Abstract
Central pulse pressure can be non-invasively derived using the radial artery tonometric methods. Knowledge of central pressure profiles has predicted cardiovascular morbidity and mortality in several populations of patients, particularly those with known coronary artery disease and those receiving dialysis. Few data exist characterizing central pressure profiles in patients with mild-moderate chronic kidney disease who are not on dialysis. We measured central pulse pressure cross-sectionally in 2531 participants in the Chronic Renal Insufficiency Cohort study to determine correlates of the magnitude of central pulse pressure in the setting of chronic kidney disease. Tertiles of central pulse pressure (CPP) were < 36 mmHg, 36–51 mmHg and > 51 mmHg with an overall mean (± S.D.) of 46 ± 19 mmHg. Multivariable regression identified the following independent correlates of central pulse pressure: age, gender, diabetes mellitus, heart rate (negatively correlated), glycosylated hemoglobin, hemoglobin, glucose and PTH concentrations. Additional adjustment for brachial mean arterial pressure and brachial pulse pressure showed associations for age, gender, diabetes, weight and heart rate. Discrete intervals of brachial pulse pressure stratification showed substantial overlap within the associated central pulse pressure values. The large size of this unique chronic kidney disease cohort provides an ideal situation to study the role of brachial and central pressure measurements in kidney disease progression and cardiovascular disease incidence.
Keywords: Elasticity, epidemiology, diabetic nephropathies, hemodynamics, gender
Introduction
Chronic kidney disease (CKD) confers a substantial risk of cardiovascular target organ damage, especially when kidney function falls below 60 mL/min/1.73m2 corresponding to National Kidney Foundation stages 3, 4 and 5 1 2 that appears inadequately explained by traditional cardiovascular risk factors 3. One goal of the Chronic Renal Insufficiency Cohort (CRIC) study is to examine traditional and novel risk factors for cardiovascular target organ damage and for progressive loss of kidney function in a diverse population with CKD 4. High blood pressure is known to influence the course of kidney disease progression 5. In recent years some data suggest that the pulse pressure (the difference of systolic and diastolic blood pressure) as derived from the standard brachial blood pressure measurement is better correlated than traditional blood pressure measures (systolic, diastolic) to the rate at which estimated glomerular filtration rates (eGFR) decline in CKD 6.
Measurements of central pulse pressure have been used for more than two decades in an attempt to improve further upon the predictive value of standard brachial blood pressure measurements 7. Studies have shown that there is substantial variability in the level of blood pressure in the aorta between people with similar brachial blood pressure measurements that is difficult to estimate without performing either an invasive or a non-invasive assessment of aortic pressure 8. The increasing use of validated non-invasive devices that estimate central blood pressure profiles based on radial artery tonometry has facilitated the incorporation of these measurements into prospective cohort studies such as the Anglo-Cardiff Collaborative Trial 9, the Strong Heart Study 10 and CRIC (which included measurements of central (aortic) pulse pressure using radial artery tonometry as an ancillary study beginning in 2005).
Aging has a marked effect upon the relationship between central and brachial pulse pressure, as does female gender 11. However, little is known about the determinants of central aortic pressure pulse in the setting of CKD. Thus we aimed to determine clinical factors independently associated with central pulse pressure in CKD, and to evaluate how well brachial pulse pressure correlates with central pulse pressure in a large, ethnically diverse population of men and women with CKD.
Methods
Participants
Enrollment characteristics of the CRIC study have been previously described in detail 12. Central aortic pulse pressure measurements were adopted into the CRIC protocol beginning at the second annual follow-up visit and all participants enrolled in the CRIC study were invited to become part of this ancillary protocol. The procedures were approved by the Institutional Review Boards at the 7 clinical centers and all participants provided written informed consent.
