Abstract
The prefrontal cortex (PFC) mediates a range of higher order ‘executive functions’ that subserve the selection and processing of information in such a way that behavior can be planned, controlled and directed according to shifting environmental demands. Impairment of executive functions typifies many forms of psychopathology, including schizophrenia, mood and anxiety disorders and addiction, that are often associated with a history of trauma and stress. Recent research in animal models demonstrates that exposure to even brief periods of intense stress is sufficient to cause significant structural remodeling of the principle projection neurons within the rodent PFC. In parallel, there is growing evidence that stress-induced alterations in PFC neuronal morphology are associated with deficits in rodent executive functions such as working memory, attentional set-shifting and cognitive flexibility, as well as emotional dysregulation in the form of impaired fear extinction. Although the molecular basis of stress-induced changes in PFC morphology and function are only now being elucidated, an understanding of these mechanisms could provide important insight into the pathophysiology of executive dysfunction in neuropsychiatric disease and foster improved strategies for treatment.
Keywords: Prefrontal cortex, Infralimbic, Prelimbic, Orbitofrontal, Stress, Neuron, Dendrite, Executive function, Working memory, Cognitive flexibility, Attentional set-shifting, Glucocorticoid, Mouse, Rat
1. Introduction
The prefrontal cortex (PFC) plays an integral role in mediating a range of executive functions that subserve the selection and processing of information necessary to plan, control and direct behavior in a manner appropriate to current environmental demands (Bush et al., 2000; Goldman-Rakic, 1996; Miller and Cohen, 2001; Robbins, 2005; Rolls, 1996; Tremblay and Schultz, 1999). A growing literature from studies in laboratory animals demonstrates that the PFC not only plays a major role in orchestrating the behavioral and systemic response to stress, but that neurons in the rodent PFC are highly sensitive to stress and undergo significant remodeling following stress exposure. These findings support the notion that stress-induced alterations in PFC function represent a principle neural insult underlying deficits in executive function observed in stressed rodents, and the executive component of many neuropsychiatric diseases.
In this article, we review this emerging field of research. We begin with a note on the anatomy and connectivity of the rodent PFC and current views about its functional homology with the corresponding anatomical region’s in the primate brain. We then describe evidence demonstrating the important role of the PFC in regulating rodent neuroendocrine and autonomic responses to stress, and modulating anxiety- and depression-related behaviors. Next, we turn to the intriguing finding that the morphology of rodent PFC neurons is highly sensitive to stress and speculate on how this might impact PFC functions. Finally, we address how such stress-induced changes might manifest in terms of impairment of three forms of rodent behavior related to executive function (working memory, cognitive flexibility and fear extinction).
2. Anatomy and connectivity of the rodent PFC
The rodent provides an invaluable model system for studying neural processes underlying complex behaviors including higher order cognitive and executive functions. However, given the evolutionary differentiation of the primate and rodent PFC, a discussion of the utility of rodent models for studying the PFC must first acknowledge the issue of the cross-species functional and anatomical homology of this region. On the basis of criteria including granular cytoarchitecture and connectivity with the mediodorsal thalamus, there is general agreement that rodents have a frontal region that is an anatomical representation of the primate PFC (Divac et al., 1993; Groenewegen, 1988; Leonard, 1969; Uylings et al., 2003; van Eden et al., 1990). The degree of functional homology is more difficult to establish, however, and as a result has been more controversial. Consistent with the evolution of the dorsolateral (dl) division of the PFC from motor cortex and its close anatomical connections with striatum, human neuroimaging studies suggest a role for the dlPFC in directing ‘cognitive actions’ (Fuster, 2000; Wood and Grafman, 2003). In contrast, the ventromedial portion of human PFC is more tightly coupled to limbic regions and regulates emotion and responses to reward (Bechara, 2005; Everitt and Robbins, 2005; Hyman, 2005; Ressler and Mayberg, 2007). While the primate dlPFC does not have a direct anatomical homologue in the rodent, functions of the dlPFC and vmPFC are thought to be integrated within the phylogenetically ancient medial PFC (mPFC) (Conde et al., 1995; Heidbreder and Groenewegen, 2003; Preuss, 1995; Uylings et al., 2003; Uylings and van Eden, 1990; Vertes, 2002). Furthermore, the orbital division of the rodent PFC (OFC) appears to have functional homology with the primate orbital PFC (Floyd et al., 2001; Uylings et al., 2003).
The mPFC and OFC together encompass quite a large area of the rodent forebrain. This area is anterior to the genu of the corpus callosum and extends rostrally as far as the olfactory bulbs. These areas are further subdivided into different subregions (Paxinos and Franklin, 2001) (Fig. 1). The mPFC is comprised of the anterior cingulate (AC), prelimbic (PL), and infralimbic (IL) cortices, while the OFC is made up of the medial (MO), ventral (VO) and lateral orbital (LO) subregions. We will attempt to refer to specific subregions in cases where they were specifically studied and this is made clear in the primary source. However, there are many instances, especially in the older literature, where studies use the more generic terms ‘mPFC’ or ‘OFC’ and in these cases our description remains faithful to the original citation.
Fig. 1.
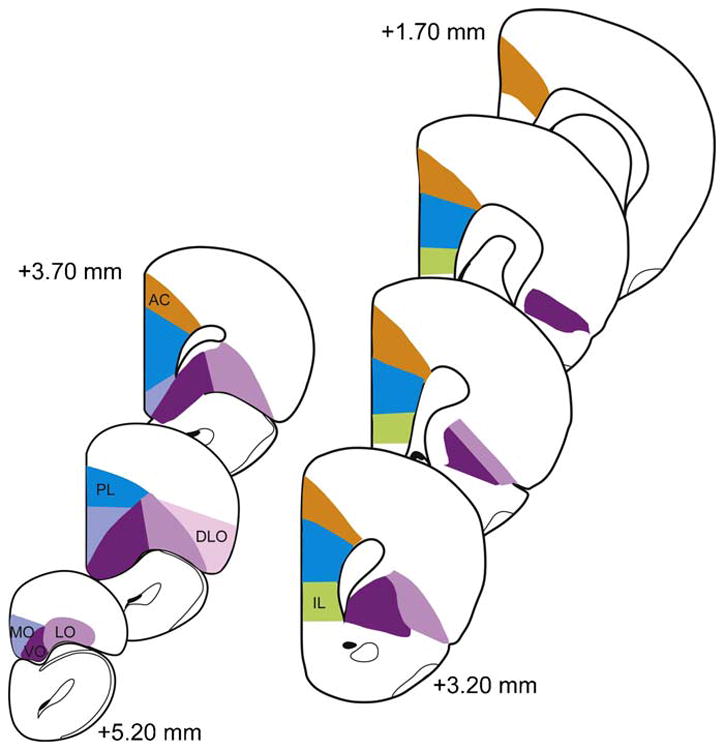
Schematic diagram of coronal sections through PFC, with major subdivisions of rodent PFC orchestrating stress responses and mediating executive function identified. Coordinates given are relative to Bregma in mouse brain. AC = anterior cingulate; PL = prelimbic; IL = infralimbic; MO = medial orbitofrontal; VO = ventral orbitofrontal; LO = Lateral Orbitofrontal; DLO = dorsolateral orbitofrontal (adapted from Paxinos and Franklin, 2001).
