Abstract
The P2X7 receptor is associated with the death of many cell types, and growing evidence supports its presence on neurons. Activation of the P2X7 receptor on isolated retinal ganglion cells increases intracellular calcium levels and can kill the cells. Within the intact eye, however, glia and other cell types surrounding the ganglion cells may provide protection and attenuate the effects of receptor stimulation. This investigation thus asks whether stimulation of the P2X7 receptor can actually kill retinal ganglion cells in vivo. Drugs were injected intravitreally into the superior/nasal region of Long Evans rats. Cell survival was determined by counting the number of remaining ganglion cells labeled with aminostilbamidine. The P2X7 receptor agonist BzATP reduced ganglion cell survival as compared to eyes injected with saline solution. Ganglion cell death was inhibited by co-injection of the P2X7 antagonists Brilliant Blue G and MRS 2540. The loss of ganglion cells following activation of the P2X7 receptor was also prevented by the adenosine A3 adenosine receptor agonist MRS 3558. In conclusion, stimulation of the P2X7 receptor can kill retinal ganglion cells in vivo. The neuroprotective effects of A3 activation identified in isolated ganglion cells are also receptor apparent in vivo. This implies that the balance between extracellular ATP and its protective metabolite adenosine can influence ganglion cell survival in the living eye.
Keywords: P2X receptor, A3 adenosine receptor, purinergic signaling, retinal ganglion cells, neuroprotection, in vivo models
1. Introduction
The purines ATP and adenosine act as transmitters throughout the nervous system, with ATP stimulating metabotropic P2Y and ionotropic P2X receptors, while adenosine acts at metabotropic A1, A2A, A2B or A3 receptors (Ribeiro et al. 2002; Abbracchio et al. 2009). The dephosphorylation of ATP into adenosine by extracellular enzymes can consequently influence signaling (Zimmermann 2000; Zuo et al. 2008), and retinal ganglion cells highlight a particularly extreme example of these interactions. Previous work has demonstrated that stimulation of the P2X7 receptor killed isolated retinal ganglion cells in vitro (Zhang et al. 2005) while stimulation of the A3 adenosine receptor enhanced their survival (Zhang et al. 2006b). In these isolated ganglion cells, the lethal action of P2X7 receptor stimulation corresponded with elevations in cytoplasmic Ca2+, while A3 receptor activation reduced the Ca2+ response.
While these in vitro experiments clearly demonstrated the lethal potential of P2X7 receptors and the contrasting neuroprotective potential of adenosine A3 receptors on isolated ganglion cells, it is not currently known whether these findings can be reproduced in an intact animal. The mechanical and chemical dissociation of neuronal cells leaves them considerably more fragile than they are in their native environment. Even the removal of the inner limiting membrane may decrease ganglion cell survival (Halfter et al. 2005). Moreover, retinal ganglion cell soma lie close to Müller and astrocytic glial cells in addition to other cells that can release protective compounds (Newman 2003; Bringmann et al. 2009). It is thus possible that ganglion cells may be more resistant to stress in vivo than in vitro.
This study asked whether stimulation of the P2X7 receptor kills retinal ganglion cells in vivo as it does in vitro. In addition, the ability of the A3 adenosine receptor to prevent ganglion cell death in vivo was examined. We demonstrate that in vitro findings are relevant to the intact retina, and that the balance of extracellular purines can alter ganglion cell health in vivo.
2. Methods
2.1 Materials
MRS 3558 (1′S,2′R,3′S,4′R,5′S)-4-(2-chloro-6-(3-chlorobenzylamino)-9H-purin-9-yl)-2,3-dihydroxy-N-methylbicyclo[3.1.0]hexane-1-carboxamide) and MRS 2540 ((S)-4-(3-(4-benzoylpiperazin-1-yl)-2-(benzyloxycarbonylamino)-3-oxopropyl)-3,5-dimethylphenyl 4-methylbenzenesulfonate) were developed at the Laboratory of Bioorganic Chemistry NIDDK, National Institutes of Health (Jacobson et al. 2005; Tchilibon et al. 2005). All other materials are from Sigma Chemical Corp (St. Louis, MO), unless otherwise indicated.
2.2 Animal Handling
All procedures were performed in accordance with University of Pennsylvania IACUC approved protocols and National Institutes of Health guide for the care and use of laboratory animals. All efforts were made to minimize animal suffering, and to reduce the number of animals used. All experiments were performed on Long Evans rats. The majority of trials were performed using adolescent rats aged postnatal day (PD) 14 to PD26 from untimed pregnant rats (Harlan Laboratories Inc; experiments in Figures 1-4). At these ages, retinal ganglion cells are past the major stage of post-natal attrition, while access and manipulation are more straightforward. Results were confirmed with adults (Figure 5). Adults were mothers of pups that had been weaned; although the exact age was not provided by the vendor, mothers were reportedly shipped at 8-12 weeks and used approximately 4 weeks later, for a final age of 12-16 weeks.