Procedures
Central pulse pressure measurements were performed supine after at least 5 minutes of rest using the Sphygmocor PVx System (AtCor Medical, West Ryde, Australia) via the right radial artery at all CRIC sites. All personnel were trained and certified to take blood pressure measurements in the dominant arm with a Tyco aneroid sphygmomanometer using American Heart Association standards and to perform the central pressure measurements using radial artery tonometry 13 14. The operator captured 10 seconds of stable radial artery waveform. Pulse pressure was defined as the difference between the systolic and the diastolic blood pressure in mmHg. When this data was derived from standard blood pressure measurement it was brachial PP; when derived by algorithm from the radial artery waveform it was CPP.
Laboratory measures
Hemoglobin values were measured directly at the laboratories of each of the centers. Standard laboratory testing (e.g. serum creatinine, glucose, uric acid, calcium and phosphorous, etc.) was performed at the CRIC central laboratory in the University of Pennsylvania. The estimated glomerular filtration rate (eGFR) was determined according to the abbreviated MDRD formula using creatinine values calibrated to the Cleveland Clinic Laboratory 15. Some laboratory results were only available at the baseline visit and are noted in Table 1.
Table 1.
Characteristics of CRIC participants
| Central Pulse Pressure available | ||||
|---|---|---|---|---|
| Variable | All Eligible N = 3277 |
No N=746 |
Yes N=2531 |
P value |
| Male | 1796 (55.0%) | 360 (48.3%) | 1436 (57.1%) | <.0001 |
| White | 1601 (49.1%) | 339 (45.4%) | 1262 (50.1%) | <.0001 |
| Black | 1367 (41.9%) | 364 (48.8%) | 1003 (39.8%) | <.0001 |
| Other | 295 (9.04%) | 43 (5.8%) | 252 (10%) | <.0001 |
| Diabetes (Yes) | 1592 (48.8%) | 404 (54.2%) | 1188 (47.2%) | 0.0008 |
| Age, years | 59.71 (10.82) | 58.26 (10.47) | 60.14 (10.88) | <.0001 |
| *eGFR, mL/min/1.73m2 | 41.32 (15.34) | 41.22 (12.87) | 41.34 (16) | 0.8534 |
| Weight, kg | 91.23 (23.02) | 98.85 (27.09) | 88.97 (21.14) | <.0001 |
| Systolic BP, mmHg | 127.38 (22.11) | 128.36 (21.5) | 127.09 (22.29) | 0.1677 |
| Diastolic BP, mmHg | 70.16 (12.87) | 71.34 (12.92) | 69.82 (12.83) | 0.0047 |
| Pulse Pressure, mmHg | 57.22 (19.47) | 57.05 (19.06) | 57.27 (19.59) | 0.7800 |
| Augmentation Index, % (M(SD)/F(SD)) |
--- | --- | 25(12)/31(11) | --- |
| Amplification Ratio (M(SD)/F(SD)) |
--- | --- | 1.33(0.23)/1.25(0.19) | --- |
| Seated Heart Rate, beats/min |
67.99 (11.35) | 68.97 (11.41) | 67.7 (11.32) | 0.0072 |
| Hemoglobin, g/dL | 12.7 (1.78) | 12.47 (1.84) | 12.77 (1.75) | <.0001 |
| Creatinine, mg/dL | 1.95 (1.17) | 1.77 (0.55) | 2 (1.3) | <.0001 |
| Triglycerides, mg/dL | 152.87 (116.19) | 162.01 (121.96) |
149.95 (114.16) | 0.0138 |
| Calcium, mg/dL | 9.28 (0.51) | 9.2 (0.52) | 9.3 (0.5) | <.0001 |
| Phosphate, mg/dL | 3.7 (0.66) | 3.81 (0.67) | 3.67 (0.65) | <.0001 |
| Ca*Phosphate product | 33.95 (6.24) | 34.99 (6.22) | 33.64 (6.21) | <.0001 |
| †PTH, pg/mL | 73.11 (67.64) | 80.97 (77.37) | 70.76 (64.27) | 0.0004 |
| †Urine Protein, g/24H | 0.94 (2.09) | 1.19 (2.63) | 0.85 (1.86) | 0.0002 |
| †Hemoglobin A1C, % | 6.61 (1.54) | 6.76 (1.64) | 6.56 (1.51) | 0.0032 |