The majority of neural connections within the PFC are between layers and subregions – reflecting a high degree of intrinsic activity and regulation within PFC (Jones et al., 2005). The anatomical connectivity of the PFC with the rest of the brain makes it ideally positioned to orchestrate higher order behavioral functions. We will not attempt to describe this extensive network in detail (for primary references, see (Bacon et al., 1996; Brog et al., 1993; Carr and Sesack, 1996; Cassell and Wright, 1986; Conde et al., 1995; Degenetais et al., 2003; Ferino et al., 1987; Fisk and Wyss, 2000; Gabbott et al., 2002, 2005; Hurley et al., 1991; Jankowski and Sesack, 2004; Jay et al., 1989, 1992; Jay and Witter, 1991; Laroche et al., 1990; Leonard, 1969; McDonald et al., 1996; Neafsey, 1990; Ottersen, 1982; Owens and Verberne, 1996; Resstel and Correa, 2006; Sesack et al., 1989; Swanson, 1981; Takagishi and Chiba, 1991; Thierry et al., 2000; Tierney et al., 2004; Vertes, 2004). However, four pathways are particularly worth emphasizing in the context of PFC modulation of stress. First is a bidirectional connection between PFC and the amygdala (a major neural locus subserving emotion- and reward-related processes among other behaviors), and a descending projection from the PFC to the hypothalamus and brainstem nuclei that mediate neuroendocrine and autonomic responses to stress, respectively. Second is the reciprocal connection between the PFC and the major monoamine systems arising from the midbrain and brainstem that are activated by stress and which are known modulators of executive functions. Third is the reciprocal pathway between the mPFC and hippocampus, which provides a channel for the transfer of complex environmental information between the two regions. Fourth, the PFC is highly interconnected with dorsal and ventral striatal regions that control reward-related behaviors and neuroadaptive responses to drugs of abuse. Collectively, this profile of regional connectivity is consistent with the PFC being a major neural node both for the modulation of the stress response and top-down executive functions. In the next two sections we summarize evidence that the rodent PFC modulates neuroendocrine and autonomic responses to stress, and some of the behavioral correlates of stress exposure in the forms of anxiety- and depression-related behaviors.
3. PFC modulation of rodent neuroendocrine and autonomic responses to stress
By quantifying the expression of immediate-early genes such as c-Fos (Singewald, 2007), the rodent PFC, particularly the medial subregions (IL, PL, and AC), has been shown to be strongly activated by exposure to various forms of stress. These stressors include acute exposure to restraint (Cullinan et al., 1995; Ostrander et al., 2003), footshock (Morrow et al., 2000), forced swimming (Cullinan et al., 1995), loud noise (Campeau et al., 2002, 1997), tests for anxiety-like behavior (Duncan et al., 1996), and treatment with anxiety-provoking drugs (Schroeder et al., 2003; Singewald et al., 2003). Activation likely reflects the engagement of the PFC as a modulator of the endocrine and autonomic response to stress (for reviews see Herman et al., 2005; Sullivan, 2004). This is supported by the finding that lesions of the PL and AC can augment acute restraint stress-induced glucocorticoid release and c-Fos activation in the paraventricular nucleus of the hypothalamus (PVN) and medial amygdala (Brake et al., 2000; Diorio et al., 1993; Figueiredo et al., 2003; Radley et al., 2006a; Spencer et al., 2005) (cf. Crane et al., 2003; Sullivan and Gratton, 1999). An opposite effect is seen with corticosterone implants in the mPFC, which serve to inhibit acute restraint or cold stress-induced glucocorticoid release (Akana et al., 2001; Diorio et al., 1993). These effects likely occur via actions on glucocorticoid and mineralocorticoid receptors abundantly expressed in the rodent mPFC (Ahima and Harlan, 1990; Chao et al., 1989; Fuxe et al., 1985; Meaney and Aitken, 1985; Patel et al., 2000) and, as such, might be attenuated with repeated exposure to stress due to glucocorticoid receptor downregulation. In support of this, Mizoguchi et al. found that prolonged stress (4 weeks of daily restraint or water immersion) decreased glucocorticoid receptor protein and mRNA expression in the PFC and attenuated PFC-mediated negative feedback control over the HPA axis in rats (Mizoguchi et al., 2003).
An important but as yet unresolved issue is the degree to which different PFC subregions play functionally distinct roles in modulating autonomic and HPA-axis responses to stress. Early reports were mixed. Lesions of AC/dorsal PL reduced gastric ulcers resulting from acute stress (Sullivan and Henke, 1986). Ablation or temporary inactivation of the rat IL and ventral PL reduced conditioned autonomic responses (heart rate and blood pressure) to shock and shock-associated context (Frysztak and Neafsey, 1991, 1994; Resstel et al., 2006), while stimulation of rabbit IL elicited sympathetic-like changes in heart rate and blood pressure (Powell et al., 1994). While these data suggest a role for IL in mediating sympathetic activation during stress, IL lesions failed to alter some autonomic components of the conditioned emotional response (Powell et al., 1994). The contradictory nature of these studies may stem in part from lack of regional specificity of the lesions (which were relatively diffuse and included parts of both IL and PL in these early studies), differential effects of electrolytic versus fiber-sparing excitotoxic lesions, and potentially differential roles of the subdivisions of mPFC in learned versus unlearned autonomic responses (e.g., Powell et al., 1994).
Radley et al. have conducted the most direct and comprehensive assessment of the regional specificity of mPFC modulation of sympathetic and HPA axis responsivity to date (Radley et al., 2006a). These authors found that IL lesions attenuated restraint stress-induced HPA axis activation reflected in plasma ACTH and corticosterone levels and decreased c-Fos and CRF mRNA expression in PVN nuclei mediating glucocorticoid release, while PL/AC lesions had opposite effects (Radley et al., 2006a). A subsequent study went on to show that stress-induced activation of PL but not IL, as assayed by c-Fos expression, was negatively correlated with c-Fos and CRF mRNA expression in the PVN (Radley et al., 2008a). Conversely, IL lesions increased activation of PVN neurons mediating sympathetic activation.
One interpretation of these data is that IL and PL have somewhat opposing modulatory effects on stress-induced activation of the HPA-axis, with PL primarily having an inhibitory role and IL predominantly excitatory. This is likely to be an over-simplification however. For example, IL lesions also increase stress-induced c-Fos in a subregion of PVN involved in sympathetic regulation (Radley et al., 2006a), suggesting IL plays an additional inhibitory role in sympathetic activation. Moreover, there is evidence for hemispheric specialization in IL’s modulation of HPA axis responses, with lesions of right but not left IL altering HPA axis activity (Sullivan and Gratton, 1999). Clearly, further work will be needed to dissect the precise role of PFC subregions in mediating the neuroendocrine and autonomic response to stress. Notwithstanding, the available data establish an important role for the PFC in mediating the autonomic and HPA-axis response to stress.
4. PFC modulation of rodent anxiety- and depression-related behaviors
Stress responsivity and anxiety are not synonymous, but are intimately linked in terms of rodent behavior and clinical pathology. For example, one consequence of stress exposure in rodents can be heightened anxiety (Cryan and Holmes, 2005). The PFC appears to have a rather complex role in mediating rodent anxiety-like behavior. Electrolytic lesions of the rodent IL have been shown to decrease state anxiety-like behavior in the elevated plus-maze, shock-probe burying and Vogel conflict tests (Lacroix et al., 2000; Maaswinkel et al., 1996; Shah and Treit, 2003; Sullivan and Gratton, 2002). Similar effects are produced by fiber of passage-sparing ibotenic acid lesions or temporary inactivation of the mPFC (Resstel et al., 2007; Shah and Treit, 2004; Wall et al., 2004). However, lesions or inactivation of AC plus dorsal part of PL are more variable in their effects, with decreases (Deacon et al., 2003; Gonzalez et al., 2000; Maaswinkel et al., 1996), increases (Holson, 1986; Jaskiw and Weinberger, 1990; Jinks and McGregor, 1997) and no change (Bissiere et al., 2006; Corcoran and Quirk, 2007; Lacroix et al., 1998) all being reported in various tests for anxiety-like behavior following ablation of these areas. Thus, if a tentative conclusion can be drawn from these data, it is that the IL appears to promote anxiety-like behavior, while the more dorsal portion of mPFC has a less well-defined role.