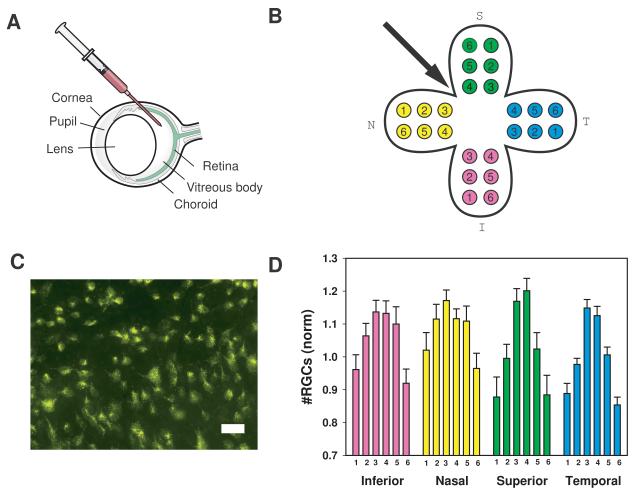
Figure 1. Intravitreal injections and ganglion cell counts.
A) Intravitreal injections were performed under an ophthalmic operating microscope with the micropipette connected to a microsyringe. The glass pipette filled with drug was passed through the sclera approximately 1 mm posterior to the limbus into the vitreous cavity. Injections were made between the nasal and superior quadrants.
B) Digital images were obtained from six areas in each of the inferior (I), nasal (N), superior (S), and temporal (T) quadrants. Regions were located circumferentially one-sixth, one half and five-sixths from the retinal center at one third and two thirds across the quadrant.
C) Representative image demonstrating labeled cells in a field. Typical images contained 35-60 cells each. Cells were labeled 2-3 days after injection and imaged 2-3 days after labeling. Bar = 30 μm.
D) The mean number of cells in each sample area in control eyes (injected with saline) from adolescent rats. Counts were normalized to the mean level of all regions within the temporal quadrant to control for variation among preparations. Here and elsewhere, bars represent mean ± SEM. The numbers along the abscissa correspond to the region numbers in B). Cell numbers were lower in the retinal periphery. n= 26.
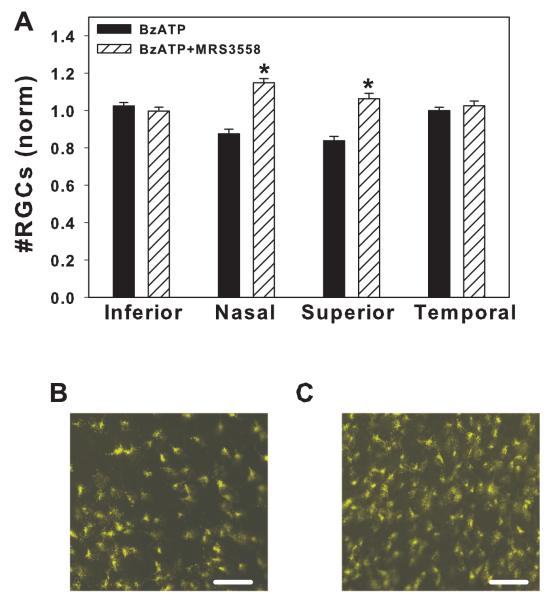
Figure 4. Adenosine A3 receptor agonist MRS 3558 prevents the lethal effect of BzATP.
A) Contralateral eyes were injected with either 250 μM BzATP or 250 μM BzATP + 10 μM A3 receptor agonist MRS3558. Co-injection of MRS3558 significantly increased the number of surviving ganglion cells in the nasal and superior quadrants (* p<0.001, paired t-test, n=48 corresponding regions from 8 adolescent rats).
B) Representative image from peripheral nasal region of eye injected with BzATP.
C) Corresponding region from contralateral eye injected with BzATP + MRS3558. In panels C and D, bar = 50 μm.
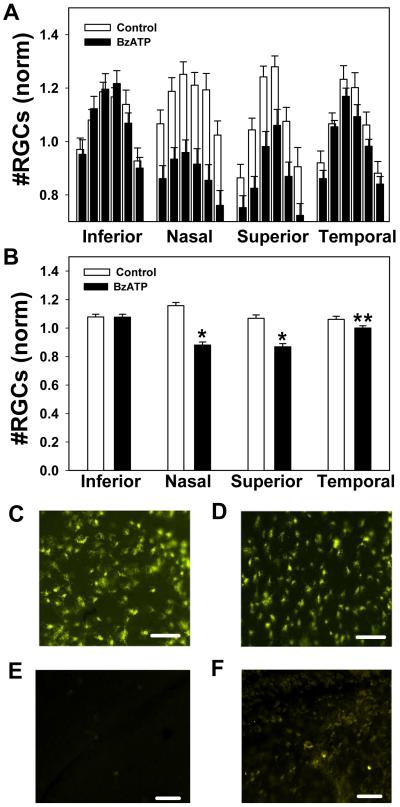
Figure 5. BzATP kills retinal ganglion cell in adult retinas.
A) The mean ganglion cell numbers in each of six regions from the inferior, nasal, superior and temporal quadrants following injection of control saline (white bars) or 250 μM BzATP (black bars) into the vitreal chamber of contralateral eyes from adult rats. Injection of BzATP reduced the number of ganglion cells, particularly in the nasal quadrant. n=6 rats.
B) Mean number of ganglion cells per quadrant following injection of control saline or 250 μM BzATP. BzATP significantly reduced the number of ganglion cells in the nasal, superior and temporal quadrants. (* p<0.05; paired t-test; n=36 corresponding regions from 6 adult rats).
C) Representative image from peripheral nasal region of adult eye injected with saline.
D) Corresponding region from contralateral eye injected with BzATP. In panels C and D, bar = 50 μm.