| †Uric Acid, mg/dL | 7.37 (1.9) | 7.72 (1.91) | 7.27 (1.89) | <.0001 |
| Medication: ACE-inhibitor | 1601 (49.2%) | 388 (52.2%) | 1213 (48.3%) | 0.0629 |
| Medication: ARB | 851 (26.1%) | 204 (27.4%) | 647 (25.7%) | 0.3615 |
| Medication: Calcium Antagonist |
1310 (40.2%) | 340 (45.7%) | 970 (38.6%) | 0.0005 |
| Medication: Beta-blocker | 1569 (48.2%) | 422 (56.7%) | 1147 (45.6%) | <.0001 |
| Medication: Diuretic | 1921 (59.0%) | 523 (70.3%) | 1398 (55.6%) | <.0001 |
| Medication: Other BP Med |
614 (18.9%) | 174 (23.4%) | 440 (17.5%) | 0.0003 |
Estimated Glomerular Filtration Rate;
Available at Baseline only
Race
Race was classified as American Indian/Alaskan Native, Asian/Asian American, Black/African American, Native Hawaiian/Other Pacific Islander or White/Caucasian based on participant self-report.
Central Pulse Pressure Data
The data reported here represent central aortic pulse pressure measures obtained on participants whose second annual follow up visit occurred on or before March 31, 2009. The augmentation index is a ratio that reflects the portion of the central pulse pressure derived from pulse wave reflection.
Statistical Analyses
Continuous data are presented as mean ± standard deviation (S.D.). Categorical variables are expressed as proportions. Independent variables were pre-specified for analyses based on previous studies showing a relation to central pulse pressure (such as age, systolic blood pressure) or because they may affect pulse pressure and are known to be affected by kidney disease (such as calcium, hemoglobin). A plot of central pulse pressure in our population showed a substantial rightward skew and analyses were performed on both raw CPP data and natural-log-transformed data. Univariable regression models for central pulse pressure were used to assess the relationship between CPP and the selected variable. We performed multivariable linear regression to examine the associations between variables of interest and central pulse pressure 16. All parameters significant at a p≤0.20 level in univariable regression were entered into both forward and backward selection algorithms. Where variables were known to be strongly correlated with each other (e.g. serum creatinine and eGFR) only the one with the stronger association was entered. Variables significant at the p≤0.05 level in the multivariable model were retained in the final model.
We considered two multivariable regression models: one with LnCPP as the outcome, without measures of brachial blood pressure as predictor; and one with LnCPP as the outcome, including measures of natural log-transformed brachial pulse pressure and brachial mean arterial pressure as predictors. All multivariable models were adjusted for clinical site. Analyses were executed in SAS 9.1 (SAS Institute, Cary, NC).
Results
Demographic characteristics of all participants eligible for central pulse pressure measurement at the year 2 follow up visit of the CRIC cohort are shown in Table 1, overall and stratified by those who did or did not have a successful central pulse pressure measurement. We anticipated data loss on approximately 20% of participants (due to arrhythmia and other difficulties with waveform capture) and we were unable to obtain or use measurements on 746 of 3277 eligible participants (22.7%).
