Abstract
Cartilage/chondrocyte transplantation is frequently utilized in the repair of focal chondral defects. It has been proposed that failure of subchondral bone maintenance or restoration is a factor contributing to the failure of cartilage-forming transplants. Some studies reveal that the transplant is associated with subchondral bone resorption, often leading to deep pits beneath the presumptive cartilage repair site. Thus, the question is raised as to the utility of agents, such as bisphosphonates, to inhibit bone remodeling at the transplant site. In the present study we show that oral administration (three times weekly) of the bisphosphonate, risedronate, inhibited the subchondral bone loss deep to the cultured allogeneic graft tissue site in attempted repair of surgically created chondral defects in a minipig model. In addition, the graft tissue, characterized by type II collagen was retained in the majority of treated animals. Untreated minipigs displayed a deep bone resorption pit, beneath the graft region, filled with type I collagen tissue as determined through immunohistochemical staining. This fibrous tissue appeared well integrated with the host tissue in the majority of cases. In the transplanted cartilage region, the overall histological score for tissue quality was significantly (p<0.05) better for the treated animals which displayed better matrix staining, cell clustering, tidemark integrity, and subchondral bone integrity (p<0.05 in each category). However, the integration of allograft with host tissue did not always occur completely. Thus, bisphosphonates might be considered in clinical treatment strategies for such procedures.
Introduction
The surgical repair of localized chondral and osteochondral lesions is an increasingly frequent clinical procedure. Among the surgical techniques currently utilized are autologous chondrocyte transplantation (ACT), autologous osteochondral transplantation, allograft transplantation, and microfracture. The outcomes generally show promise, but have also produced some negative results. 1–4 Importantly, the actual success rate, along with possible reasons for failures, have never been followed in a large blinded clinical trial and thus remain to be fully established. However, in one magnetic resonance imaging study on a small sample of patients with ACT, the clinical outcome was reported to be correlated to the filling of the defect, structure of the repair tissue, and changes in the subchondral bone. 5
Several large animal models of cartilage/chondrocyte transplantation in goats and pigs have been developed for translation to humans. 6,7 These studies revealed that the transplant was associated with subchondral bone resorption, often leading to deep pits beneath the presumptive cartilage repair site. It has been proposed that failure of subchondral bone maintenance or restoration is a factor contributing to the failure of cartilage-forming transplants. These results raise the question of the utility of agents, such as bisphosphonates, to inhibit bone remodeling at the transplant site thus allowing the maintenance of a stable base of subchondral bone. Bisphosphonates modulate remodeling in a number of skeletal tissues. They reduce markers of cartilage degradation and bone resorption 8 in osteoarthritis patients and inhibit the bone resorption associated with bone graft. 9 They have also been shown to be efficacious in inhibiting the bone remodeling associated with experimentally-produced cartilage lesions. 10,11 The question then arises as to whether bisphosphonates might be used to inhibit the bone resorption associated with cartilage transplantation.
Another variable affecting graft outcome is the graft tissue type, such as in autograft vs. allograft. The use of either of these tissues presents a unique set of drawbacks. Autografts require the retrieval of tissue from a donor site as well as a second surgery for transplantation of the cultured donor cells. Allografts have the advantage of requiring only one surgical procedure and no donor site morbidity, but the availability of fresh donor tissue can be problematic. 12 Recent developments in tissue engineering have thus sought to produce a construct with the properties of native articular cartilage, but with the advantage of ready availability. These constructs can be manufactured with a scaffold, which seeks to mimic the biomechanical properties of the collagen backbone of hyaline cartilage, or without a scaffold. An example of the latter is the tissue developed through the alginate-recovered-chondrocyte (ARC) method, which does not require an exogenous synthetic matrix.13 This product is ideally suited for studies of transplantable cartilage, including those in animal models.14 Thus, we hypothesized that, used in combination, articular cartilage transplantation and bisphosphonate treatment, to prevent subchondral bone remodeling, will lead to a successful defect repair.