2.3 Intravitreal Injections
Rats were anesthetized by intraperitonial injection of ketamine/xylazine (50/5 mg/kg for adolescents, 100/10 mg/kg for adults). Micropipettes were fabricated from glass capillaries pulled on a pipette puller and beveled using a Dremel drill with a sander attachment. Injections were performed under an ophthalmic operating microscope with the micropipette connected to a microsyringe (Drummond Scientific Co., Broomall, PA). The glass pipette filled with drug was passed through the sclera at a point approximately 1 mm behind the limbus into the vitreous cavity (Figure 1A). Injections were made in a superonasal quadrant (Figure 1B). A total volume of 1μl was injected into adolescent rats and 3-5 μl into adult rats over a 20-sec time period. Sterile balanced saline solution (BSS) was injected as a control, with drugs dissolved into the saline. Animals showing signs of cataract or other damage after injection were excluded from the study, although such occurrences were rare.
2.4 Fluorescent Labeling of Retinal Ganglion Cells
Two-three days after the intravitreal injection, ganglion cells were back-labeled by the injection of FluoroGold derivative aminostilbamidine (Invitrogen Corp., Carlsbad, CA) as previously described (Zhang et al. 2005; Zhang et al. 2006a; Zhang et al. 2006b). Following anesthetization as described above, an incision exposed the skull. Two 1 mm holes were drilled on either side, 0.8 mm and 1.0 mm anterior to Bregma’s line, each 0.8 mm lateral from the midline, exposing the cortex overlying each superior colliculus. Using a Hamilton syringe affixed to a micromanipulator, a needle was inserted and a total of 2.5 μl dye was delivered to each point at a depth of 2 mm. The needle remained in place for 2 min after each injection to allow dye absorption. The wound was closed with 2-3 sutures. For the adult rats, a 3 × 4 mm patch of skull was removed anterior to the intersection of Bregma’s line and the midline. Three injections were made on each side of the midline equidistant from each other, avoiding blood vessels. Each injection of 2.3 μl was given at a depth of 3.5 mm, with a 2 min delay before retraction. Examination of labeled retinal whole mounts confirmed that this procedure led to an even distribution of dye across the retina.
2.5 Retinal Ganglion Cell Counts
Rats were sacrificed with CO2 or overdose of ketamine/xylazine 2-3 days after labeling. This allowed sufficient time for the retrograde transport of dye from the superior colliculus to the retinal ganglion cells. Eyes were enucleated and a mark was made in the nasal position to identify ocular orientation. The eyes were fixed for 45 min with 4% paraformaldehyde and the retina was removed by dissection. The excised retina was flat-mounted on a microscope slide and fluorescent cells excited at 360 ± 20 nm and emitting >515 nm were detected with a Nikon Eclipse E600 microscope (Nikon USA Inc., Melville, NY). The superior, nasal, inferior and temporal quadrants were inspected, and six areas per retinal quadrant were examined with a x40 objective. The regions were located circumferentially one-sixth, one half and five-sixths from the retinal center, as indicated in Figure 1B. The areas were digitally photographed with a 3-CCD digital camera (Toshiba America Inc., New York, NY) or a Retiga-2000R digital camera (QImaging Inc., Surrey, B.C. Canada) and analyzed in a masked fashion using Image Pro Plus software (Media Cybernetics Inc., Silver Spring, MD). The different cameras led to slight differences in the color of the images but did not alter results.
2.6 Annexin V staining
BzATP (250 μM) was injected into the nasal/superior region of one eye, with BSS injected into the corresponding region of the contralateral eye as described above. Twenty four hours later, 3 μl of FITC-conjugated annexin-V (Invitrogen, Inc) was injected into nasal-superior region of the vitreous cavity of anesthetized rats. The animals were allowed to recover for 1 hour, sacrificed, and the eyes removed. The whole eyes were fixed for 30 min in 4% paraformaldehyde after which the retina was dissected out. Retinas were whole mounted on microscope slides and FITC was imaged with a Retiga-2000R digital camera, at 488 nm excitation and > 520 nm emission.
2.7 Data Analysis
All data are expressed as mean ± SEM. Significance was determined using Sigmastat software (Systat Software Inc., San Jose, CA) with appropriate tests as indicated. Ganglion cells counts were normalized to the mean number in the temporal region of rats injected with BzATP. The BzATP response was chosen because it was the common treatment given to binocular animals injected with either BSS vs. BzATP or BzATP vs. BzATP plus modifying agents. Care was taken to ensure that BzATP was effective in all trials. In particular, experiments were designed so that some rats from a litter received BzATP in one eye and saline in another, while other received BzATP in one eye and BzATP + blocked. This was to ensure that BzATP was effective in all cases. The BzATP vs. BSS results were used for the data in Figure 2. Statistical comparisons between treatments were performed by normalizing each measurement to the mean temporal BzATP value, taking the mean for each quadrant and comparing matched regions from each animal with a paired Student’s t-test. Normalization was performed because the absolute numbers varied considerable with preparation. For example, counts in the central area of the temporal region from rats injected with control saline ranged from 31 to 66. This corresponds to 518-1102 cells/mm2. There are likely several causes of this variation including differences in labeling strength, operator variation, and actual differences in cell number between animals. While each source of variation may be minor, they combine to create a considerable spread. As such, it was necessary to normalize data from multiple trials before combining, and not express them as RGC/mm2, All data with p<0.05 were defined as significantly different.
