-actin accumulated more slowly than β-actin (Fig. 3A-B, Fig. S11-12; Table S1). In agreement with this, ribosome translocation speeds measured by incorporation of labeled amino acids into nascent peptides (11) were faster for β– than for γ–actin (Fig. 3C; Table S2). Patterns of the ribosome-bound nascent peptides (12) showed at least one prominent low molecular band in γ-actin that was not present in β-actin (Fig. 3D), suggesting that the ribosome paused during translation of the first short stretch of the γ-actin sequence.
Figure 3. Slower translation of γ-actin is linked to arginylation-mediated degradation.
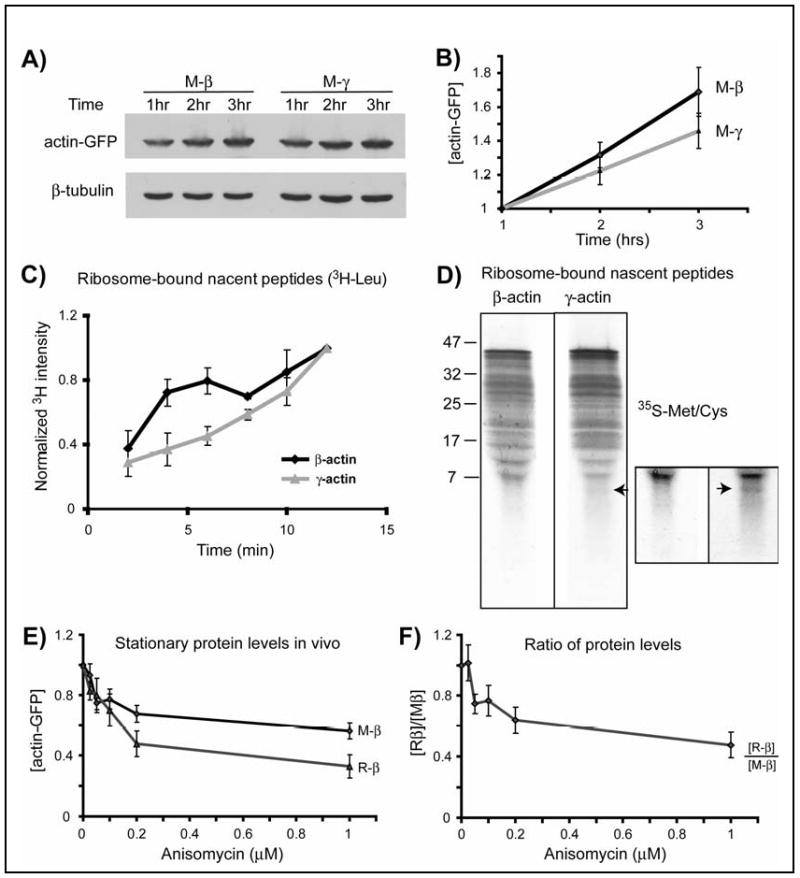
A. Representative immunoblots of the lysates of HEK 293T cells transfected with actin-GFP constructs and treated with MG132, probed with anti-GFP and β-tubulin as a loading control. B. Quantification of protein levels for M-β and M-γ-actin adjusted to the starting mRNA level and normalized to the 1 hr time point in each set (n=8). C. Incorporation of 3H-Leu into ribosome-bound nascent peptides of β- and γ-actin over time, normalized to 12′ time point (n=3). D. Pattern of 35S-labeled nascent peptides. Boxed area represents the bottom part of the same gel, contrasted to emphasize the lower molecular weight bands using Adobe Photoshop ‘brightness/contrast’ function applied equally to the entire inset image. Arrow in both images indicates the additional low molecular weight band present in γ-actin. Similar results were observed in two independent repeats. E. Stationary protein levels of actin proteins plotted against the increasing concentrations of anisomycin. F. Stationary levels of actin proteins from the experiment shown in E, quantified by independently performed Western blots by loading M-β and R-β side by side and plotted as ratio of [R-β]/[M-β] against anisomycin concentration. Curves in E and F are normalized to the sample with 0 μM of anisomycin in each set (n=3). Numbers in all panels represent mean +/- SEM.