With regards studies of the role of the PFC in modulating rodent ‘depression-related’ behaviors, Muigg and colleagues found that rats selectively bred for high anxiety-like behavior exhibited an increased depression-related phenotype in the forced swim test, coupled with increased swim-induced PL activation, as compared to low anxiety counterparts (Muigg et al., 2007). Moreover, both the behavioral and neural abnormalities displayed by these rats were normalized by chronic antidepressant treatment. Another recent study found that lesions of the AC increased depression-related passive floating behavior in rats repeatedly subjected to forced swimming (Bissiere et al., 2006). This finding is reminiscent of work by Maier et al. which has demonstrated the importance of the rat mPFC in regulating the perception of stress ‘controllability.’ Their studies utilize an elegant task in which one group of rats has control over the cessation of shock while another, yoked, group passively receives an equivalent number of shocks. Rats exposed to the inescapable stress regime develop a passive behavioral response even when escape from shock becomes available: a phenomenon termed ‘learned helplessness’ (Seligman, 1972). The inescapable condition selectively elicits a number of neural responses including activation of the ascending serotonergic system and release of serotonin in the ventral part of mPFC (Grahn et al., 1999; Maswood et al., 1998). As already mentioned, there is a large projection from the IL/PL area to the serotonergic dorsal raphe nucleus (Gabbott et al., 2005; Jankowski and Sesack, 2004). Stimulation of this pathway inhibits activity of serotonin neurons via local GABAergic interneurons (Celada et al., 2001; Hajos et al., 1998). Amat et al. have shown that temporary inactivation of the IL/PL area is sufficient to induce learned helplessness in response to stress – i.e., lesioned rats given control over stress still develop a passive behavioral response and persistent serotonin activation akin to that produced by uncontrollable conditions (Amat et al., 2005). Conversely, pharmacological stimulation of the IL/PL region prevents the learned helplessness and persistent serotonin activation induced by inescapable stress (Amat et al., 2005, 2008). These intriguing findings suggest that one major consequence of stress-induced mPFC dysfunction is to produce a bias towards perceived lack of control and as a result a passive, maladaptive response to stress (for further discussion, see Maier et al., 2006; Maier and Watkins, 2005). In the next section we discuss recent evidence that these changes may in part result from stress-induced structural remodeling in PFC.
5. Stress effects on rodent PFC neuronal morphology
The seminal work of McEwen, Sapolsky, de Kloet and others has shown how prolonged exposure to glucocorticoids or stress produces significant neuronal atrophy in the hippocampus, characterized by a retraction of dendrites on pyramidal neurons in the CA3 subregion (de Kloet et al., 2005; McEwen and Milner, 2007; Sapolsky, 2003). It was subsequently found that neurons in other limbic regions also undergo morphological changes following stress. Of particular note, Chattarji and co-workers have found increased dendritic growth and spine density in pyramidal neurons of the basolateral nucleus of the amygdala in rats and mice exposed to chronic restraint stress (Govindarajan et al., 2006; Mitra et al., 2005; Vyas et al., 2006, 2002).
In the first demonstration that pyramidal neurons in the rodent medial PFC exhibit morphological changes in response to stress, Wellman found that 3 weeks of corticosterone administration reorganized dendrites of pyramidal neurons in layer II–III of the dorsal part of the PL as well as the AC (Wellman, 2001). In a follow-up study, a marked reduction in dendritic material in this area was seen following exposure to stress itself; in this case, 3 weeks of 3 h daily restraint (Cook and Wellman, 2004). In these studies, dendritic remodeling was specific to the apical but not basilar branches, with the most dramatic reductions in branch number and length occurring in terminal branches relatively distal to the soma. A similar pattern of apical dendritic retraction and debranching in mPFC neurons following chronic restraint stress or corticosterone treatment has been independently documented in a series of excellent studies by Liston et al. (2006), Radley et al. (2005a, 2006b, 2004), and Cerqueira et al. (2005a,b, 2007a,b) (see also recent work by Czeh et al., 2008). Importantly, this work has shown that stress-induced debranching of apical dendrites is also associated with a significant decrease in the density of dendritic spines, particularly in their mature functional form (Michelsen et al., 2007; Radley et al., 2006a, 2008b; Silva-Gomez et al., 2003).
The restriction of remodeling and spine loss to apical dendrites provides some clues as to the mechanisms driving these alterations. Cortical pyramidal neurons segregate their inputs. In piriform cortex for instance, distal apical dendrites of layer III pyramidal neurons receive extrinsic inputs, while more proximal portions of the apical dendrite, as well as the basilar dendrites, receive intrinsic inputs (Price, 1973). While the segregation of inputs to pyramidal neurons in neocortex appears to be less straightforward, mPFC pyramidal neurons also tend to segregate inputs. For example, there is some evidence that extra-cortical afferents (e.g., from mediodorsal thalamus and CA3 region of hippocampus) predominantly synapse on layer I distal dendrites (Groenewegen, 1988; Swanson and Cowan, 1977) while local cortical afferents tend to cluster more on proximal portions of the apical and basilar arbor (Scheibel and Scheibel, 1970). Thus, the stress-induced retraction of relatively distal apical dendrites could be a response to alterations in activity of subcortical inputs. Because stimulation of the apical tufts of cortical pyramidal neurons disproportionately excites the cell (Rhodes and Llinas, 2001), apical retraction would serve as a relatively effective adaptive response to over-excitation. However, precisely how stress-induced morphological alterations in mPFC neurons translates into changes in their physiological properties remains a critical but largely unanswered question. Interestingly in this context, one recent study found that stress-induced reductions in branch length of apical tufts in layer V mPFC neurons correlated with reduced neuronal responses (i.e., excitatory post-synaptic currents) to serotonin application (Liu and Aghajanian, 2008). Further work along these lines will be an important area for future study.
5.1. Sensitivity, reversibility and subregion specificity of stress effects on rodent PFC neurons
One somewhat unexpected finding from Wellman’s initial finding of dendritic remodeling after corticosterone treatment was that control rats receiving subcutaneous vehicle injections showed the same pattern of changes, albeit to a lesser extent (Wellman, 2001). Earlier data had shown that a similar regime of vehicle injections did not alter dendritic morphology in hippocampal CA3 pyramidal neurons (Woolley et al., 1990). This suggested that mPFC neurons might be relatively more ‘susceptible’ to stress than neurons in other brain regions. Subsequent findings have borne this out. For example, repeated exposure to brief restraint (10 min per day for 7 days) has been shown to cause dendritic retraction in PL and AC (Brown et al., 2005) similar in pattern but lesser in extent to that produced by lengthier (3–6 h) restraint protocols (Cook and Wellman, 2004; Radley et al., 2004). Even more strikingly, a single 10-min forced swim followed by tone-shock Pavlovian fear conditioning was sufficient to produce apical dendritic retraction in the IL of mice (Izquierdo et al., 2006). Brief stress also affects the synaptic plasticity of mPFC neurons. In rats, a single 30-min bout of elevated platform stress impaired a form of long-term potentiation in PL produced by theta-burst stimulation of the basolateral amygdala (Maroun and Richter-Levin, 2003). Jay and co-workers have found that the same single stressor also impaired LTP in the hippocampus-mPFC pathway (Dupin et al., 2006; Jay et al., 2004; Mailliet et al., 2008; Rocher et al., 2004), mimicking the effects of chronic restraint stress (Cerqueira et al., 2007a). Collectively, these findings speak to the exquisite morphological and physiological sensitivity of mPFC neurons to stress.