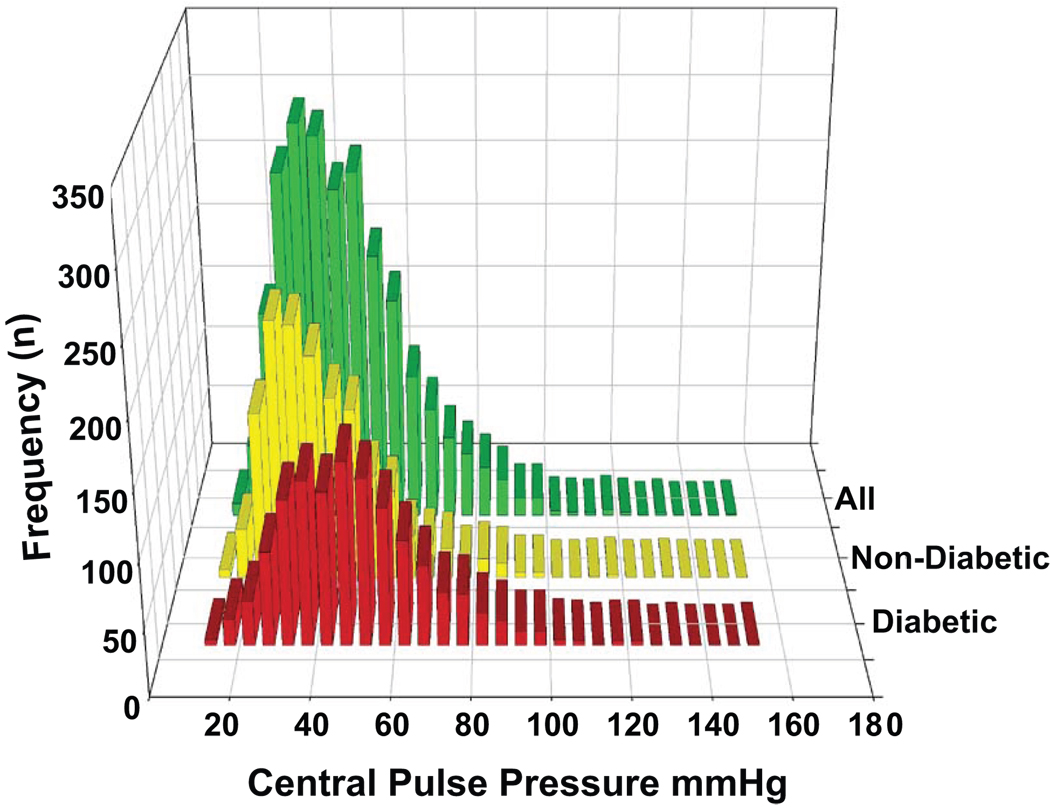
Figure 1 shows the frequency distribution of central pulse pressure determinations in the overall sample and stratified by diabetes status. The tertiles of the central pulse pressure were < 36 mmHg, 36–51 mmHg and > 51 mmHg with an overall mean value of 46 ± 19 (S.D.).
Figure 1.
Plot of Central Pulse Pressure in 5 mmHg increments along X-axis and number of participants in that increment on Y-axis. Green bars are all CRIC participants (n=2531). Yellow bars are those without (n=1343) and Ochre bars are those with (n=1188) diabetes.
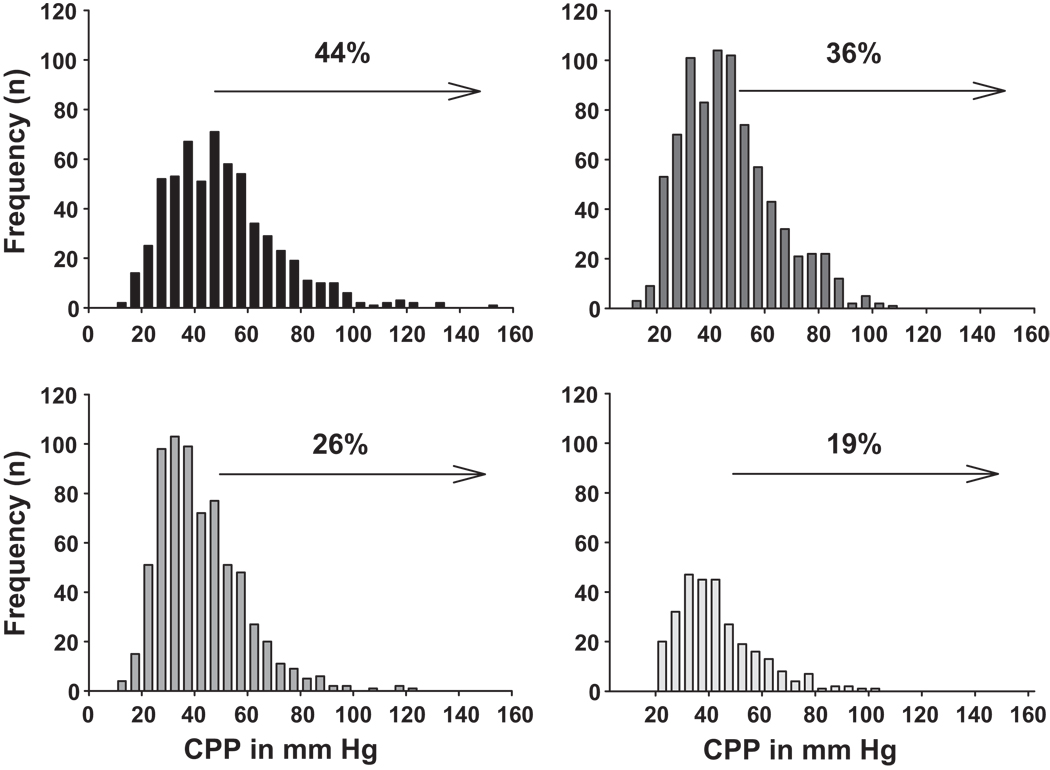
Figure 2 presents mean central pulse pressure values for the cohort by strata of renal function that correspond to CKD stages. Given the large number of participants in CKD stage 3 (30–59.9 mL/min/1.73m2 we divided this stage into stage 3A (45–59.9 mL/min/1.73m2) and 3B (30–44.9 mL/min/1.73m2). Each 10 mL/min/1.73m2 decrement in eGFR was associated with an increase in central pulse pressure of about 2.5 mmHg (Table 2). A central pulse pressure of 50 mmHg was recently shown to be a significant independent predictor of cardiovascular outcomes in the Strong Heart Study where it represented the lower boundary of the highest quartile 17. Figure 2 shows the increasing proportion (%) of those within each declining eGFR strata in CRIC that had a central pulse pressure ≥ 50 mmHg.
Figure 2.
Upper Left panel plots central pulse pressure in 10 mmHg increments among those with eGFR < 30 mL/min/1.73m2. Upper Right plots those with eGFR 30–44.9, Lower Left plots those with eGFR of 45–59.9 and the Lower Right panel depicts those with an eGFR > 60. Arrow onset marks Central Pulse Pressure (CPP) of 50 mmHg and percent (%) indicates the portion of participants within that eGFR range with a CPP > 50 mmHg.