Here, in an animal model of ARC tissue transplantation, we report that the oral administration of risedronate sodium inhibits the bone resorption often associated with deep cartilage damage and cartilage transplantation, thus maintaining the subchondral plate and improving the success of transplantation.
Methods
Animals
The animal procedures were approved by the Rush University Medical Center Institutional Animal Care and Use Committee (IACUC #04-083). Seventeen 50–60 kg skeletally mature Yucatan minipigs (15 females, 2 males) were obtained from S&S Farms (California), allowed to acclimate to positive human contact for a minimum of 5 days prior to the beginning of experimentation, and fed the Harlan Teklad Mini Pig Diet. All 17 minipigs were subjected to a surgically created chondral defect and subsequent transplantation of ARC tissue (see procedure below). The animals were divided into two groups, (1) untreated (n = 9 animals) and (2) risedronate-treated (n = 8 animals).
Risedronate treatment
Risedronate-treated animals (risedronate sodium was provided by Procter and Gamble Pharmaceuticals,) were orally administered 3cc of water containing 2.5mg Risedronate sodium/kg body weight beginning one day prior to surgery and three times per week thereafter until sacrifice. The risedronate administration was followed by oral administration of 8 oz. of water. All animals were fasted for 14 hours prior to, and one hour after, the time of risedronate administration. Water was available ad libitum.
Minipig Chondral Defect Model
Chondrocytes isolated from full-thickness minipig articular cartilage were utilized to produce the engineered cartilage substitute construct using the ARC technology, the details of which can be found in Masuda et al. 13,15 The cartilage constructs were cultured for 4 weeks until implanted into the chondral defect produced in the femoral condyle. A chondral defect measuring approximately 6 mm in diameter and 1 mm deep was made in the non-weight-bearing area of the host medial femoral condyle using a biopsy punch, and the cartilage was removed using a circular knife (2.25 mm, Visitec™, BD Bioscience) without disturbing the underlying subchondral plate. The implant was in place for 59 days prior to euthanasia. Integration of the implant with the surrounding host tissue was assessed macroscopically and by histological analysis. The surgeons were blinded as to which animals were receiving risedronate treatment. No signs of infection or abnormal swelling were noted upon daily visual inspection of the joint.
Euthanasia and cartilage collection and examination
On day 59 after implantation, animals were sacrificed under anesthesia with Telazol (4.4 mg/kg to effect) and Xylazine (2.2 mg/kg to effect) by the intravenous injection of a supersaturated Phenobarbital solution (50 mg/kg; Euthanasia B solution: Henry Schein Inc., Melville, NY). Intact joints were collected, opened, photographed and examined macroscopically for defect filling and appearance. Using a surgical saw, an area of surrounding host tissue containing the implant and underlying bone was collected from each defect site. The collected tissue was further divided into multiple portions, each containing implant tissue, adjoining host tissue, and underlying bone for histochemical and immunohistochemical analyses.
Histological preparation and analyses
Portions of the samples were prepared for histology by fixation in paraformaldehyde for a minimum of two days and then decalcified and processed for paraffin-embedding and Safranin O/Fast green staining 16. The histological scoring of these sections was carried out by two observers, in agreement, using the O’Driscoll scoring method (Table 1), with perfect healing represented by a total score of 34 points 7,17,18.
Table 1.