Given the rapid response of mPFC neurons to stress, a corollary question is how quickly these neurons recover from stress. Dendritic retraction in the hippocampus caused by chronic restraint is ameliorated within 10 days of stress cessation (Conrad et al., 1999). By comparison, chronic restraint stress-induced dendritic retraction in the dorsal potion of PL and AC was no longer evident 3 weeks after stress (Radley et al., 2005b). A detailed time course of the progression of recovery would be very useful. Notwithstanding, the more general finding that stress effects on mPFC neuronal morphology are reversible further underscores the plasticity of these neurons. Critically, it also implies that the mechanisms mediating this plasticity can be identified and manipulated to expedite the process of recovery.
Given increasing evidence that different subregions of the rodent PFC have dissociable functions in mediating stress and behavioral (including executive, as discussed below) functions, another important issue is whether stress differentially targets specific subregions. Sousa, Cerquiera et al. have demonstrated that rats chronically treated with either corticosterone or the glucocorticoid receptor agonist dexamethasone, or exposed to chronic unpredictable mild stress, results in decreases in volume and apical dendritic length (and sometimes cell loss) that are equivalent in the IL, PL and AC (but not motor or retrosplenial cortex) (Cerqueira et al., 2005a,b, 2007a,b). While this suggests that stress-induced dendritic retraction generalizes across subregions of mPFC, there is preliminary evidence that changes in OFC may actually be quite different. This stems from the finding that the same chronic restraint procedure that produces dendritic retraction in the AC actually increased dendritic material in the rat lateral OFC (Liston et al., 2006). This intriguing finding suggests that stress produces opposite effects on the morphology of mPFC and OFC neurons. That mPFC and OFC show divergent changes in response to an environmental insult is not without precedent. For example, repeated exposure to psychostimulants produces increased spine density in mPFC neurons but decreased spine density in OFC neurons (Robinson and Kolb, 2004), as well as contrasting changes in the firing properties of mPFC and OFC neurons (for further discussion, see Moghaddam and Homayoun, 2008). This raises the possibility that behavioral functions mediated by mPFC and OFC might be differentially sensitive to stress. We will take up this issue in the next section where we discuss some of the literature describing the effects of stress on rodent executive functions.
6. Stress effects on rodent executive functions
The rodent PFC subserves a range of cognitive and behavioral processes analogous to some of the executive functions mediated by the human PFC (Heidbreder and Groenewegen, 2003; Marko-witsch and Pritzel, 1977; Robbins, 2005; Uylings et al., 2003). Executive functions measurable in rats and mice include working memory (e.g., delayed alternation in the T-maze), cognitive flexibility (e.g., reversal learning and set-shifting), sustained attention (e.g., 5-choice serial reaction time task), and inhibitory response control (e.g., delay discounting, Go/No-Go). A number of laboratories have now convincingly shown that the rodent PFC mediates these processes and does so with a remarkable degree of subregional specialization (reviewed in Chudasama and Robbins, 2006; Dalley et al., 2004). However, although a significant corpus of data describes the effects of stress on various forms of rodent learning and memory, chiefly those involving the hippocampus (Kim and Diamond, 2002; McEwen, 1999; Sandi, 2004; Shors, 2006), stress effects on PFC-mediated executive functions is only now being elucidated. We will summarize some of the main findings to date.
6.1. Stress effects on rodent working memory
The T-maze delayed alternation test and the 8-arm radial maze are two of the more common rodent assays for working memory (Lalonde, 2002; Olton, 1987). Lesions encompassing most of the rodent mPFC, or the IL and PL subregions specifically, impair working memory performance on these and related tasks such as the Y-maze and appear to do so in part at least by impairing inhibitory response control (Aggleton et al., 1995; Brito and Brito, 1990; Chudasama et al., 2003; Di Pietro et al., 2004; Dias and Aggleton, 2000; Floresco et al., 1997; Granon et al., 1994; Kellendonk et al., 2006; Kolb, 1984; Ragozzino et al., 1998; Schwabe et al., 2004; Seamans et al., 1995; Sloan et al., 2006). The detrimental effects of these lesions can be mimicked by stressors including chronic exposure to restraint, cold water immersion, loud noise, high illumination, or footshock, as well as acute stressors such as handling and anxiogenic drug treatment (Anisman et al., 1985; Del Arco et al., 2007; Hahn et al., 1986; Manikandan et al., 2006; Mizoguchi et al., 2000; Murphy et al., 1996a,b; Nishimura et al., 1999; Pierard et al., 2006; Shanks and Anisman, 1988). Interestingly, either adrenalectomy (Mizoguchi et al., 2004), acute or chronic corticosterone treatment (Bardgett et al., 1994; Roozendaal et al., 2004), or infusion of a glucocorticoid receptor agonist directly into the PL and AC (Roozendaal et al., 2004) also impairs performance on working memory tasks. These effects are reminiscent of the aforementioned finding that either adrenalectomy or corticosterone administration produces dendritic retraction in mPFC (Cerqueira et al., 2007b; Wellman, 2001). Thus, there appears to be an inverted U-shaped relationship between glucocorticoid activity and both mPFC neuronal morphology and mPFC-mediated working memory, with either hypo-or hypercortisolemia causing dendritic retraction and behavioral impairment.
While the similarities between the effects of stress and mPFC lesions on working memory suggest the possibility of a causative link between stress-induced changes in mPFC neuronal morphology and concomitant impairment of the behavior, direct evidence of such a link has not yet been obtained. In fact, this issue is raised in a recent study by Cerqueira et al. Using the Morris water maze, a paradigm typically used to assess spatial reference memory, but which has also been adapted to assay spatial working memory (Steele and Morris, 1999), these authors found that 6 days of unpredictable stress impaired water maze spatial working memory and caused dendritic atrophy in the IL, PL and AC (Cerqueira et al., 2007a). As the authors note, this deficit could reflect functional changes in either the mPFC or the hippocampus (or both) given evidence that both regions mediate this behavior (de Bruin et al., 1994; Ferbinteanu et al., 2003; Lacroix et al., 2002; Steele and Morris, 1999; Sullivan and Gratton, 2002; Wolf et al., 1987). This illustrates a general caveat when attributing stress-induced changes in behavior to functional changes in PFC per se because executive functions including working memory are subserved by a distributed network of brain regions in which the PFC has a special but by no means exclusive role (e.g., Floresco et al., 1997; Kesner and Rogers, 2004). In other words, a full understanding of the effects of stress on the PFC and its associated functions will ultimately have to be at the systems level, and account for interactions between PFC and other structures that are influenced by stress and/or critical components of the circuitry subserving executive functions.