Table 2.
Factors associated with Central Pulse Pressure
| Variable | Central Pulse Pressure | *Ln Central Pulse Pressure | |||||
|---|---|---|---|---|---|---|---|
| Est (StdErr) | R2 | p | Est (StdErr) | R2 | p | ||
| Female Sex | 3.787 (0.75) | 0.010 | <.0001 | 0.089 (0.02) | 0.012 | <.0001 | |
| Race | Black | 4.847 (0.79) | 0.020 | <.0001 | 0.106 (0.02) | 0.020 | <.0001 |
| Other | 6.527 (1.28) | 0.020 | <.0001 | 0.128 (0.03) | 0.020 | <.0001 | |
| Ethnicity | Hispanic | 4.700 (2.12) | 0.025 | <.0001 | 0.091 (0.04) | 0.024 | <.0001 |
| Diabetes | 10.449 (0.72) | 0.076 | <.0001 | 0.227 (0.02) | 0.081 | <.0001 | |
| Age (/10 years) | 6.083 (0.33) | 0.123 | <.0001 | 0.147 (0.01) | 0.162 | <.0001 | |
| †eGFR (/10 mL/min/1.73m2) | −2.483 (0.23) | 0.045 | <.0001 | −0.053 (0.00) |
0.046 | <.0001 | |
| Weight (/10 Kg) | −0.769 (0.18) | 0.008 | <.0001 | −0.015 (0.00) |
0.006 | <.0001 | |
| Waist (/10 cm) | 0.262 (0.23) | 0.001 | 0.2561 | 0.010 (0.00) | 0.002 | 0.0356 | |
| ‡MAP (/10 mmHg) | 4.656 (0.26) | 0.118 | <.0001 | 0.093 (0.01) | 0.106 | <.0001 | |
| §SBP (/10 mmHg) | 6.160 (0.12) | 0.535 | <.0001 | 0.127 (0.00) | 0.512 | <.0001 | |
| ‖DBP (/10 mm Hg) | −1.200 (0.29) | 0.007 | <.0001 | −0.030 (0.01) |
0.009 | <.0001 | |
| Heart Rate (/10 beats/min) | −3.187 (0.33) | 0.037 | <.0001 | −0.073 (0.01) |
0.043 | <.0001 | |
| Brachial PP (/10 mmHg) | 8.574 (0.09) | 0.795 | <.0001 | 0.179 (0.00) | 0.778 | <.0001 | |
| Hemoglobin (g/dL) | −3.098 (0.21) | 0.083 | <.0001 | −0.069 (0.00) |
0.094 | <.0001 | |
| Glucose (/10 mg/dL) | 0.575 (0.08) | 0.021 | <.0001 | 0.012 (0.00) | 0.020 | <.0001 | |