Mean scores on O’Driscoll histological scale.
| Group (N) | Tissue morphology *(3) | Matrix staining (3) | Structural integrity (4) | Clustering (2) | Tide-mark (4) | Architect of defect (3) | Filling of defect (4) | Lateral integration (2) | Basal integration (3) | SCB integrity (2) | Inflammation (4) | Total [range] (34) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Graft Without RA (9) | 1.8 | 0.4 | 1.7 | 0 | 0 | 1.8 | 1.7 | 1.7 | 2.7 | 0 | 4 | 15.8 [0–15] |
| Graft with RA (8) | 2.6 | 1.6 | 2.9 | 1.4 | 3.2 | 1.7 | 2.9 | 1.3 | 2.3 | 2.0 | 4 | 25.9 [13–28] |
| p-value | N.S. | p < 0.05 | N.S. | p<0.01 | p<0.01 | N.S. | N.S. | N.S. | N.S. | p<0.001 | N.S. | p < 0.05 |
= maximum score attainable, SCB: subchondral bone, RA: risedronate
Immunohistochemistry for types I and II collagen
Portions of the decalcified samples were prepared for immunohistochemistry on 6 micron paraffin sections and incubated with mouse anti-human type I collagen primary antibody (Calbiochem, San Diego, CA) diluted 1:50 in PBS, or mouse anti-collagen type II collagen (Calbiochem) primary antibody diluted 1:50 in PBS overnight at 4°C in a humid chamber as previously described.7
Statistics
The Mann-Whitney U test was used to analyze the histology grading for the effect of treatment. Statistical analysis was performed using the Statview (Version 5.0, SPSS, Chicago, IL) program package with a significance level of p<0.05.
Results
Macroscopic examination
At sacrifice, no inflammation was observed in any animal. Animal weights were not significantly different between groups at any time point. Macroscopic examinations of the entire joint surfaces revealed no signs of cartilage lesions outside the grafted region and none in the contralateral control knees in any of the minipigs, nor were there any signs of osteophytes or synovial proliferation. 55% of the grafts in the untreated animals were at least 75% filled and 11% were at least 50% filled with repair tissue. 50% of the grafts in the risedronate-treated animals were at least 75% filled and 25% were at least 50% filled with repair tissue4. The nature of this repair tissue was definitively identified only upon histological examination. However, in the untreated animals, the tissue appeared fibrous from the surface, with subchondral bone often showing through (Fig. 1d). In the risedronate-treated animals, the tissue appeared smooth, and glassy-like, having an appearance similar to normal articular cartilage, but being slightly thinner and with a slight pinkish hue from the bone below (Fig. 1c).
Figure 1.
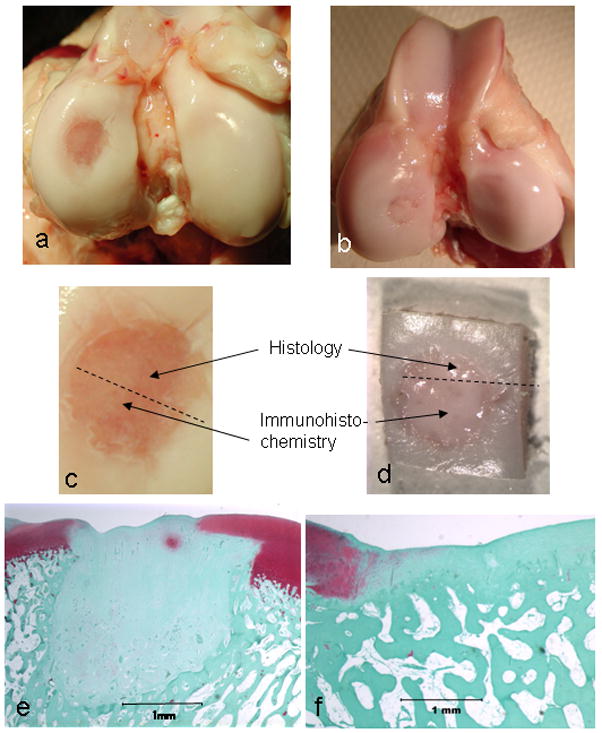
Representative gross (a–d) and histologic (e, f) views of the transplant site from untreated (a, c, e), and risedronate-treated (b, d, f) animals showing a severe subchondral bone reaction in the untreated group deep to the allograft site and the absence of a subchondral bone reaction in the risedronate-treated group.