The two major factors that can affect translation speed or dynamics are codon usage and mRNA structure. Analysis of the codon usage in β- and γ- actin revealed no prominent features within the first hundred nucleotides that could explain their difference in translation speeds (Fig. S20). However, computational prediction of the folded RNA structure within the 5′-region of the actin coding sequence showed a significant difference between γ- and β-actin mRNA within the beginning of their coding sequences (Fig. S21-25) that probably underlies their different translation speed and dynamics.
To test whether the slow translation of γ-actin is responsible for its preferential co-translational degradation upon arginylation, we used translation inhibitor anisomycin (13), and compared the levels of the normally stable R-β-actin to that of non-arginylated M-β–actin in anisomycin-treated cells. As expected, increasing doses of anisomycin progressively reduced the levels of both actin species, however R-β-actin levels decreased to a larger extent than M-β-actin in a dose-dependent manner (Fig. 3E-F, and Fig. S13). A similar effect was observed for R-γ and M-γ, while no significant difference was seen between non-arginylated actins (Fig. S14). Thus, slowing down translation indeed leads to a reduction in the actin levels that is mediated by N-terminal arginylation.
N-terminal arginylation can attract ubiquitin conjugation machinery(14, 15). Such ubiquitination often happens on the Lys nearest to the N-terminus and is believed to be an essential feature of the arginylation-dependent N-degron (9). The actin sequence has a Lys residue in position 18 from the N-terminus, which is predicted to be buried in the core of the folded actin molecule (Fig. S15). However, this Lys is probably exposed for potential modifications during translation before the folding can be completed – an effect that is expected to affect γ-actin, translated at a slower rate, much more than β-actin. To test whether Lys18 affected degradation of arginylated γ-actin, we replaced this residue with Arg (the closest Lys homolog) or Leu (naturally found in a homologous position in the prokaryotic actin homolog MreB(16); SOM Text 2 and Fig. S16). Such K18L and K18R mutations increased the stationary protein level of R-γ-actin by at least 50%, without significantly changing its posttranslational degradation dynamics (Fig. 4A, Fig. S17-18). Moreover, K18L mutation reduced ubiquitination of R-γ-actin (Fig. 4B, Fig. S26A). Actin ubiquitination levels also depended on nucleotide coding sequences, and R-γ- and R-γc-β-actin – the two actin species found to be the least stable – were ubiquitinated to a significantly higher extent than the others (Fig. 4C, Fig. S26B-C).
Figure 4. Co-translational degradation of arginylated γ-actin is achieved via a ubiquitin-dependent mechanism.
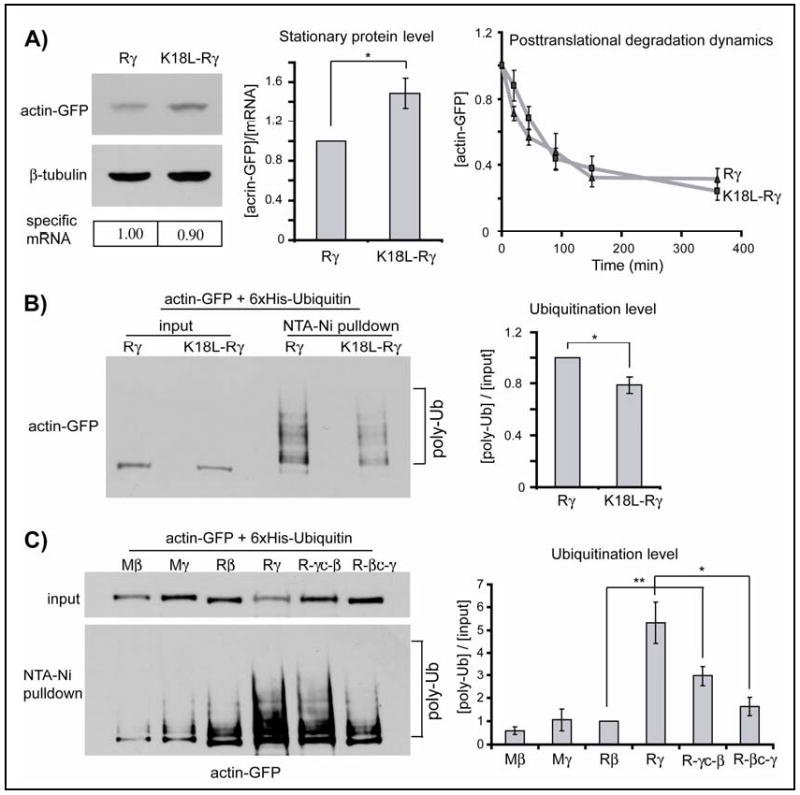