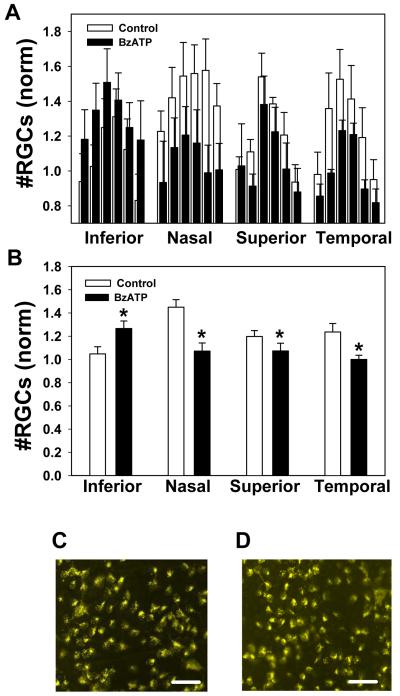
Figure 2. The P2X7R agonist BzATP kills retinal ganglion cells in vivo.
A) The mean ganglion cell numbers in each of six regions from the inferior, nasal, superior and temporal quadrants following injection of control saline (white bars) or 250 μM BzATP (black bars) into the vitreal chamber of contralateral eyes. Injection of BzATP reduced the number of ganglion cells detected. The decline was greatest in the nasal and superior quadrants closest to the injection site, with the proportional loss similar across peripheral, middle and central regions. Bars have been slightly offset to indicate all results. n=27 adolescent rats.
B) Mean number of ganglion cells per quadrant following injection of control saline or 250 μM BzATP. BzATP significantly reduced the number of ganglion cells in the nasal, superior and temporal quadrants (* p<0.001, ** p<0.01; paired t-test; n=162 corresponding regions from 27 from adolescent rats). In both panels A and B, counts were normalized to mean levels in the temporal quadrant of the eye injected with BzATP from each pair.
C) Representative image from peripheral nasal region of eye injected with saline.
D) Corresponding region from contralateral eye injected with BzATP.
E) The nasal/superior regions of a retina injected with saline and then stained with annexin V 24 hrs later. Minimal staining was detected
F) The nasal/superior regions of the contralateral retina injected with 250 μM BzATP and then stained with annexin V 24 hrs later. Clear staining for annexin V was detected throughout the nasal/superior region, although little was observed in other retinal areas. In panels C-F, bars = 50 μm.
3. Results
3.1 Ganglion Cell Distribution in Control Retinas
Initial experiments were performed to determine the number of ganglion cells in control eyes of adolescent rats PD14 to 26. Sterile saline was injected into the region between the nasal and superior quadrants, and 2-3 days later the fluorescent label aminostilbamidine was injected into the superior colliculus. After allowing several days for retrograde transport of the dye along axons into the ganglion cell soma, the retina was removed and inspected. Fluorescent ganglion cells were clearly visible throughout the retina (Figure 1C). The number of fluorescent ganglion cells was determined for each of 24 regions as indicated in Figure 1B.
Analysis demonstrated a consistent pattern of ganglion cell distribution across control saline-injected retinas (Figure 1D). Peripheral retinal regions # 1 and 6 (see Figure 1B) had fewer cells than central regions # 3 and 4. Regions in between (#s 2 and 5) had an intermediate number of cells. This pattern was generally well maintained across all 4 quadrants. The reproducibility of the pattern suggested this model was sufficiently robust to determine the effects of potentially neurotoxic compounds.
3.2 P2X7 Receptor Agonist Kills Ganglion Cells in vivo
The P2X7 receptor agonist BzATP was injected into the vitreal chamber of rats and the number of fluorescent ganglion cells was quantified to determine whether the compound was toxic in vivo as well as in vitro. Previous trials indicated that BzATP kills ganglion cells with an EC50 of 30 μM when applied to isolated cells in a dish, with most trials examining the lethal effect of 50 μM (Zhang et al. 2005). Initial in vivo experiments indicated that injection of 50 μM BzATP had a small effect on cell survival but results were variable (data not shown). As this level was close to the EC50, and as the drug is likely to be diluted within the vitreal space, the concentration of BzATP was increased 5-fold to 250 μM.
Injection of 250 μM BzATP led to a consistent reduction in the number of ganglion cells in the retinas of adolescent rats (Figure 2A). Of interest, ganglion cell loss was largely confined to the nasal and superior regions of the retina. This implies that the distribution of BzATP through the vitreal cavity to the retina was restricted. The neuronal loss was approximately equal in central, middle and peripheral regions of the nasal and superior regions. Some variation did occur, possibly arising from small fluctuations in the needle placement in addition to any altered susceptibility of individual rats.
To determine whether the decrease in ganglion cell number that accompanied injection of BzATP was statistically significant, the mean cell number from each quadrant was determined, and counts from each region were compared with a paired test to control for gradations towards the retinal periphery. There was a substantial loss of ganglion cells in the nasal and superior regions of 28% and 20%, respectively (Figure 2B). A small but significant loss also occurred in the temporal region. Representative images of the peripheral nasal regions of retina from eyes injected with BSS or BzATP are presented in Figures 2C and D.