| Triglycerides (/10 mg/dL) | −0.060 (0.03) | 0.001 | 0.0754 | −0.002 (0.00) |
0.003 | 0.0159 | |
| LDL-Cholesterol (/10 mg/dL) | −0.237 (0.11) | 0.002 | 0.0374 | −0.007 (0.00) |
0.004 | 0.0045 | |
| Calcium (mg/dL) | −3.709 (0.75) | 0.010 | <.0001 | −0.074 (0.02) |
0.009 | <.0001 | |
| #Phosphate (mg/dL) | 1.979 (1.43) | 0.005 | 0.1670 | 0.044 (0.03) | 0.005 | 0.1608 | |
| #Calcium-Phosphate (product) | 0.324 (0.06) | 0.011 | <.0001 | 0.007 (0.00) | 0.013 | <.0001 | |
|
#Parathryoid Hormone (/10 pg/mL) |
0.343 (0.06) | 0.014 | <.0001 | 0.008 (0.00) | 0.016 | <.0001 | |
| #Urine Protein (g/day) | 1.439 (0.21) | 0.021 | <.0001 | 0.027 (0.00) | 0.016 | <.0001 | |
| #HemoglobinA1C (%) | 2.870 (0.24) | 0.054 | <.0001 | 0.063 (0.01) | 0.058 | <.0001 | |
| #Uric Acid (mg/dL) | 0.848 (0.20) | 0.007 | <.0001 | 0.017 (0.00) | 0.007 | <.0001 | |
| Number of Antihypertensive Drugs |
3.495 (0.24) | 0.083 | <.0001 | 0.078 (0.00) | 0.093 | <.0001 | |
Ln = Natural logarithm transformation of Central Pulse Pressure;
eGFR=estimated Glomerular Filtration Rate;
MAP=Mean Arterial Pressure;
SBP=Systolic Blood Pressure;
DBP=Diastolic Blood Pressure;
Aortic Tr = Time to reflected wave in aortic pressure profile;
Available at baseline visit only
Table 2 displays the results of univariable regression of demographic, hemodynamic and laboratory data of our participants on central pulse pressure. The strongest univariable associations with central pulse pressure were presence of diabetes, brachial pulse pressure and brachial systolic blood pressure, decade of age, female gender, non-white ethnicity, serum calcium, the number of antihypertensive medications taken regularly and lower eGFR level.