A. Left: Representative immunoblots of the lysates of HEK 293T cells transfected with actin-GFP constructs probed with anti-GFP and β-tubulin as a loading control; specific mRNA levels are indicated below each lane. Middle: stationary protein levels generated with each construct quantified per mRNA unit and normalized to the level of arginylated γ-actin (n=4). Right: changes in actin protein levels over time in the presence of cycloheximide, normalized to the protein level at zero time point (n= 4 for K18L-Rγ and 6 for R-γ). B. Left: Total actin-GFP levels in cells co-transfected with actin constructs and His-tagged ubiquitin (input), and in the pellet after His-tag pulldown to enrich for ubiquitinated proteins (pulldown). Right: quantification of the ubiquitination levels (n=3). Levels of K18L- R-γ mutant are normalized to those of R-γ. C. Left: representative immunoblots of the input and His-tag-Ub pulldown for the constructs marked on the top. Right: quantification of the ubiquitination levels, normalized to those of R-β (n=3). Numbers in all panels represent mean +/- SEM; * P<0.05, ** P<0.01, Student's t-test.
Thus, differential N-terminal arginylation of actin isoforms in non-muscle cells is regulated by a degradation mechanism that selectively targets γ-actin, is dependent not only on the amino acid but also on the nucleotide coding sequence, and is linked to co-translational processing and ubiquitination. Such combination of mechanisms is at least partially proteasome-dependent and results in selective removal of arginylated γ-actin from the intracellular protein pool, so that only arginylated β-actin is found in vivo. Because arginylation regulates actin function at the cell leading edge (8), ensuring that only β- but not γ-actin is arginylated can constitute an important step in this regulation (SOM Text 3).
Because Lys18 involved in co-translational degradation of γ- actin is normally hidden inside the protein core, it appears likely that this Lys is ubiquitinated before the protein folds, probably early during translation, targeting actin for degradation in a proteasome-dependent or independent way. However, replacement of Lys18 had only a partial effect in reducing the ubiquitination and stabilizing the arginylated γ- actin, suggesting that other Lys residues in the actin sequence, or the molecular chaperones involved in co-translational actin processing, may also contribute to this process (17).
In addition to selective degradation, actin isoforms may also be selectively recognized by arginyltransferase, either via the specificity of the enzyme itself, or by its spatial segregation toward one actin isoform. However, arginyltransferase has fairly poor substrate specificity (7) and it can efficiently arginylate both N-terminal Asp and Glu (18, 19) found at the N-terminus of β- and γ-actin, respectively(5). Spatial segregation toward one actin isoform is also unlikely, because arginyltransferase appears to have no bias in its intracellular distribution (18, 19). Actin's N-terminal acetylation (5, 20, 21), may also be isoform-biased and regulate its degradation state, however, to date all actin isoforms appear to be equally acetylated. Thus, selective degradation appears to be the most plausible explanation for why only one of the two predominant actin isoforms is arginylated in non-muscle cells.
For some proteins N-terminal arginylation targets them for degradation (15, 22), while for others it does not (7, 8). Perhaps arginylation may be a self-regulating modification that ensures selective accumulation of some arginylated proteins and removal of others. This mechanism may be employed not only with actin isoforms but also with other closely homologous but selectively arginylated proteins.