While the reduction in the number of labeled ganglion cells near the injection site suggested cells were being killed, this was confirmed by staining the retina with annexin V conjugated to FITC. Annexin V binds to phosphatidylserine, and the movement of phosphatidylserine from the inner to outer layer of the membrane bilayer is an early sign of apoptosis (Van Engeland et al. 1997). An increase in annexin V staining has previously been associated with glaucomatous loss of ganglion cells in vivo (Guo et al. 2005). In the present study, injection of BzATP led to an increased staining for annexin V (Figure 2E-F). This increased staining was not observed following the injection of BSS, and was greatest in the nasal/superior region. The overlap between the region where ganglion cells were lost and the region highest in staining by annexin V makes it highly likely that the loss of ganglion cells is correlated with apoptotic death, as found in vitro (Zhang et al., 2005).
3.3 P2X7 Receptor Antagonists Stop Ganglion Cell Loss
While BzATP is an agonist at the P2X7 receptor, it is not specific and can also act at other P2X and P2Y receptors (Bianchi et al. 1999; Communi et al. 1999; Carrasquero et al. 2009). To confirm that stimulation of the P2X7 receptor was responsible for the death of retinal ganglion cells, the ability of P2X7 receptor antagonists to protect against cell death was examined. In order to avoid the need for multiple intravitreal injections within a given eye, BzATP was injected into one eye, and BzATP along with antagonist was given to the contralateral eye.
The number of ganglion cells in the nasal and superior quadrants was significantly higher when P2X7 antagonist Brilliant Blue G (BBG) was coinjected than with BzATP alone (Figure 3A). Cell numbers were increased by 19% and 16% in the nasal and superior quadrants, respectively, when both agonist and antagonist were injected concurrently into the eye of adolescent rats.
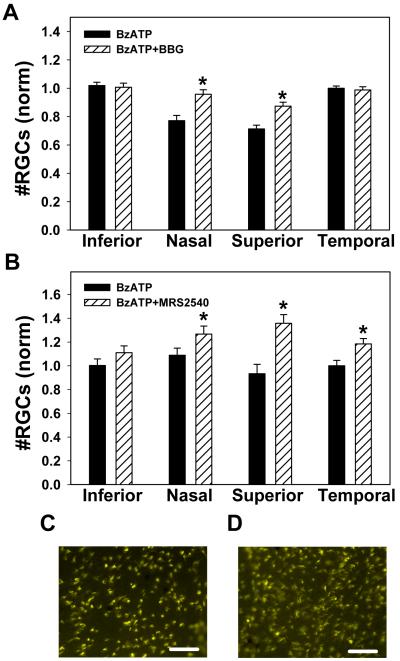
Figure 3. P2X7R antagonists block the lethal effect of BzATP.
A) Contralateral eyes were injected with either 250 μM BzATP or 250 μM BzATP + 10 mM P2X7 receptor antagonist BBG. Co-injection of BBG significantly increased the number of surviving ganglion cells in the nasal and superior quadrants (* p<0.001, paired t-test, n=48 regions from 8 from adolescent rats). The concentration of antagonist was increased as it was injected simultaneously with the BzATP agonist.
B) Co-injection of 250 μM BzATP with 10 μM of the P2X7 receptor antagonist MRS 2540 also significantly increased the number of surviving ganglion cells as compared to injection of BzATP alone (* p<0.01, paired t-test, n=18 corresponding regions from 3 from adolescent rats).
C) Representative image from peripheral nasal region of eye injected with BzATP.
D) Corresponding region from contralateral eye injected with BzATP + MRS2540. In panels C and D, bar = 50 μm.
To support a role for the P2X7 receptor in vivo, the novel antagonist (S)-4-(3-(4-benzoylpiperazin-1-yl)-2-(benzyloxycarbonylamino)-3-oxopropyl)-3,5-dimethylphenyl 4-methylbenzenesulfonate (MRS 2540) was coinjected with BzATP. MRS 2540 significantly increased the number of surviving ganglion cells by 18%, 43% and 18% in the nasal, superior and temporal regions, respectively (Figure 3B). Neither MRS 2540 nor BBG increased ganglion cell number when injected alone in the absence of BzATP, implying it was the antagonism of the P2X7 receptor that prevented cell loss. Separate pairs within the same trial confirmed that injection of BzATP reduced cell number in the nasal and superior regions as compared to injection of saline control in sets of experiments with the antagonists.
3.4 Protective Actions of Adenosine A3 Receptor
Extracellular ATP can be rapidly dephosphorylated to adenosine, which in turn can stimulate its own receptors. In isolated ganglion cells, stimulation of the adenosine A3 receptor reduced the Ca2+ rise and cell death triggered by P2X7 receptor activation (Zhang et al. 2006b). To determine whether this protection occurred in vivo, BzATP was injected into one eye and a combination of BzATP and the highly selective A3 adenosine receptor agonist MRS 3558 was injected into the contralateral eye. In the presence of MRS 3558, the number of retinal ganglion cells was significantly increased in the nasal and superior quadrants by 27% and 22%, respectively (Figure 4). This increase in ganglion cell survival suggests that stimulation of the A3 receptor can protect against the neurotoxic actions of the P2X7 receptor in vivo as they do in vitro. Again, separate pairs within the same trial confirmed that injection of BzATP reduced cell number in the nasal and superior regions as compared to injection of saline control.
3.5 P2X7 Receptor Kills Ganglion Cells in Adult Retina
The experiments above were performed on adolescent animals for a variety of reasons. The placement of the needle delivering drugs was more reliable in adolescent eyes, while the use of siblings also enhanced reproducibility. The developmental culling of ganglion cells is largely complete by PD10 (Provis and Penfold 1988), and the pressure-dependent damage to retinal ganglion cells mediated by the P2X7 receptor was similar at PD14 and PD25, when cells are functionally mature (Resta et al. 2007). The activity of sodium channels does change over this period, however (Wang et al. 1997). It was thus important to determine whether the loss of ganglion cells was influenced by developmental age.