6.2. Stress effects on rodent cognitive flexibility (reversal learning, attentional set-shifting)
There is growing evidence that stress-induced impairments extend to executive functions other than working memory. The Morris water maze task has been used to test for cognitive flexibility in the form of spatial reversal learning; by measuring the ability to learn to swim to a submerged platform after its trained location has been switched. A number of studies have now shown that spatial reversal learning on this task is impaired by exposure to stressors including chronic unpredictable stress, postnatal maternal separation and acute foot shock (Enthoven et al., 2007; Francis et al., 1995; Hill et al., 2005; Szuran et al., 2000). In studying the link of such deficits to mPFC neuronal changes, Cerqueira et al. demonstrated that 6 days of unpredictable stress impaired spatial reversal learning but did not alter the volume of IL, PL or AC (Cerqueira et al., 2005a). On the other hand, these authors found that either chronic corticosterone treatment or adrenalectomy was sufficient to impair spatial reversal learning (not working or reference memory) in a manner that correlated with reduced mPFC volume (Cerqueira et al., 2007a, 2005a). They also showed that their unpredictable stress paradigm impaired plasticity in the hippocampal-mPFC pathway (Cerqueira et al., 2007a). This latter observation is particularly noteworthy given the finding that chronic restraint stress produces spatial maze reversal deficits that parallel morphological changes in the CA3 region of the hippocampus (Luine et al., 1994). The conclusion from these data therefore is that while stress effects on spatial working memory are coupled to morphological alterations in mPFC neuronal morphology, they likely stem from changes at the level of the mPFC-hippocampus circuit.
Another way to ask how stress affects rodent cognitive flexibility is via the use of assays for attentional set-shifting. An illustrative example of set-shifting in rodents is an assay developed by Birrell and Brown (2000). In this task, rodents first learn to dig for food reward using sand texture and sand smell and then to reverse the learned association (e.g., if smooth sand previously signaled reward, now dig in rough sand). There is then a subsequent phase known as an extra-dimensional shift in which the subject must use a different stimulus ‘set’ to guide their digging behavior (e.g., if sand texture previously discriminated reward, now use sand smell to discriminate). In a now classic study, Birrell and Brown found that lesions of most of the rat mPFC selectively impaired the extra-dimensional component on this task. Similarly, combined IL and PL lesions impairs extra-dimensional set-shifting on various exploration-based tasks (Delatour and Gisquet-Verrier, 2000; Ragozzino et al., 1999a,b). Using the Birrell and Brown digging task, Bondi et al. demonstrated that 2 weeks of unpredictable stress impaired reversal learning and extra-dimensional set-shifting (Bondi et al., 2008). Along similar lines, Liston et al. (2006) found that 3 weeks of restraint stress produced a (in this case selective) deficit in extra-dimensional set-shifting and, furthermore, that the magnitude of the impairment correlated with the extent of dendritic retraction in the AC (Liston et al., 2006). Although it again should be emphasized that correlations alone cannot demonstrate that the dendritic atrophy in AC neurons caused the stress-induced disruption in set-shifting, this is the best evidence to date that the two are related.
Lesion studies have shown that, in contrast to the effects of mPFC lesions, ablation of the lateral OFC impaired reversal performance in the Birrell and Brown digging task while leaving the extra-dimensional set-shifting component intact (McAlonan and Brown, 2003). This effect is highly reminiscent of the finding that ventral and lateral OFC lesions disrupt the ability of rats to shift their responses for reward when stimulus-reinforcement contingencies are reversed (Chudasama et al., 2003; Ferry et al., 2000; Kim and Ragozzino, 2005; Schoenbaum et al., 2002, 2003; Stalnaker et al., 2007), and work by Schoenbaum and co-workers showing that the activity of OFC neuronal activity tracks reversal performance (Saddoris et al., 2005; Schoenbaum et al., 1999, 2000). Collectively, these observations support a model in which the OFC (in concert with subcortical regions including BLA and ventral striatum) guides behavioral responses according to their current incentive value of reward-related stimuli (Bechara et al., 2000; Clark et al., 2004; Rolls, 1996; Schoenbaum et al., 2006). This in turn provides a conceptual framework for explaining how OFC dysfunction might underlie the cognitive and behavioral rigidity characteristic of disorders ranging from drug addiction to obsessive compulsive disorder (Kalivas et al., 2005; Schoenbaum and Shaham, 2008).
A better understanding of how stress might affect OFC neuronal morphology and the behavioral functions supported by this region is of great topical interest. However, potential effects of stress-induced changes in OFC for measures of rodent cognitive flexibility such as reversal learning have not yet been well studied. One study found that 14 days of cold stress impaired reversal (but not extra-dimensional set-shifting) in the Birrell and Brown task (Lapiz-Bluhm et al., 2008) – i.e., mimicking the effects of OFC lesions. In addition, as discussed above, there is one report that chronic restraint stress produced increased dendritic length in lateral OFC neurons (Liston et al., 2006). However, this study did not find any correlation between the extent of the morphological changes and intra-dimensional set-shifting on the Birrell and Brown task. Although it is not certain that dendritic elongation of OFC would necessarily lead to an improvement (as opposed to an impairment) in this behavior, the authors speculate that their negative finding may have been due to the fact that stress improved rather than impaired performance but the task was unable to detect this due to a ‘ceiling effect’. Thus, precisely how stress might affect cognitive flexibility and other executive functions subserved by the OFC (e.g., certain forms of impulsivity Cardinal et al., 2001; Mobini et al., 2002) remains another important avenue for future work.
6.3. Stress effects on rodent fear extinction
Extinction of fear memories does not fit the definition of an executive function, but this behavior does have certain features that make it pertinent to the current discussion. First, fear extinction requires the ability to flexibly alter behavior by inhibiting a previously learned response – with extinction deficits manifesting as a form of ‘emotional perseveration’ somewhat akin to the ‘cognitive perseveration’ caused by OFC lesions (Morgan et al., 2003; Sotres-Bayon et al., 2004). Second, the evidence implicating the rodent mPFC in fear extinction is compelling. For example, IL is strongly activated (as assayed by immediate-early gene expression and neuronal firing) during extinction, while IL lesions or inactivation impair fear extinction learning and/or memory (reviewed in Quirk and Mueller, 2008).
How stress affects fear extinction is not yet fully clear. In part this is because stress can enhance the acquisition or expression of conditioned fear memories per se (Rau et al., 2005), making it difficult to disentangle a selective deficit in the ability to extinguish the fear memory from increased fear itself. However, stress-induced deficits in fear extinction that are dissociable from any apparent increase in fear learning have been observed. Izquierdo et al. (2006) showed that 1 or 3 days of forced swimming prior to fear conditioning retarded fear extinction learning in mice, concomitant with significant retraction of apical dendrites in IL. In rats, 1 week of restraint was also found to impair extinction memory (Miracle et al., 2006), which is interesting in light of evidence that IL is preferentially recruited during extinction recall (Milad and Quirk, 2002). Moreover, Maroun and co-workers have shown that a single bout of elevated platform stress both impaired fear extinction and caused a shift in plasticity (LTP to long-term depression) in the IL-amygdala pathway subserving extinction (Akirav and Maroun, 2007; Maroun, 2006; Maroun and Richter-Levin, 2003). Together, these findings demonstrate that stress can selectively impair fear extinction, and may do so via alterations in PFC function.
Current models posit that fear extinction occurs in part by IL inhibition of amygdala output via activation of the intercalated cell masses (ITC) found in the amygdala (Hefner et al., 2008; Pare et al., 2004). One model is that stress-induced deficits in fear extinction result from loss of ITC-mediated IL inhibitory control over the amygdala. The situation is likely to be more complex, however. For example, recent work from Quirk and co-workers has shown that PL appears to play an opposing role to IL in fear extinction (i.e., PL stimulation impairs extinction and PL inactivation increases fear (reviewed in Quirk and Mueller, 2008). Thus, because, as discussed above, most stressors produce morphological changes in both PL and IL, the net effect of these changes for fear extinction is unclear. Another consideration is that the basolateral amygdala is also an important locus for fear extinction (Barad, 2005; Herry et al., 2008) and chronic restraint stress causes dendritic elongation and increased spine density in this region (Mitra et al., 2005; Vyas et al., 2002). How changes at the level of the amygdala contribute to stress-induced impairment of fear extinction is another issue that awaits clarification.