Multivariable analyses are described in Table 3a–3c. In the absence of an adjustment for brachial blood pressure (Table 3a), there were independent contributions to the natural log-transformed central pulse pressure (LnCPP) that included age (10 years), gender, diabetes, heart rate, glycosylated hemoglobin (HbA1C), hemoglobin, glucose and serum PTH concentration at baseline. When brachial pulse pressure and brachial mean arterial pressure were incorporated into the model (Table 3b) gender was the strongest non-blood pressure predictor term for LnCPP followed by age (10 years), diabetes, weight (per 10 kg) and heart rate. Weight and heart rate had negative influences on LnCPP. Glucose, glycosylated hemoglobin, hemoglobin and PTH concentrations were no longer independently associated with LnCPP. Table 3c shows that most of the variability in the multivariable model predicting LnCPP is explained from the natural log-transformed brachial pulse pressure. In the supplemental Table (online) we expanded our multivariable regression incorporating the time to central wave reflection (Tr) and augmentation index into the model to pursue these avenues as possible mechanisms by which these clinical factors might influence CPP, demonstrating that addition of augmentation index may mediate the effect of several clinical predictors of CPP, while aortic Tr was not a predictor of CPP in univariate or multivariable analysis. We refer the reader to the supplemental data section available at http://hyper.ahajournals.org for further details.
Table 3.
| a: Multivariable regression model for *LnCPP (no adjustment for brachial blood pressure) | ||
|---|---|---|
| Variable | Estimate (StdErr) | p value |
| Age (/10 years) | 0.13 (0.01) | <.0001 |
| Diabetes (Yes) | 0.09 (0.02) | <.0001 |
| Heart Rate (beats/minute) | −0.01 (0.00) | <.0001 |
| Sex (Male) | −0.08 (0.01) | <.0001 |
| HemoglobinA1C (%) | 0.03 (0.01) | <.0001 |
| Hemoglobin (gm/dL) | −0.04 (0.00) | <.0001 |
| Glucose (mg/dL) | 0.00 (0.00) | 0.0079 |
| †PTH Baseline (pg/mL) | 0.00 (0.00) | 0.0019 |
| b: Multivariable regression model for *LnCPP (adjusted for brachial pulse and mean arterial pressure) | ||
|---|---|---|
| Variable | Estimate (StdErr) | p value |
| †LnBPP (mmHg) | 0.98 (0.01) | <.0001 |
| Mean arterial pressure (mmHg) | 0.00 (0.00) | <.0001 |
| Age (/10 years) | 0.03 (0.00) | <.0001 |
| Sex (Male) | −0.06 (0.01) | <.0001 |
| Diabetes (Yes) | 0.02 (0.01) | 0.0087 |
| Heart Rate (beats/minute) | −0.01 (0.00) | <.0001 |
| Weight (/10 kg) | −0.012 (00) | <.0001 |
| c: Stepwise change in multivariable model R2 (from Table 3b) | |
|---|---|
| Variable | Model R2 |
| *LnBPP | 0.8395081 |
| LnBPP + †MAP | 0.8396461 |
| LnBPP + MAP + Age(/10yrs) | 0.8444102 |
| LnBPP + MAP + Age(/10yrs) + Diabetes (Y) | 0.8447113 |