The proportion of ganglion cells killed by BzATP in the nasal and superior region was not influenced by the age of the animals over the range examined (PD14-32, n=27, r2 0.11), suggesting that the loss was not developmentally related. To confirm this, the effect of BzATP was examined in adult rats. Injection of BzATP into the vitreous of adult rats significantly reduced the number of ganglion cells. (Figure 5A). This reduction was greatest in the nasal quadrant, with 38% fewer cells, although cell number was also less in superior and temporal quadrants (Figure 5B). The percentage of cells killed by BzATP was not significantly different between adult and adolescent rats (p>0.25). These findings confirm that BzATP can kill retinal ganglion cells from adult rats.
4. Discussion
This study has demonstrated that in vivo injection of the P2X7 receptor agonist BzATP into the vitreal chamber reduces ganglion cell number, and that cell loss could be prevented by P2X7 receptor antagonists. In addition, ganglion cell loss was prevented by co-stimulation of an A3 adenosine receptor. On this basis, we conclude that the neurochemical pathways elucidated on isolated ganglion cells in vitro also function in vivo. In particular, these findings suggest that the balance between extracellular ATP and adenosine may influence the health of retinal ganglion cells.
Several technical points are worth consideration. Analysis of 6 regions from each retinal quadrant provided a clear assessment of the distribution of cell loss. This is twice the number of regions typically analyzed with this technique, but the increased effort provided greater spatial resolution. The concentration of cell loss in nasal and superior quadrants suggested that the distribution of the agonist may have a restricted its impact, as the areas of greatest ganglion cell loss were closest to the injection site.
In this study, the number of ganglion cells was determined by quantifying the fluorescent cells in retinas from animals where aminostilbamidine was injected into the superior colliculus and transported along the axons into the soma. As such, changes in the rate of axonal transport could have contributed to the differential staining seen in eyes treated with BzATP. Although the simplest explanation for the findings is that overstimulation of the P2X7 receptors on the ganglion cell membrane within the retina kills the cells, effects of P2X7 receptor stimulation on the transport of aminostilbamidine along the axon cannot be ruled out. However, the ability of P2X7 receptors to reduce the number of fluorescent cells and of the adenosine A3 receptor to prevent this reduction is of interest regardless of whether the ultimate cause is neuronal death, impaired axonal transport, or a combination of both factors. In addition, the regionally constricted increase in staining with FITC-conjugated annexin V suggests the reduction in labeled ganglion cells may well be associated with enhanced apoptosis, as found in vitro (Zhang et al., 2005).
The number of ganglion cells killed after injection of BzATP was less than the loss found in vitro. In the adolescent rats, loss was generally 20-28%, and in adults it reached only 38%. In contrast, in vitro exposure of dissociated retinal cells to BzATP for 24 hrs led to a loss of half the total ganglion cells (Zhang et al. 2005). This increased survival in vivo may be due to enhanced protection by nearby Müller cells. However, the final concentration reaching the ganglion cells in vivo is unknown and may also have affected the result. While BzATP is generally considered stable, it can be converted into Bz-adenosine by ecto-enzymes (Kukley et al. 2004), so the decreased lethality in vivo may also reflect dephosphorylation of the agonist. It is worth emphasizing that even in vitro, stimulation of the P2X7 receptor never killed much more than 50% of the ganglion cells, implying that only a particular subset of ganglion cells may be susceptible.
While BzATP is not considered specific for the P2X7 receptor, multiple lines of evidence supported the idea that BzATP acted at P2X7 receptor in vitro, including increased efficacy of BzATP over ATP, enhanced response in the absence of Mg2+, and block by low levels of BBG (Zhang et al. 2005). The ability of both BBG and MRS 2540 to prevent the actions of BzATP in vivo also implicate the P2X7 receptors. Although BBG blocks P2X7 receptors at a lower concentration than P2X5 receptors (Bianchi et al. 1999; Bo et al. 2003), the final concentration of BBG after dilution through the vitreous to the ganglion cells cannot be determined precisely. However, the ability of relatively low levels of MRS 2540 to inhibit cell loss supports the theory that BzATP is acting at the P2X7 receptor. MRS 2540 is based upon the structure of KN-62 and inhibited the human P2X7-expressing HEK cells with an IC50 of 219 ± 69 nM (Lee et al. 2008; Lee et al. 2009). This present study is the first indication that MRS 2540 acts at rodent neuronal P2X7 receptors.
Although the in vivo retinal model used here has an obvious enhanced relevance over an isolated neuronal preparation, multiple retinal cell types express the P2X7 receptor (Pannicke et al. 2000; Puthussery et al. 2006). It is thus not possible to determine whether the death of ganglion cells in this study directly caused by P2X7 receptors on the membrane of ganglion cells or indirectly by receptors on other cell types. The previous demonstrations that BzATP elevates Ca2+ in immunopurified isolated ganglion cells, and that stimulation of the A3 adenosine receptor attenuates this rise (Zhang et al. 2005; Zhang et al. 2006b) implies that ganglion cells are themselves at least capable of the neurochemical interactions observed in vitro. While it is likely that the processes observed in isolated cells are responsible for the parallel responses seen in vivo, contributions from other retinal cells cannot be ruled out. In this regard, the A3 adenosine receptor agonist MRS 3558 was recently shown to prevent the Ca2+ rise induced by NMDA in isolated ganglion cells (Zhang et al. 2010), suggesting complex interactions may indeed take place.