Supplementary Material
Acknowledgments
We thank R. Dominguez for help with actin structure analysis and the preparation of Fig. S15, D. Volgin for helpful suggestions on quantitative PCR, S. Fuchs, Y. Goldman, and J. M. Murray for helpful discussions, and S. Kurosaka for critical reading of the manuscript. This work was supported by NIH grant 5R01HL084419, W.W.Smith Charitable Trust, and Philip Morris Research Management Group awards to A.K.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Materials and Methods
SOM Text 1, 2, 3
References
References and Notes
- 1.Otey CA, Kalnoski MH, Lessard JL, Bulinski JC. J Cell Biol. 1986 May;102:1726. doi: 10.1083/jcb.102.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otey CA, Kalnoski MH, Bulinski JC. J Cell Biochem. 1987 Jun;34:113. doi: 10.1002/jcb.240340205. [DOI] [PubMed] [Google Scholar]
- 3.Hofer D, Ness W, Drenckhahn D. J Cell Sci. 1997 Mar;110(Pt 6):765. doi: 10.1242/jcs.110.6.765. [DOI] [PubMed] [Google Scholar]
- 4.Condeelis J, Singer RH. Biol Cell. 2005 Jan;97:97. doi: 10.1042/BC20040063. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein PA, Martin DJ. J Biol Chem. 1983 Mar 25;258:3961. [PubMed] [Google Scholar]
- 6.Balzi E, Choder M, Chen WN, Varshavsky A, Goffeau A. J Biol Chem. 1990 May 5;265:7464. [PubMed] [Google Scholar]
- 7.Wong CCL, et al. PLoS Biology. 2007 September 01;5:e258. [Google Scholar]
- 8.Karakozova M, et al. Science. 2006 Jul 14;313:192. doi: 10.1126/science.1129344. [DOI] [PubMed] [Google Scholar]
- 9.Varshavsky A. Cell. 1992 May 29;69:725. doi: 10.1016/0092-8674(92)90285-k. [DOI] [PubMed] [Google Scholar]
- 10.Kramer G, Boehringer D, Ban N, Bukau B. Nat Struct Mol Biol. 2009 Jun;16:589. doi: 10.1038/nsmb.1614. [DOI] [PubMed] [Google Scholar]
- 11.Lopo AC, Lashbrook CC, Hershey JW. Biochem J. 1989 Mar 1;258:553. doi: 10.1042/bj2580553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komar AA, Lesnik T, Reiss C. FEBS Lett. 1999 Dec 3;462:387. doi: 10.1016/s0014-5793(99)01566-5. [DOI] [PubMed] [Google Scholar]
- 13.Grollman AP. J Biol Chem. 1967 Jul 10;242:3226. [PubMed] [Google Scholar]
- 14.Elias S, Ciechanover A. J Biol Chem. 1990 Sep 15;265:15511. [PubMed] [Google Scholar]
- 15.Ferber S, Ciechanover A. Nature. 1987 Apr 23-29;326:808. doi: 10.1038/326808a0. [DOI] [PubMed] [Google Scholar]
- 16.van den Ent F, Amos LA, Lowe J. Nature. 2001 Sep 6;413:39. doi: 10.1038/35092500. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg AL. Nature. 2003 Dec 18;426:895. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 18.Rai R, Kashina A. Proc Natl Acad Sci U S A. 2005 Jul 19;102:10123. doi: 10.1073/pnas.0504500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon YT, Kashina AS, Varshavsky A. Mol Cell Biol. 1999 Jan;19:182. doi: 10.1128/mcb.19.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandekerckhove J, Weber K. Arch Int Physiol Biochim. 1978 Oct;86:891. [PubMed] [Google Scholar]
- 21.Redman KL, Rubenstein PA. Methods Enzymol. 1984;106:179. doi: 10.1016/0076-6879(84)06018-3. [DOI] [PubMed] [Google Scholar]
- 22.Gonda DK, et al. J Biol Chem. 1989 Oct 5;264:16700. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


