Abstract
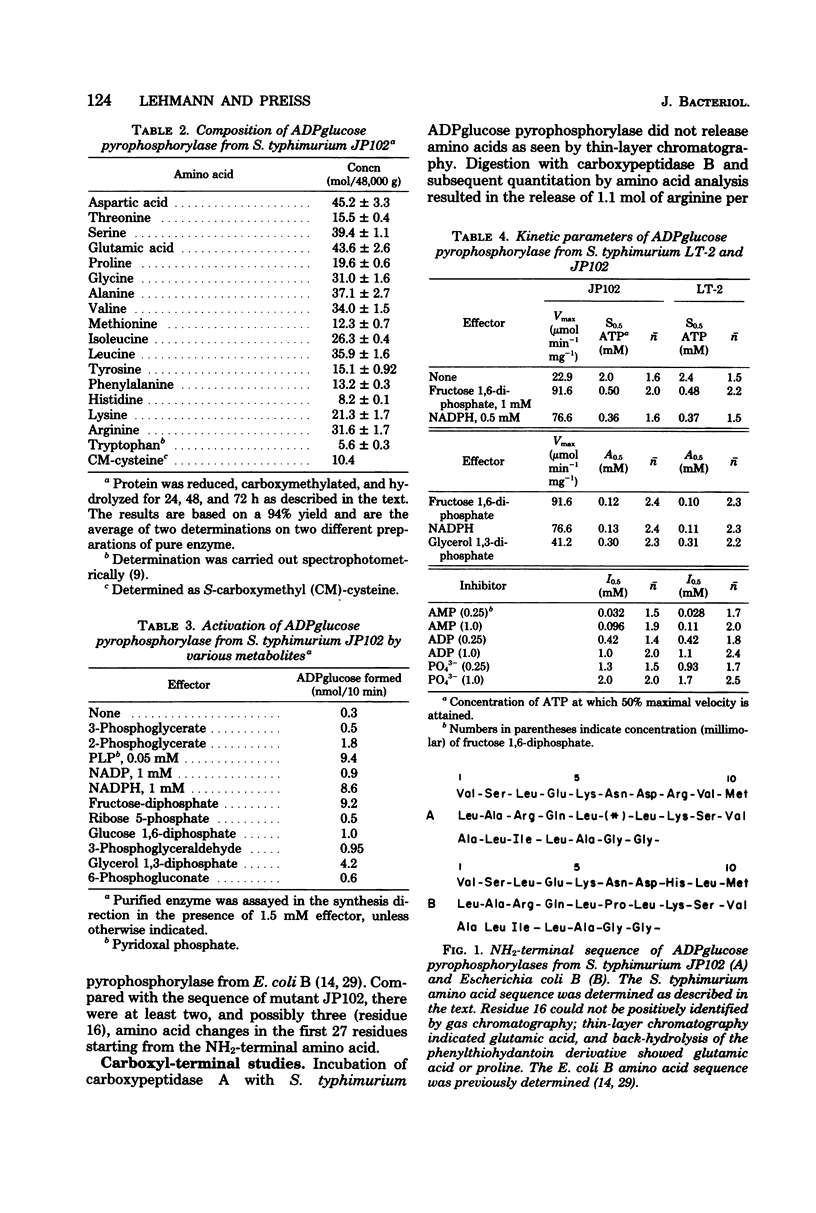
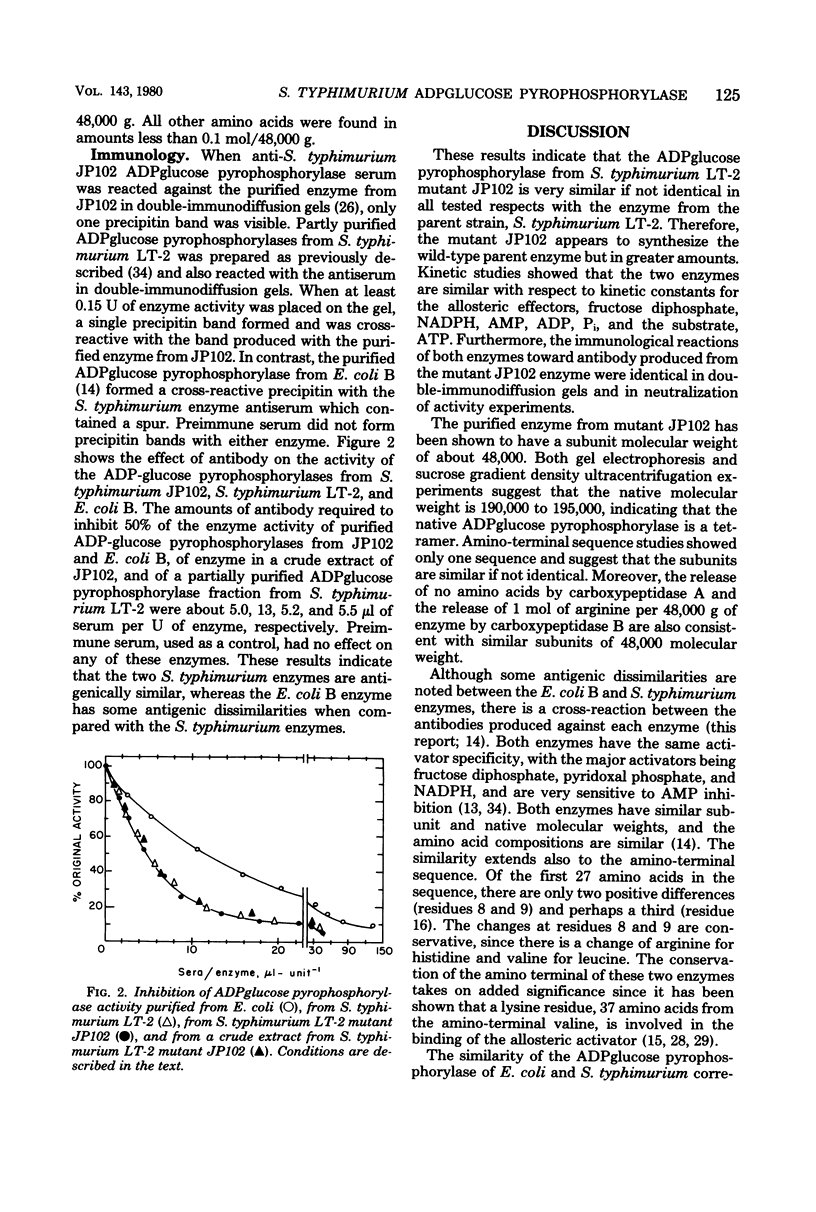
The adenosine diphosphate glucose pyrophosphorylase from a Salmonella typhimurium LT-2 mutant, JP102, derepressed in the glycogen biosynthetic enzymes was purified to homogeneity. The enzyme was found to be identical with the parent wild-type enzyme with respect to regulatory properties, immunological reactivity, and kinetic constants for the allosteric effectors and for the substrate, adenosine triphosphate. The JP102 enzyme was composed of four identical subunits, each with a molecular weight of about 48,000. This was supported by the findings that (i) gel electrophoresis under denaturing conditions showed only one component; (ii) digestion with carboxypeptidase B released stoichiometric amounts of arginine, and (iii) amino-terminal sequencing showed a single sequence for the first 27 residues. The properties of the purified S. typhimurium enzyme were compared with the properties of the previously purified Escherichia coli B enzyme.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Damotte M., Cattanéo J., Sigal N., Puig J. Mutants of Escherichia coli K 12 altered in their ability to store glycogen. Biochem Biophys Res Commun. 1968 Sep 30;32(6):916–920. doi: 10.1016/0006-291x(68)90114-9. [DOI] [PubMed] [Google Scholar]
- Dietzler D. N., Leckie M. P., Lais C. J., Magnani J. L. Evidence for the allosteric regulation of bacterial glycogen synthesis in vivo. Arch Biochem Biophys. 1974 Jun;162(2):602–606. doi: 10.1016/0003-9861(74)90221-5. [DOI] [PubMed] [Google Scholar]