Physiological Implications
The literature is replete with lists of compounds which kill retinal ganglion cells when injected into the eye, and the identification of yet another such compound is not in itself of particular interest. However, the endogenous agonist for the P2X7 receptor is extracellular ATP, and accumulating evidence suggests excess extracellular ATP is released in response to an elevated intraocular pressure. Patients with acute glaucoma had 9-fold more ATP in the aqueous humor (Zhang et al. 2007). Increased ocular pressure led to ATP release from the bovine retina (Reigada et al. 2008), and preliminary findings suggest that ATP may be elevated in chronic models of glaucoma (Lu et al. 2008). Of particular relevance, the study by Resta et al. (2007) demonstrated that transient elevation of pressure increased ATP levels in rat eyes and damaged ganglion cells through P2X7 receptor stimulation.
Whether moderate elevations in IOP are able to trigger the release of sufficient ATP to stimulate P2X7 receptors in vivo is not currently known. Müller cells posses NTPDases and other extracellular enzymes capable of degrading extracellular ATP (Iandiev et al. 2007; Ricatti et al. 2009), and the ATP surrounding ganglion cell soma can be dephosphorylated into adenosine to reduce neuronal excitability (Newman 2001; Newman 2003). It should be stressed that BzATP was used to stimulate the P2X7 receptor in this study rather than the endogenous agonist ATP because we had previously demonstrated that addition of ATP was protective following extracellular dephosphorylation into adenosine (Zhang et al. 2006b). The P2X7 receptor channel requires a relatively high concentration of ATP to open, and thus the kinetics of release and the proximity of the release site is likely to determine whether ATP levels rise sufficiently after pressure rises to stimulate the receptor. While the abundance of P2X7 receptors on the ganglion cells implies they can be stimulated endogenously, the details of such activation remain to be determined. If overstimulation of P2X7 receptors is shown to damage ganglion cells in glaucoma, the protective actions of BBG, MRS 2540 and MRS 3558 may be of interest. In this regard, it is worth noting that BBG has been injected into the vitreous to enhance visualization of the inner limiting membrane with no contraindications in humans or rats (Enaida et al. 2006; Remy et al. 2008).
Preliminary versions of some of these data have been presented in abstract form (Hu et al. 2008).
Acknowledgements
This work is supported by grants from the NIH EY015537 and EY013434 (CHM), Vision Research Core Grant EY001583 (CHM and AML), Research to Prevent Blindness (AML), the Paul and Evanina Bell Mackall Foundation Trust (AML), the Jody Sack Fund (HH, MZ and XZ), the National Natural Science Foundation of China 30872831(XZ), by support from the Intramural Research Program of NIDDK, NIH, Bethesda, MD (KAJ), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (YCK: grant number 2009-0074289). The authors would like to thank Mary Leonard and Gabriel Baltazar for help with the illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Of Note: The University of Pennsylvania owns a patent on some of the concepts presented here on which C.H. Mitchell and A.M. Laties are listed as inventors.
References
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Tr Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Bianchi BR, Lynch KJ, Touma E, Niforatos W, Burgard EC, Alexander KM, Park HS, Yu H, Metzger R, Kowaluk E, Jarvis MF, Van Biesen T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- Bo X, Jiang LH, Wilson HL, Kim M, Burnstock G, Surprenant A, North RA. Pharmacological and biophysical properties of the human P2X5 receptor. Mol Pharmacol. 2003;63:1407–1416. doi: 10.1124/mol.63.6.1407. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Müller cell gliosis: Neuroprotective and detrimental effects. Prog Ret Eye Res. 2009;28:423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Carrasquero LMG, Delicado EG, Bustillo D, Gutiérrez-Martín Y, Artalejo AR, Miras-Portugal MT. P2X7 and P2Y13 purinergic receptors mediate intracellular calcium responses to BzATP in rat cerebellar astrocytes. J Neurochem. 2009;110:879–889. doi: 10.1111/j.1471-4159.2009.06179.x. [DOI] [PubMed] [Google Scholar]
- Communi D, Robaye B, Boeynaems J-M. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enaida H, Hisatomi T, Goto Y, Hata Y, Ueno A, Miura M, Kubota T, Ishibashi T. Preclinical investigation of internal limiting membrane staining and peeling using intravitreal brilliant blue G. Retina. 2006;26:623–630. doi: 10.1097/01.iae.0000236470.71443.7c. [DOI] [PubMed] [Google Scholar]
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma Is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Willem M, Mayer U. Basement membrane-dependent survival of retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2005;46:1000–1009. doi: 10.1167/iovs.04-1185. [DOI] [PubMed] [Google Scholar]
- Hu H, Lu W, Laties AM, Mitchell CH. Stimulation of P2X7 receptor kills rat retinal ganglion cells in vivo. Invest. Ophthalmol. Vis. Sci. 2008;49 ARVO E abstract:2063. [Google Scholar]