Microscopic examination
The mean histological scores for both groups of animals are shown in Table 1. The overall score for the risedronate-treated animals was significantly better than that for the untreated animals. The categories which most distinguished the two groups from each other were: matrix staining, cell clustering, presence of a tidemark, and subchondral bone integrity. The most striking difference was a pronounced resorption of subchondral bone, which extended the width of the articular cartilage defect and extended down to as much as six times the depth of the cartilage in the untreated group and was not present in the risedronate-treated group (Fig. 1e-f). This subchondral bone reaction took place in every animal within the untreated group but was not seen in any animal of the risedronate-treated group.
Also, in the untreated group, none of the animals displayed any remnants of the originally implanted ARC tissue. Rather, the defect was not filled at all in one animal and the defect was partially filled with a disorganized fibrous tissue in the other eight animals. In the risedronate-treated group, the defect in every animal displayed either partial or full filling with the implanted ARC tissue. This tissue was characterized by either an intact or a slightly irregular superficial zone and a cartilage morphology characteristic of articular hyaline cartilage.
The integration of the tissue that filled the defect was at times better for the untreated group; there was no discernable tissue space at the lateral or basal borders between the filled defect and surrounding cartilage (Fig. 2a). However, the lateral borders were readily identifiable by the abrupt absence of Safranin O staining and by the change in tissue organization. In the risedronate-treated group there was a slight but visible space between the implanted ARC tissue and the native surrounding cartilage in four of the animals, thus implicating incomplete integration (Fig. 2b). In the remaining 4 animals in this group there was complete lateral integration. At the basal border of the defect region, the untreated animals showed a deep subchondral bone resorption associated with numerous osteoclasts (Fig. 2c) and an abrupt change from fibrous tissue to trabecular bone with no visible space between the two tissues, whereas those of the risedronate-treated animals displayed a normal tidemark deep to the cartilage lying on typical subchondral bone. No osteoclasts were observed in or near the subchondral bone beneath the defect site in the risedronate-treated animals.
Figure 2.
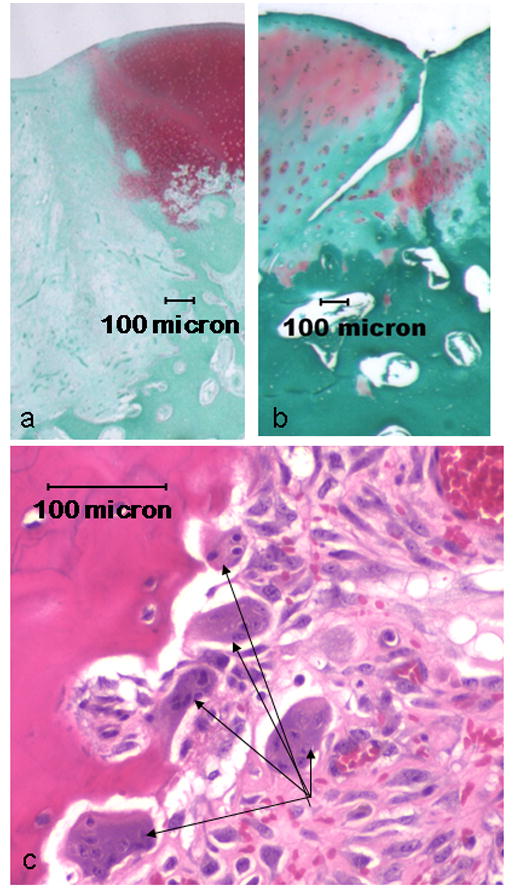
Representative microscopic views of a successful lateral integration site from an untreated animal (a) and an incomplete lateral integration site from a risedronate-treated animal (b). The integration of the specimen in (a), although good, shows a pronounced distinction between the fibrous fill tissue and the surrounding articular cartilage, while the fill tissue in (b) (from risedronate-treated animal) is normal-appearing articular hyaline cartilage. Numerous osteoclasts (arrows) were found adjacent to the deep subchondral bone “pit” deep to the defect site in the untreated animal.