- Dietzler D. N., Leckie M. P., Lais C. J., Magnani J. L. Evidence for the allosteric regulation of glycogen synthesis in the intact Escherichia coli cell. Agreement of the values of the parameters of the Hill equation fitted to data for glycogen synthesis in vivo with the abailable values obtained in vitro with adenosine diphosphoglucose synthetase. J Biol Chem. 1975 Mar 25;250(6):2383–2387. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Govons S., Gentner N., Greenberg E., Preiss J. Biosynthesis of bacterial glycogen. XI. Kinetic characterization of an altered adenosine diphosphate-glucose synthase from a "glycogen-excess" mutant of Escherichia coli B. J Biol Chem. 1973 Mar 10;248(5):1731–1740. [PubMed] [Google Scholar]
- Haugen T., Ishaque A., Chatterjee A. K., Preiss J. Purification of Escherichia coli ADPglucose pyrophosphorylase by affinity chromatography. FEBS Lett. 1974 Jun 1;42(2):205–208. doi: 10.1016/0014-5793(74)80786-6. [DOI] [PubMed] [Google Scholar]
- Haugen T., Ishaque A., Preiss J. ADPGlucose pyrophosphorylase: evidence for a lysine residue at the activator site of the Escherichia coli B enzyme. Biochem Biophys Res Commun. 1976 Mar 22;69(2):346–353. doi: 10.1016/0006-291x(76)90528-3. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Kulbe K. D. Micropolyamide thin-layer chromatography of phenylthiohydantoin amino acids (PTH) at subnanomolar level. A rapid microtechnique for simultaneous multisample identification after automated Edman degradations. Anal Biochem. 1974 Jun;59(2):564–573. doi: 10.1016/0003-2697(74)90310-8. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Parsons T. F., Preiss J. Biosynthesis of bacterial glycogen. Incorporation of pyridoxal phosphate into the allosteric activator site and an ADP-glucose-protected pyridoxal phosphate binding site of Escherichia coli B ADP-glucose synthase. J Biol Chem. 1978 Sep 10;253(17):6197–6202. [PubMed] [Google Scholar]
