Abstract
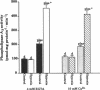
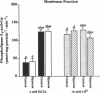
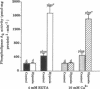
Although the activation of calcium-independent phospholipase A2 (PLA2) enzymes has been described in the heart, the pathogenetic role of this enzyme(s) in hypoxic cell injury has not been previously examined in any tissue. Therefore, we characterized the time course of activation of calcium-independent PLA2 using both plasmalogen and diacylglycerophospholipid substrates during hypoxia in rabbit proximal tubules and examined whether inhibition of calcium-independent PLA2 activity is associated with a cytoprotective effect. Subjecting rabbit proximal tubules to hypoxia for 5 min resulted in at least a threefold increase in cytosolic calcium-independent PLA2, which was selective for plasmalogen substrates (control 444 +/- 69 vs hypoxia 1,675 +/- 194 pmol.mg protein-1.min-1, n = 5). In contrast, no changes in PLA2 activity were observed in the presence of 4 mM EGTA in the membrane fraction using plasmenylcholine substrates. 20 min of hypoxia resulted in an increase in arachidonate from 3 +/- 1 to 28 +/- 4 ng/mg protein and lactate dehydrogenase release from 7.5 +/- 2% to 38 +/- 5%, n = 4. Pretreatment of proximal tubules with 10 microM Compound I, a specific inhibitor of calcium-independent PLA2, resulted in reduction in the magnitude of both hypoxia-induced arachidonic acid release (11 +/- 3 ng/mg protein) and lactate dehydrogenase release (18 +/- 4%). Our data indicate that a significant fraction of PLA2 activity in the proximal tubule is calcium-independent and selective for plasmalogen substrates. Furthermore, the activation of this enzyme plays an important role in the pathogenesis of membrane injury during hypoxia in the proximal tubule.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V. Phospholipase A2 and signal transduction. J Am Soc Nephrol. 1992 Aug;3(2):128–150. doi: 10.1681/ASN.V32128. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest. 1988 Jul;82(1):168–176. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Ozgür L. E., Conway T. M., Dispoto J., Crooke S. T., Bomalaski J. S. Cloning of a phospholipase A2-activating protein. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5418–5422. doi: 10.1073/pnas.88.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. A., Hazen S. L., Saffitz J. E., Gross R. W. The rapid and reversible activation of a calcium-independent plasmalogen-selective phospholipase A2 during myocardial ischemia. J Clin Invest. 1991 Jul;88(1):331–335. doi: 10.1172/JCI115296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Identification and characterization of a hormonally regulated form of phospholipase A2 in rat renal mesangial cells. J Biol Chem. 1988 Nov 15;263(32):16645–16651. [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Identification and characterization of a hormonally regulated form of phospholipase A2 in rat renal mesangial cells. J Biol Chem. 1988 Nov 15;263(32):16645–16651. [PubMed] [Google Scholar]
- Hazen S. L., Ford D. A., Gross R. W. Activation of a membrane-associated phospholipase A2 during rabbit myocardial ischemia which is highly selective for plasmalogen substrate. J Biol Chem. 1991 Mar 25;266(9):5629–5633. [PubMed] [Google Scholar]
- Hazen S. L., Stuppy R. J., Gross R. W. Purification and characterization of canine myocardial cytosolic phospholipase A2. A calcium-independent phospholipase with absolute f1-2 regiospecificity for diradyl glycerophospholipids. J Biol Chem. 1990 Jun 25;265(18):10622–10630. [PubMed] [Google Scholar]
- Hazen S. L., Zupan L. A., Weiss R. H., Getman D. P., Gross R. W. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. J Biol Chem. 1991 Apr 15;266(11):7227–7232. [PubMed] [Google Scholar]
- Hirashima Y., Farooqui A. A., Mills J. S., Horrocks L. A. Identification and purification of calcium-independent phospholipase A2 from bovine brain cytosol. J Neurochem. 1992 Aug;59(2):708–714. doi: 10.1111/j.1471-4159.1992.tb09426.x. [DOI] [PubMed] [Google Scholar]
- Hirashima Y., Mills J. S., Yates A. J., Horrocks L. A. Phospholipase A2 activities with a plasmalogen substrate in brain and in neural tumor cells: a sensitive and specific assay using pyrenesulfonyl-labeled plasmenylethanolamine. Biochim Biophys Acta. 1990 Oct 22;1047(1):35–40. doi: 10.1016/0005-2760(90)90257-x. [DOI] [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993 Jan 29;72(2):269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Hoilien C. A., Dahl R., Ahnen D. J., Wilson P. D., Kim J. Characterization of ischemia-induced loss of epithelial polarity. J Membr Biol. 1988 Dec;106(3):233–242. doi: 10.1007/BF01872161. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Simon F. R. Renal cortical brush-border and basolateral membranes: cholesterol and phospholipid composition and relative turnover. J Membr Biol. 1985;83(3):207–215. doi: 10.1007/BF01868695. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Nemenoff R. A., Gronich J. H., Bonventre J. V. Subcellular characteristics of phospholipase A2 activity in the rat kidney. Enhanced cytosolic, mitochondrial, and microsomal phospholipase A2 enzymatic activity after renal ischemia and reperfusion. J Clin Invest. 1991 May;87(5):1810–1818. doi: 10.1172/JCI115202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Ohara O., Teraoka H., Arita H. Glucocorticoids suppress group II phospholipase A2 production by blocking mRNA synthesis and post-transcriptional expression. J Biol Chem. 1990 Jul 25;265(21):12745–12748. [PubMed] [Google Scholar]
- Nemenoff R. A., Winitz S., Qian N. X., Van Putten V., Johnson G. L., Heasley L. E. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J Biol Chem. 1993 Jan 25;268(3):1960–1964. [PubMed] [Google Scholar]
- Nguyen V. D., Cieslinski D. A., Humes H. D. Importance of adenosine triphosphate in phospholipase A2-induced rabbit renal proximal tubule cell injury. J Clin Invest. 1988 Sep;82(3):1098–1105. doi: 10.1172/JCI113666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K., Browning J. L., Mattaliano R. J., Smart J. E., Chow E. P., Falbel T., Ribolini A., Garwin J. L., Wallner B. P. Purification and partial sequence analysis of a 37-kDa protein that inhibits phospholipase A2 activity from rat peritoneal exudates. J Biol Chem. 1986 Mar 25;261(9):4239–4246. [PubMed] [Google Scholar]
- Portilla D., Mandel L. J., Bar-Sagi D., Millington D. S. Anoxia induces phospholipase A2 activation in rabbit renal proximal tubules. Am J Physiol. 1992 Mar;262(3 Pt 2):F354–F360. doi: 10.1152/ajprenal.1992.262.3.F354. [DOI] [PubMed] [Google Scholar]
- Scherrer L. A., Gross R. W. Subcellular distribution, molecular dynamics and catabolism of plasmalogens in myocardium. 1989 Jun 27-Jul 24Mol Cell Biochem. 88(1-2):97–105. doi: 10.1007/BF00223430. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- Wetzels J. F., Wang X., Gengaro P. E., Nemenoff R. A., Burke T. J., Schrier R. W. Glycine protection against hypoxic but not phospholipase A2-induced injury in rat proximal tubules. Am J Physiol. 1993 Jan;264(1 Pt 2):F94–F99. doi: 10.1152/ajprenal.1993.264.1.F94. [DOI] [PubMed] [Google Scholar]
- Wolf R. A., Gross R. W. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985 Jun 25;260(12):7295–7303. [PubMed] [Google Scholar]