For untreated animals, collagen type I was present in bone and in the fibrous fill tissue of the defect region (Fig. 3a). For risedronate-treated animals, collagen type I was present only in bone (Fig 3b). For untreated animals, collagen type II was present in the native articular cartilage but not in the defect region (Fig. 3c). For risedronate-treated animals, collagen type II was found throughout the articular cartilage, including the defect region that was filled with transplanted cartilage (Fig. 3d).
Figure 3.
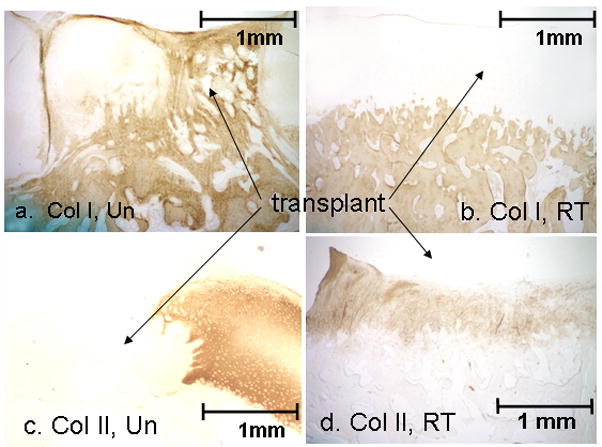
Representative microscopic sections of immunohistochemical staining for type I collagen (a, b) and type II collage (c, d) from untreated (a, c) and risedronate-treated (b, d) animals. Note the presence of type I collagen staining in the fibrous fill tissue of the untreated animal while the risedronate-treated animal displayed type II collagen in the allograft.
Discussion
In the present study we demonstrate that the use of the bisphosphonate, risedronate, inhibits the subchondral bone resorption associated with surgically created cartilage lesions and improves the success of a graft of engineered cartilaginous tissue. The graft tissue of choice was ARC tissue of an allogeneic source which: 1. serves as a clinically relevant model for cartilage grafting and, 2. has previously been characterized for cellular phenotype, cell proliferation, and biochemical constituents 13,14,19. The focus of this study, however, was not the graft tissue itself, bur rather the prevention of subchondral bone resorption associated with any grafting procedure of choice. Focal articular cartilage damage following injury is commonly seen in orthopedic practices. Because of the extremely limited ability of cartilage to repair, these lesions, which can be either partial or full-thickness, have been traditionally addressed with debridement, abrasion, or microfracture of the subchondral bone to allow for subsequent filling with fibrous tissue. More current techniques include the transplantation of chondral and osteochondral plugs into the debrided defect site. Although there have been no blinded clinical trial data, such procedures are reported to have varying levels of success, often with failed healing being associated with alterations in the subchondral bone. 4,20 Although bone stimulating techniques depend upon rejuvenating cells derived from the bone marrow for defect repair, any technique which removes the cartilage defect without penetrating the bone is dependent upon maintenance and subsequent attachment of the subchondral bone to the chondral graft.
Increased subchondral bone remodeling has also been shown in experimental models following the removal of a small segment of overlying articular cartilage in preparation for cartilage transplantation. A subchondral bone reaction, characterized by a significant loss of bone matrix and distortion of the trabecular structure extending several millimeters into the bone, has previously been reported to be associated with chondral defects and attempted repair in a goat model, 6,21 and equine model 22. The pathologic changes were suggestive of structural bone remodeling beneath the lesion. As in the goat model study of Vasara et al. 6, the current study also employed methods to avoid injury to the subchondral plate during cartilage transplant surgery. Upon creation of the defect, the deep aspect of the removed cartilage, as well as the defect site, was inspected to insure that the subchondral bone was not removed or damaged in the process. However, it is inevitable that the calcified cartilage and perhaps the most superficial aspect of the subchondral bone were damaged. Because the surgeon was blinded to the treatment, the same surgical technique was used for both treated and risedronate-untreated animals. Despite these efforts a strong subchondral reaction, substantiated by the presence of numerous osteoclasts, took place in the absence of risedronate treatment. Furthermore, the graft was unsuccessful in the untreated animals. This is not surprising as it would not be possible for a transplant to take hold while the tissue upon which it must be stabilized undergoes remodeling and resorption.