- Parsons T. F., Preiss J. Biosynthesis of bacterial glycogen. Isolation and characterization of the pyridoxal-P allosteric activator site and the ADP-glucose-protected pyridoxal-P binding site of Escherichia coli B ADP-glucose synthase. J Biol Chem. 1978 Nov 10;253(21):7638–7645. [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Preiss J., Greenberg E., Sabraw A. Biosynthesis of bacterial glycogen. Kinetic studies of a glucose-1-phosphate adenylyltransferase (EC 2.7.7.27) from a glycogen-deficient mutant of Escherichia coli B. J Biol Chem. 1975 Oct 10;250(19):7631–7638. [PubMed] [Google Scholar]
- Preiss J., Lammel C., Greenberg E. Biosynthesis of bacterial glycogen. Kinetic studies of a glucose-1-P adenylyltransferase (EC 2.7.7.27) from a glycogen-excess mutant of Escherichia coli B. Arch Biochem Biophys. 1976 May;174(1):105–119. doi: 10.1016/0003-9861(76)90329-5. [DOI] [PubMed] [Google Scholar]
- Preiss J. Regulation of adenosine diphosphate glucose pyrophosphorylase. Adv Enzymol Relat Areas Mol Biol. 1978;46:317–381. doi: 10.1002/9780470122914.ch5. [DOI] [PubMed] [Google Scholar]
- Ribéreau-Gayon G., Sabraw A., Lammel C., Preiss J. Biosynthesis of bacterial glycogen IX: regulatory properties of the adenosine diphosphate glucose pyrophosphrylases of the Enterobacterieae. Arch Biochem Biophys. 1971 Feb;142(2):675–692. doi: 10.1016/0003-9861(71)90534-0. [DOI] [PubMed] [Google Scholar]
- Steiner K. E., Preiss J. Biosynthesis of bacterial glycogen: genetic and allosteric regulation of glycogen biosynthesis in Salmonella typhimurium LT-2. J Bacteriol. 1977 Jan;129(1):246–253. doi: 10.1128/jb.129.1.246-253.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]