- Iandiev I, Wurm A, Pannicke T, Wiedemann P, Reichenbach A, Robson SC, Zimmermann H, Bringmann A. Ectonucleotidases in Muller glial cells of the rodent retina: Involvement in inhibition of osmotic cell swelling. Puriner Signal. 2007;3:423–433. doi: 10.1007/s11302-007-9061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao Z-G, Tchilibon S, Duong HT, Joshi BV, Sonin D, Liang BT. Semirational design of (north)-methanocarba nucleosides as dual acting A1 and A3 adenosine receptor agonists: Novel prototypes for cardioprotection. J Med Chem. 2005;48:8103–8107. doi: 10.1021/jm050726b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Stausberg P, Adelmann G, Chessell IP, Dietrich D. Ecto-Nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2′-3′-O-(4-benzoylbenzoyl)-ATP. J. Neurosci. 2004;24:7128–7139. doi: 10.1523/JNEUROSCI.2093-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GE, Joshi BV, Chen W, Jeong LS, Moon HR, Jacobson KA, Kim Y-C. Synthesis and structure-activity relationship studies of tyrosine-based antagonists at the human P2X7 receptor. Bioorgan Med Chem Letts. 2008;18:571–575. doi: 10.1016/j.bmcl.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GE, Joshi BV, Chen W, Jeong LS, Moon HR, Jacobson KA, Kim Y-C. Corrigendum to “Synthesis and structure-activity relationship studies of tyrosine-based antagonists at the human P2X7 receptor”. Bioorgan Med Chem Letts. 2009;19:556. doi: 10.1016/j.bmcl.2007.11.077. Bioorg. Med. Chem. Lett. 18 (2008) 571-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Hu H, Laties AM, Sevigney J, Mitchell CH. Upregulation of retinal NTPDase 1 and vitreal ATP levels in an experimental rat glaucoma model. Invest. Ophthalmol. Vis. Sci. 2008;49 ARVO E abstract:869. [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannicke T, Fischer W, Biedermann B, Schädlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A. P2X7 receptors in Muller glial cells from the human retina. J Neurosci. 2000;20:5965–5972. doi: 10.1523/JNEUROSCI.20-16-05965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provis JM, Penfold PL. Cell death and the elimination of retinal axons during development. Prog Neurobiol. 1988;31:331–347. doi: 10.1016/0301-0082(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Yee P, Vingrys AJ, Fletcher EL. Evidence for the involvement of purinergic P2X7 receptors in outer retinal processing. Eur J Neurosci. 2006;24:7–19. doi: 10.1111/j.1460-9568.2006.04895.x. [DOI] [PubMed] [Google Scholar]
- Reigada D, Lu W, Zhang M, Mitchell CH. Elevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannels. Neurosci. 2008;157:396–404. doi: 10.1016/j.neuroscience.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy M, Thaler S, Schumann R, May C, Fiedorowicz M, Schuettauf F, Grüterich M, Priglinger S, Nentwich M, Kampik A, Haritoglou C. An in vivo evaluation of Brilliant Blue G in animals and humans. Br J Ophthalmol. 2008;92:1142–1147. doi: 10.1136/bjo.2008.138164. [DOI] [PubMed] [Google Scholar]
- Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;25:2741–2754. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM, de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2002;68:377–392. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Ricatti MJ, Alfie LD, Lavoie ÃG, Sevigny J, Schwarzbaum PJ. Faillace MP Immunocytochemical localization of NTPDases1 and 2 in the neural retina of mouse and zebrafish. Synapse. 2009;63:291–307. doi: 10.1002/syn.20605. [DOI] [PubMed] [Google Scholar]
- Tchilibon S, Joshi BV, Kim SK, Duong HT, Gao ZG, Jacobson KA. (N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists. J Med Chem. 2005;48:1745–1758. doi: 10.1021/jm049580r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Engeland M, Kuijpers HJH, Ramaekers FCS, Reutelingsperger CPM, Schutte B. Plasma membrane alterations and cytoskeletal changes in apoptosis. Exp Cell Res. 1997;235:421–430. doi: 10.1006/excr.1997.3738. [DOI] [PubMed] [Google Scholar]
- Wang G-Y, Ratto G-M, Bisti S, Chalupa LM. Functional development of intrinsic properties in ganglion cells of the mammalian retina. J Neurophysiol. 1997;78:2895–2903. doi: 10.1152/jn.1997.78.6.2895. [DOI] [PubMed] [Google Scholar]
- Zhang M, Budak MT, Lu W, Khurana TS, Zhang X, Laties AM, Mitchell CH. Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol Vis. 2006a;12:937–948. [PubMed] [Google Scholar]
- Zhang M, Hu H-L, Zhang X, Lu W, Lim J, Eysteinsson T, Jacobson KA, Laties AM, Mitchell CH. The A3 adenosine receptor attenuates the calcium rise triggered by NMDA receptors in retinal ganglion cells. Neurochem Int. 2010;56:35–41. doi: 10.1016/j.neuint.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li A, Ge J, Reigada D, Laties AM, Mitchell CH. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007;85:637–643. doi: 10.1016/j.exer.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH. Balance of purines may determine life or death of retinal ganglion cells as A3 adenosine receptors prevent loss following P2X7 receptor stimulation. J Neurochem. 2006b;98:566–575. doi: 10.1111/j.1471-4159.2006.03900.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- Zuo P, Picher M, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC. Mathematical model of nucleotide regulation on airway epithelia: IImplications for airway homeostasis. J. Biol. Chem. 2008;283:26805–26819. doi: 10.1074/jbc.M801516200. [DOI] [PMC free article] [PubMed] [Google Scholar]