In a porcine model of ACT, Vasara et al. 7 reported a marked subchondral bone reaction in 65% of the pigs that had a 6 mm diameter cartilage lesion in the patellar groove, without penetration of the subchondral bone. The repair tissue, characterized by toluidine blue and type II collagen staining, was observed to spread downwards through the subchondral bone plate.
Although the exact biomechanical and physiologic influences of subchondral bone in relation to the integrity of the overlying articular cartilage are controversial, certainly, as a basic function, the subchondral bone provides a mechanical base upon which the overlying articular cartilage can be seated to provide a smoothly contoured and lubricated interface for the opposing cartilage surface. Additionally, there is evidence that bone remodeling does have some influence, either directly or indirectly, on joint cartilage. Examples include the inhibition of bone remodeling through the use of bisphosphonates in an attempt to improve cartilage integrity after either enzymatic treatment 10 or surgically-induced instability. 11 But at a very basic biomechanical level, an irregular subchondral plate leads to an imbalance in stress transduction in the overlying articular cartilage, thus promoting cartilage degradation. 23 Elevated bone turnover and remodeling of the subchondral bone may precede, or be concomitant with, the etiology of osteoarthritis in humans and in animal models. 24,25
That the integrity of subchondral bone is an important issue in cartilage health is substantiated by several clinical studies demonstrating that local bone loss is associated with osteoarthritis.26 The subchondral bone loss could be reduced through the use of bisphosphonate,27 but most importantly, although signs, symptoms, or progression of osteoarthritis did not improve, a reduction in the level of a marker of cartilage degradation8,27 and bone resorption 8,28 was observed.
A limitation of the present study is that the minipigs were allowed load-bearing activity immediately upon recovery from anesthesia, which is not consistent with the usual post-surgical practice in the human. This may be of significance if load-bearing increases bone remodeling immediately after disruption of the subchondral plate. However, it has already been reported that changes in the subchondral bone, as determined through magnetic resonance imaging, can occur upon matrix-associated ACT in patients; these changes were correlated to clinical outcome. 4 In addition, the inflammatory responses due to the surgical procedure, such as intra-articular bleeding and stimulation of the synovial membrane, may induce proinflammatory cytokines and result in activation of osteoclasts. This point needs to be elucidated in order to improve the efficacy of transplantation because post-traumatic and post-surgical inflammation is unavoidable.
Additionally, an experimental time of only 59 days post-implantation may not provide sufficient time to determine the full effect of bisphosphonate treatment on the integrity of the cartilage transplant and bone. It may be of interest to extend the experimental time in excess of 4–6 months post-implantation for determination of long-term effects of bisphosphonate on a model such as this. However, it might be suggested here that it is the initial post-surgical period which is most important as it is during this time that the transplant is seated and begins its integration with surrounding tissue. As we have shown in the untreated animals of the present study, if the subchondral bone is not maintained during the first couple of months or less, the transplant is inevitably lost. It is also possible that the lack of complete lateral integration we observed with a few of our allografts may improve with time during bisphosphonate treatment.
Finally, it has previously been shown by us29 and others30 that bisphosphonate treatment has a positive effect on cartilage integrity following enzyme treatment or induction of inflammatory arthritis, respectively. Thus, we must at least consider that the risedronate may have acted to improve allograft success through a mechanism other than through the maintenance of the subchondral bone alone.