7. Concluding remarks
In this review, we have described how the PFC plays a significant, if as yet not fully clarified, role in regulating rodent neuroendocrine and autonomic responses to stress, and anxiety-and depression-related behaviors. We also discussed the important discovery that pyramidal neurons in several regions of the rodent PFC undergo dramatic remodeling with exposure to stressors, even those of brief or ostensibly mild nature. These pronounced structural changes likely result in important functional changes in PFC neurons and the behavioral processes they support, including higher-order executive functions. Although these functional changes have also to be fully elucidated, there is accumulating evidence that stress can impair measures of working memory, cognitive flexibility and fear extinction in rodents.
These findings open up a number of avenues for future research. One key objective is to uncover the mechanisms mediating stress-induced changes in PFC structure and function. A review of the large literature describing the neurotransmitters and molecular signaling pathways within the PFC that have been shown to be altered by stress is beyond the scope of this article. Nonetheless, the available evidence supports a potential role in this regard for the mono-aminergic transmitters serotonin (Holmes, 2008; Lapiz-Bluhm et al., 2008; Maier et al., 2006; Robbins, 2005), dopamine (Mizoguchi et al., 2004, 2000; Murphy et al., 1996a,b; Pani et al., 2000) and norepinephrine (Ramos and Arnsten, 2007), as well as glutamate (Brann, 1995; Moghaddam, 2002; Moghaddam and Jackson, 2004) and glucocorticoids (Liu and Aghajanian, 2008). Parsing how these and other mechanisms cause stress-induced alterations in PFC structure and function will be a massive undertaking, but ultimately a worthy one that could provide important insights into the pathophysiology of neuropsychiatric illness
Risk for a number of neuropsychiatric disease states is strongly linked to psychological trauma and stress. For example, stress is associated with increased prevalence and poorer long-term prognosis in anxiety disorders (Yehuda and LeDoux, 2007), bipolar disorder (Agid et al., 1999), depression (Brown and Harris, 1989), schizophrenia (Corcoran et al., 2002; Ventura et al., 1989), and drug addiction (Koob et al., 2004; Piazza and Le Moal, 1998; Stewart, 2000). Moreover, many of these same conditions are associated with abnormalities in PFC structure and function, including anxiety disorders (Davidson et al., 2002; Engels et al., 2007; Rauch et al., 2006), depression (Drevets et al., 1998; Rajkowska, 2000; Ressler and Mayberg, 2007), drug addiction (Hyman, 2007; Hyman et al., 2006; Kalivas and Volkow, 2005), Attention Deficit Hyperactivity Disorder (Arnsten, 2006), and schizophrenia (Harrison and Weinberger, 2005; Lewis and Gonzalez-Burgos, 2006). It is extremely difficult to disentangle a causative effect of stress from other factors, including genetic predisposition, on PFC dysfunction and risk for complex disease; in all likelihood these factors interact to determine disease risk (Caspi and Moffitt, 2006; Holmes and Hariri, 2003; Uher and McGuffin, 2008). However, the emerging data obtained from rodents tested under controlled laboratory conditions reviewed herein demonstrate that the PFC is a key target of stress, and supports the hypothesis that stress-induced PFC dysfunction is a major pathophysiological factor underlying these disorders. Further insights into the role of the PFC in stress-related disorders will improve our understanding of these devastating conditions and could ultimately foster the development of novel therapeutic approaches that serve to protect and promote the functional integrity of the PFC.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program (A.H.) and National Institute of Mental Health Grant MH067607 (C.L.W.).
References
- Aggleton JP, Neave N, Nagle S, Sahgal A. A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: evidence of a double dissociation between frontal and cingulum bundle contributions. J Neurosci. 1995;15:7270–7281. doi: 10.1523/JNEUROSCI.15-11-07270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Akana SF, Chu A, Soriano L, Dallman MF. Corticosterone exerts site-specific and state-dependent effects in prefrontal cortex and amygdala on regulation of adrenocorticotropic hormone, insulin and fat depots. J Neuroen-docrinol. 2001;13:625–637. doi: 10.1046/j.1365-2826.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Anisman H, Hahn B, Hoffman D, Zacharko RM. Stressor invoked exacerbation of amphetamine-elicited perseveration. Pharmacol Biochem Behav. 1985;23:173–183. doi: 10.1016/0091-3057(85)90552-0. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67 (Suppl 8):7–12. [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 1996;720:211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Taylor GT, Csernansky JG, Newcomer JW, Nock B. Chronic corticosterone treatment impairs spontaneous alternation behavior in rats. Behav Neural Biol. 1994;61:186–190. doi: 10.1016/s0163-1047(05)80074-3. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S, McAllister KH, Olpe HR, Cryan JF. The rostral anterior cingulate cortex modulates depression but not anxiety-related behaviour in the rat. Behav Brain Res. 2006;175:195–199. doi: 10.1016/j.bbr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsycho-pharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Brake WG, Flores G, Francis D, Meaney MJ, Srivastava LK, Gratton A. Enhanced nucleus accumbens dopamine and plasma corticosterone stress responses in adult rats with neonatal excitotoxic lesions to the medial pre-frontal cortex. Neuroscience. 2000;96:687–695. doi: 10.1016/s0306-4522(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Brann DW. Glutamate: a major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology. 1995;61:213–225. doi: 10.1159/000126843. [DOI] [PubMed] [Google Scholar]
- Brito GN, Brito LS. Septohippocampal system and the prelimbic sector of frontal cortex: a neuropsychological battery analysis in the rat. Behav Brain Res. 1990;36:127–146. doi: 10.1016/0166-4328(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Life events and illness. In: Brown GW, Harris TO, editors. Depression. Guilford; New York: 1989. pp. 49–93. [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. c-fos mRNA induction in acute and chronic audiogenic stress: possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78:1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Wright DJ. Topography of projections from the medial pre-frontal cortex to the amygdala in the rat. Brain Res Bull. 1986;17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007a;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007b;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005a;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Catania C, Sotiropoulos I, Schubert M, Kalisch R, Almeida OF, Auer DP, Sousa N. Corticosteroid status influences the volume of the rat cingulate cortex—a magnetic resonance imaging study. J Psychiatr Res. 2005b;39:451–460. doi: 10.1016/j.jpsychires.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Chao HM, Choo PH, McEwen BS. Glucocorticoid and mineralocorticoid receptor mRNA expression in rat brain. Neuroendocrinology. 1989;50:365–371. doi: 10.1159/000125250. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;17:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Mujica-Parodi L, Yale S, Leitman D, Malaspina D. Could stress cause psychosis in individuals vulnerable to schizophrenia? CNS Spectr. 2002;7 (33–38):32–41. doi: 10.1017/s1092852900022240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JW, Ebner K, Day TA. Medial prefrontal cortex suppression of the hypothalamic-pituitary-adrenal axis response to a physical stressor, systemic delivery of interleukin-1beta. Eur J Neurosci. 2003;17:1473–1481. doi: 10.1046/j.1460-9568.2003.02568.x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Czeh B, Perez-Cruz C, Fuchs E, Flugge G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere location matter? Behav Brain Res. 2008;190:1–13. doi: 10.1016/j.bbr.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, Sanchez-Santed F, Heinsbroek RP, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res. 1994;652:323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Penny C, Rawlins JN. Effects of medial prefrontal cortex cytotoxic lesions in mice. Behav Brain Res. 2003;139:139–155. doi: 10.1016/s0166-4328(02)00225-5. [DOI] [PubMed] [Google Scholar]
- Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: an in vivo intracellular recording study. Cereb Cortex. 2003;13:782–792. doi: 10.1093/cercor/13.7.782. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Garrido P, de Blas M, Mora F. Stress, prefrontal cortex and environmental enrichment: studies on dopamine and acetylcholine release and working memory performance in rats. Behav Brain Res. 2007;176:267–273. doi: 10.1016/j.bbr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Delatour B, Gisquet-Verrier P. Functional role of rat prelimbic-infralimbic cortices in spatial memory: evidence for their involvement in attention and behavioural flexibility. Behav Brain Res. 2000;109:113–128. doi: 10.1016/s0166-4328(99)00168-0. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Complementary tasks to measure working memory in distinct prefrontal cortex subregions in rats. Behav Neurosci. 2004;118:1042–1051. doi: 10.1037/0735-7044.118.5.1042. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur J Neurosci. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divac I, Mogensen J, Petrovic-Minic B, Zilles K, Regidor J. Cortical projections of the thalamic mediodorsal nucleus in the rat. Definition of the prefrontal cortex. Acta Neurobiol Exp (Wars) 1993;53:425–429. [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3 (220–226):190–221. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- Dupin N, Mailliet F, Rocher C, Kessal K, Spedding M, Jay TM. Common efficacy of psychotropic drugs in restoring stress-induced impairment of pre-frontal plasticity. Neurotox Res. 2006;10:193–198. doi: 10.1007/BF03033356. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Enthoven L, de Kloet ER, Oitzl MS. Effects of maternal deprivation of CD1 mice on performance in the water maze and swim stress. Behav Brain Res. 2007;187:195–199. doi: 10.1016/j.bbr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Ray C, McDonald RJ. Both dorsal and ventral hippocampus contribute to spatial learning in Long–Evans rats. Neurosci Lett. 2003;345:131–135. doi: 10.1016/s0304-3940(03)00473-7. [DOI] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon’s horn to the medial prefrontal cortex in the rat. Exp Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal–thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Exp Brain Res. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Wyss JM. Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain Res. 2000;859:83–95. doi: 10.1016/s0006-8993(00)01935-1. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial pre-frontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Francis DD, Zaharia MD, Shanks N, Anisman H. Stress-induced disturbances in Morris water-maze performance: interstrain variability. Physiol Behav. 1995;58:57–65. doi: 10.1016/0031-9384(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on respiration, “freezing and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb Cortex. 1991;1:418–425. doi: 10.1093/cercor/1.5.418. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Prefrontal neurons in networks of executive memory. Brain Res Bull. 2000;52:331–336. doi: 10.1016/s0361-9230(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Wikstrom AC, Okret S, Agnati LF, Harfstrand A, Yu ZY, Granholm L, Zoli M, Vale W, Gustafsson JA. Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology. 1985;117:1803–1812. doi: 10.1210/endo-117-5-1803. [DOI] [PubMed] [Google Scholar]
- Gabbott P, Headlam A, Busby S. Morphological evidence that CA1 hippo-campal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res. 2002;946:314–322. doi: 10.1016/s0006-8993(02)02487-3. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B: Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Rujano M, Tucci S, Paredes D, Silva E, Alba G, Hernandez L. Medial prefrontal transection enhances social interaction. I: behavioral studies. Brain Res. 2000;887:7–15. doi: 10.1016/s0006-8993(00)02931-0. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- Granon S, Vidal C, Thinus-Blanc C, Changeux JP, Poucet B. Working memory, response selection, and effortful processing in rats with medial prefrontal lesions. Behav Neurosci. 1994;108:883–891. doi: 10.1037//0735-7044.108.5.883. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the medio-dorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Hahn B, Zacharko RM, Anisman H. Alterations of amphetamine elicited perseveration and locomotor excitation following acute and repeated stressor application. Pharmacol Biochem Behav. 1986;25:29–33. doi: 10.1016/0091-3057(86)90225-x. [DOI] [PubMed] [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacol-ogy. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Hariri AR. The serotonin transporter gene-linked polymorphism and negative emotionality: placing single gene effects in the context of genetic background and environment. Genes Brain Behav. 2003;2:332–335. doi: 10.1046/j.1601-1848.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- Holson RR. Mesial prefrontal cortical lesions and timidity in rats. I. Reactivity to aversive stimuli. Physiol Behav. 1986;37:221–230. doi: 10.1016/0031-9384(86)90224-6. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE. The neurobiology of addiction: implications for voluntary control of behavior. Am J Bioeth. 2007;7:8–11. doi: 10.1080/15265160601063969. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Jaskiw GE, Weinberger DR. Ibotenic acid lesions of the medial prefrontal cortex potentiate FG-7142-induced attenuation of exploratory activity in the rat. Pharmacol Biochem Behav. 1990;36:695–697. doi: 10.1016/0091-3057(90)90276-n. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 1989;505:337–340. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. Contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci. 1992;4:1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Spedding M. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases. Neurotox Res. 2004;6:233–244. doi: 10.1007/BF03033225. [DOI] [PubMed] [Google Scholar]
- Jinks AL, McGregor IS. Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 1997;772:181–190. doi: 10.1016/s0006-8993(97)00810-x. [DOI] [PubMed] [Google Scholar]
- Jones BF, Groenewegen HJ, Witter MP. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience. 2005;133:193–207. doi: 10.1016/j.neuroscience.2005.01.063. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Rogers J. An analysis of independence and interactions of brain substrates that subserve multiple attributes, memory systems, and underlying processes. Neurobiol Learn Mem. 2004;82:199–215. doi: 10.1016/j.nlm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kolb B. Functions of the frontal cortex of the rat: a comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Lacroix L, White I, Feldon J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- Lacroix L, Broersen LM, Weiner I, Feldon J. The effects of excitotoxic lesion of the medial prefrontal cortex on latent inhibition, prepulse inhibition, food hoarding, elevated plus maze, active avoidance and locomotor activity in the rat. Neuroscience. 1998;84:431–442. doi: 10.1016/s0306-4522(97)00521-6. [DOI] [PubMed] [Google Scholar]
- Lacroix L, Spinelli S, Heidbreder CA, Feldon J. Differential role of the medial and lateral prefrontal cortices in fear and anxiety. Behav Neurosci. 2000;114:1119–1130. doi: 10.1037//0735-7044.114.6.1119. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Soto-Pina AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche S, Jay TM, Thierry AM. Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci Lett. 1990;114:184–190. doi: 10.1016/0304-3940(90)90069-l. [DOI] [PubMed] [Google Scholar]
- Leonard CM. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res. 1969;12:321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Gispen WH, Spruijt BM. Effects of an electrolytic lesion of the prelimbic area on anxiety-related and cognitive tasks in the rat. Behav Brain Res. 1996;79:51–59. doi: 10.1016/0166-4328(95)00261-8. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailliet F, Qi H, Rocher C, Spedding M, Svenningsson P, Jay TM. Protection of stress-induced impairment of hippocampal/prefrontal LTP through blockade of glucocorticoid receptors: implication of MEK signaling. Exp Neurol. 2008;211:593–596. doi: 10.1016/j.expneurol.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Manikandan S, Padma MK, Srikumar R, Jeya Parthasarathy N, Muthuvel A, Sheela Devi R. Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neurosci Lett. 2006;399:17–22. doi: 10.1016/j.neulet.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Pritzel M. Comparative analysis of prefrontal learning functions in rats, cats, and monkeys. Psychol Bull. 1977;84:817–837. [PubMed] [Google Scholar]
- Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur J Neurosci. 2006;24:2917–2922. doi: 10.1111/j.1460-9568.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdale. Proc Natl Acad Sci USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1574. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Jackson M. Effect of stress on prefrontal cortex function. Neurotox Res. 2004;6:73–78. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Homayoun H. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Lee EJ, Roth RH. Divergent effects of putative anxiolytics on stress-induced fos expression in the mesoprefrontal system of the rat. Synapse. 2000;36:143–154. doi: 10.1002/(SICI)1098-2396(200005)36:2<143::AID-SYN7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Muigg P, Hoelzl U, Palfrader K, Neumann I, Wigger A, Landgraf R, Singewald N. Altered brain activation pattern associated with drug-induced attenuation of enhanced depression-like behavior in rats bred for high anxiety. Biol Psychiatry. 2007;61 (6):782–796. doi: 10.1016/j.biopsych.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996a;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J Neurosci. 1996b;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Prog Brain Res. 1990;85:147–165. doi: 10.1016/s0079-6123(08)62679-5. (discussion 165–146) [DOI] [PubMed] [Google Scholar]
- Nishimura J, Endo Y, Kimura F. A long-term stress exposure impairs maze learning performance in rats. Neurosci Lett. 1999;273:125–128. doi: 10.1016/s0304-3940(99)00645-x. [DOI] [PubMed] [Google Scholar]
- Olton DS. The radial arm maze as a tool in behavioral pharmacology. Physiol Behav. 1987;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- Ostrander MM, Richtand NM, Herman JP. Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptors. Brain Res. 2003;990:209–214. doi: 10.1016/j.brainres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horse-radish peroxidase. J Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Owens NC, Verberne AJ. An electrophysiological study of the medial prefrontal cortical projection to the nucleus of the solitary tract in rat. Exp Brain Res. 1996;110:55–61. doi: 10.1007/BF00241374. [DOI] [PubMed] [Google Scholar]
- Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Paxinos KBJ, Franklin G. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; London: 2001. [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pierard C, Liscia P, Valleau M, Drouet I, Chauveau F, Huart B, Bonneau D, Jouanin JC, Beaumont M, Beracochea D. Modafinil-induced modulation of working memory and plasma corticosterone in chronically-stressed mice. Pharmacol Biochem Behav. 2006;83:1–8. doi: 10.1016/j.pbb.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Powell DA, Watson K, Maxwell B. Involvement of subdivisions of the medial prefrontal cortex in learned cardiac adjustments in rabbits. Behav Neurosci. 1994;108:294–307. doi: 10.1037//0735-7044.108.2.294. [DOI] [PubMed] [Google Scholar]
- Price JL. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J Comp Neurol. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Do rats have a prefrontal cortex? The Rose–Woolsey–Akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006a;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008a;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005a;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005b;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006b;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008b;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999a;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999b;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Histopathology of the prefrontal cortex in major depression: what does it tell us about dysfunctional monoaminergic circuits? Prog Brain Res. 2000;126:397–412. doi: 10.1016/S0079-6123(00)26026-3. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, Decola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Correa FM. Involvement of the medial prefrontal cortex in central cardiovascular modulation in the rat. Auton Neurosci. 2006;126–127:130–138. doi: 10.1016/j.autneu.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Souza RF, Guimaraes FS. Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Guimaraes FG, Correa FM. Involvement of medial prefrontal cortex neurons in behavioral and cardiovascular responses to contextual fear conditioning. Neuroscience. 2006;143:377–385. doi: 10.1016/j.neuroscience.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Rhodes PA, Llinas RR. Apical tuft input efficacy in layer 5 pyramidal cells from rat visual cortex. J Physiol. 2001;536:167–187. doi: 10.1111/j.1469-7793.2001.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of pre-frontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 (Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex. Philos Trans R Soc Lond B: Biol Sci. 1996;351:1433–1443. doi: 10.1098/rstb.1996.0128. (discussion 1443–1434) [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochem Res. 2003;28:1735–1742. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Organization of spinal motoneuron dendrites in bundles. Exp Neurol. 1970;28:106–112. doi: 10.1016/0014-4886(70)90165-2. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BE, Schiltz CA, Kelley AE. Neural activation profile elicited by cues associated with the anxiogenic drug yohimbine differs from that observed for reward-paired cues. Neuropsychopharmacology. 2003;28:14–21. doi: 10.1038/sj.npp.1300007. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Enkel T, Klein S, Schutte M, Koch M. Effects of neonatal lesions of the medial prefrontal cortex on adult rat behaviour. Behav Brain Res. 2004;153:21–34. doi: 10.1016/j.bbr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Seligman ME. Learned helplessness. Annu Rev Med. 1972;23:407–412. doi: 10.1146/annurev.me.23.020172.002203. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- Shah AA, Treit D. Infusions of midazolam into the medial prefrontal cortex produce anxiolytic effects in the elevated plus-maze and shock-probe burying tests. Brain Res. 2004;996:31–40. doi: 10.1016/j.brainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Shanks N, Anisman H. Stressor-provoked behavioral changes in six strains of mice. Behav Neurosci. 1988;102:894–905. doi: 10.1037//0735-7044.102.6.894. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. Annu Rev Psychol. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in post-weaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Singewald N. Altered brain activity processing in high-anxiety rodents revealed by challenge paradigms and functional mapping. Neurosci Biobehav Rev. 2007;31:18–40. doi: 10.1016/j.neubiorev.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 2004;11:525–535. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM. Hemispheric asymmetry in stress processing in rat prefrontal cortex and the role of mesocortical dopamine. Stress. 2004;7:131–143. doi: 10.1080/102538900410001679310. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Henke PG. The anterior midline cortex and adaptation to stress ulcers in rats. Brain Res Bull. 1986;17:493–496. doi: 10.1016/0361-9230(86)90216-9. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Behavioral effects of excitotoxic lesions of ventral medial prefrontal cortex in the rat are hemisphere-dependent. Brain Res. 2002;927:69–79. doi: 10.1016/s0006-8993(01)03328-5. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon’s horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tierney PL, Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci. 2004;20:514–524. doi: 10.1111/j.1460-9568.2004.03501.x. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Kros JM, Uylings HB. The development of the rat prefrontal cortex. Its size and development of connections with thalamus, spinal cord and other cortical areas. Prog Brain Res. 1990;85:169–183. doi: 10.1016/s0079-6123(08)62680-1. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Lukoff D, Hardesty JP. A prospective study of stressful life events and schizophrenic relapse. J Abnorm Psychol. 1989;98:407–411. doi: 10.1037//0021-843x.98.4.407. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PM, Blanchard RJ, Yang M, Blanchard DC. Differential effects of infralimbic vs. ventromedial orbital PFC lidocaine infusions in CD-1 mice on defensive responding in the mouse defense test battery and rat exposure test. Brain Res. 2004;1020:73–85. doi: 10.1016/j.brainres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wolf C, Waksman D, Finger S, Almli CR. Large and small medial frontal cortex lesions and spatial performance of the rat. Brain Res Bull. 1987;18:1–5. doi: 10.1016/0361-9230(87)90025-6. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]