In summary, the success rate of cartilage/chondrocyte transplantation in the clinic shows room for improvement, with sometimes unexplainable negative results. Here we have shown that the use of the bisphosphonate, risedronate, inhibits the subchondral bone reaction associated with cartilage transplantation surgery and augments the seating of the transplanted tissue.
Acknowledgments
This work was supported, in part, by NIH P50 AR39239, NIH PO1 AR048152 (U.A., B.F., K.M.) and NIH RO1 AR048292 (C.M., J.L.). Roger Phipps is an employee of Procter and Gamble, the source of the Risedronate used in the study.
References
- 1.Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med. 1999;18:13–44. v–vi. doi: 10.1016/s0278-5919(05)70128-9. [DOI] [PubMed] [Google Scholar]
- 2.Peterson L, Brittberg M, Kiviranta I, et al. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 3.Henderson IJ, Tuy B, Connell D, et al. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85:1060–1066. doi: 10.1302/0301-620x.85b7.13782. [DOI] [PubMed] [Google Scholar]
- 4.Marlovits S, Striessnig G, Resinger CT, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52:310–319. doi: 10.1016/j.ejrad.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Marlovits S, Singer P, Zeller P, et al. Magnetic resonance observation of cartilage repair tissue (mocart) for the evaluation of autologous chondrocyte transplantation: Determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Vasara AI, Hyttinen MM, Lammi MJ, et al. Subchondral bone reaction associated with chondral defect and attempted cartilage repair in goats. Calcif Tissue Int. 2004;74:107–114. doi: 10.1007/s00223-002-2153-8. [DOI] [PubMed] [Google Scholar]
- 7.Vasara AI, Hyttinen MM, Pulliainen O, et al. Immature porcine knee cartilage lesions show good healing with or without autologous chondrocyte transplantation. Osteoarthritis Cartilage. 2006;14:1066–1074. doi: 10.1016/j.joca.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Spector TD, Conaghan PG, Buckland-Wright JC, et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: Results of the brisk randomized, controlled trial [isrctn01928173] Arthritis Res Ther. 2005;7:R625–633. doi: 10.1186/ar1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tagil M, Aspenberg P, Astrand J. Systemic zoledronate precoating of a bone graft reduces bone resorption during remodeling. Acta Orthop. 2006;77:23–26. doi: 10.1080/17453670610045650. [DOI] [PubMed] [Google Scholar]
- 10.Muehleman C, Green J, Williams JM, et al. The effect of bone remodeling inhibition by zoledronic acid in an animal model of cartilage matrix damage. Osteoarthritis Cartilage. 2002;10:226–233. doi: 10.1053/joca.2001.0506. [DOI] [PubMed] [Google Scholar]
- 11.Hayami T, Pickarski M, Wesolowski GA, et al. The role of subchondral bone remodeling in osteoarthritis: Reduction of cartilage degeneration and prevention of osteophyte formation by alendronate in the rat anterior cruciate ligament transection model. Arthritis Rheum. 2004;50:1193–1206. doi: 10.1002/art.20124. [DOI] [PubMed] [Google Scholar]
- 12.Shelton WR, Treacy SH, Dukes AD, Bomboy AL. Use of allografts in knee reconstruction: Ii. Surgical considerations. J Am Acad Orthop Surg. 1998;6:169–175. doi: 10.5435/00124635-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Masuda K, Sah RL, Hejna MJ, Thonar EJ. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: The alginate-recovered-chondrocyte (arc) method. J Orthop Res. 2003;21:139–148. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 14.Chawla K, Klein TJ, Schumacher BL, et al. Short-term retention of labeled chondrocyte subpopulations in stratified tissue-engineered cartilaginous constructs implanted in vivo in mini-pigs. Tissue Eng. 2007;13:1525–1537. doi: 10.1089/ten.2007.0044. [DOI] [PubMed] [Google Scholar]
- 15.Masuda K, Sah RL. Tissue engineering of articular cartilage. In: Vunjak-Novakovic G, Freshney RI, editors. Culture of cells for tissue engineering. New York: John Wiley & Sons; 2006. pp. 157–189. [Google Scholar]
- 16.Rosenberg L. Chemical basis for the histological use of safranin o in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- 17.O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am. 1988;70:595–606. [PubMed] [Google Scholar]
- 18.Mainil-Varlet P, Rieser F, Grogan S, et al. Articular cartilage repair using a tissue-engineered cartilage-like implant: An animal study. Osteoarthritis Cartilage. 2001;9 (Suppl A):S6–15. doi: 10.1053/joca.2001.0438. [DOI] [PubMed] [Google Scholar]
- 19.Chawla K, Klein TJ, Schumacher BL, et al. Tracking chondrocytes and assessing their proliferation with pkh26: Effects on secretion of proteoglycan 4 (prg4) J Orthop Res. 2006;24:1499–1508. doi: 10.1002/jor.20116. [DOI] [PubMed] [Google Scholar]
- 20.Alparslan L, Winalski CS, Boutin RD, Minas T. Postoperative magnetic resonance imaging of articular cartilage repair. Semin Musculoskelet Radiol. 2001;5:345–363. doi: 10.1055/s-2001-19044. [DOI] [PubMed] [Google Scholar]
- 21.Breinan HA, Minas T, Hsu HP, et al. Autologous chondrocyte implantation in a canine model: Change in composition of reparative tissue with time. J Orthop Res. 2001;19:482–492. doi: 10.1016/S0736-0266(00)90015-9. [DOI] [PubMed] [Google Scholar]
- 22.Howard RD, McIlwraith CW, Trotter GW, et al. Long-term fate and effects of exercise on sternal cartilage autografts used for repair of large osteochondral defects in horses. Am J Vet Res. 1994;55:1158–1167. [PubMed] [Google Scholar]
- 23.Shibakawa A, Yudoh K, Masuko-Hongo K, et al. The role of subchondral bone resorption pits in osteoarthritis: Mmp production by cells derived from bone marrow. Osteoarthritis Cartilage. 2005;13:679–687. doi: 10.1016/j.joca.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Lahm A, Kreuz PC, Oberst M, et al. Subchondral and trabecular bone remodeling in canine experimental osteoarthritis. Arch Orthop Trauma Surg. 2006;126:582–587. doi: 10.1007/s00402-005-0077-2. [DOI] [PubMed] [Google Scholar]
- 25.Quasnichka HL, Anderson-MacKenzie JM, Bailey AJ. Subchondral bone and ligament changes precede cartilage degradation in guinea pig osteoarthritis. Biorheology. 2006;43:389–397. [PubMed] [Google Scholar]
- 26.Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C. Tibial cancellous bone changes in patients with knee osteoarthritis. A short-term longitudinal study using fractal signature analysis. Osteoarthritis Cartilage. 2005;13:463–470. doi: 10.1016/j.joca.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Bingham CO, 3rd, Buckland-Wright JC, Garnero P, et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: Results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54:3494–3507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- 28.Buckland-Wright JC, Messent EA, Bingham CO, 3rd, et al. A 2 yr longitudinal radiographic study examining the effect of a bisphosphonate (risedronate) upon subchondral bone loss in osteoarthritic knee patients. Rheumatology (Oxford) 2007;46:257–264. doi: 10.1093/rheumatology/kel213. [DOI] [PubMed] [Google Scholar]
- 29.Muehleman C, Green J, Williams JM, et al. The effect of bone remodeling inhibition by zoledronic acid in an animal model of cartilage matrix damage. Osteoarthritis Cartilage. 2001;10:226–233. doi: 10.1053/joca.2001.0506. [DOI] [PubMed] [Google Scholar]
- 30.Podworny NV, Kandel RA, Renlund, et al. Partial chondroprotective effect of zoledronic acid in a rabbit model of inflammatory arthritis. J Rheumatol. 1999;26:1972–1982. [PubMed] [Google Scholar]