| LnBPP + MAP + Age(/10yrs) + Diabetes (Y) + Heart Rate | 0.8589313 |
| LnBPP + MAP + Age(/10yrs) + Diabetes (Y) + Heart Rate + Sex | 0.8668658 |
| LnBPP + MAP + Age(/10yrs) + Diabetes (Y) + Heart Rate + Sex + Weight (/10 kg) | 0.8705188 |
Natural logarithm of Central Pulse Pressure;
Available Baseline only
Natural Logarithm of Brachial Pulse Pressure
Natural Logarithm of Brachial Pulse Pressure;
Mean Arterial Pressure in mmHg
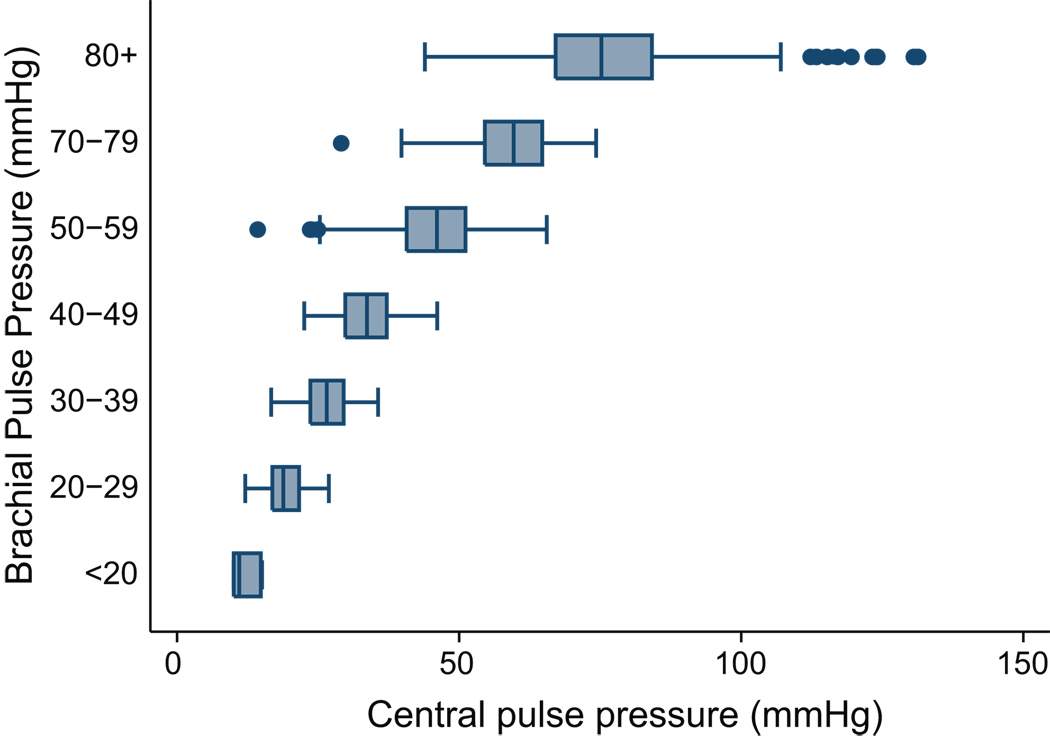
When discrete intervals of brachial pulse pressure were plotted against their corresponding levels of central pulse pressure there was a substantial overlap within the associated central pulse pressure values (Figure 3).
Figure 3.
shows discrete intervals of brachial pulse pressure in 10 mmHg increments on Y-axis and corresponding range of central pulse pressure measured on the X-axis. Box represents 25–75 percentile, whiskers indicate 95th percentile, line inside box is median value.
Discussion
We performed central aortic pressure measurements on a population of 2531 participants recruited specifically with impaired kidney function but not on dialysis, of whom approximately half were diabetic. Our results indicate that that central pulse pressure values are positively and independently correlated with increasing brachial pulse pressure, older age, female gender and the presence of diabetes in a population of participants with CKD. In addition we found a significant, though weaker, negative correlation between weight and heart rate, as noted in some other studies of central blood pressure 18. Our results confirm a substantial overlap in central pulse pressures when participants are stratified by discrete levels of brachial-derived pulse pressures (Figure 3). This report adds to the literature because it examined a uniquely large cohort with a spectrum of kidney dysfunction at high cardiovascular risk, a population infrequently studied using central pressure measures. Reproducibility of central blood pressure measurements has been shown by others in non-CKD populations 19 20. In addition our prior work in this CKD population as well as that of others21 22 shows good reproducibility supporting the validity of these findings.
Central pressure measurements offer the opportunity to estimate the pulse pressure that the left ventricle and aorta actually “see” 7. As the pressure wave travels from the elastic central vessels into the muscular arterial conduits there is a varying degree of increase (‘amplification’) in the systolic pressure whereas there is little change in diastolic or mean arterial pressure in the circulation. This rise in blood pressure is often expressed as an amplification ratio (defined as the pulse pressure in the brachial artery divided by the central aortic pulse pressure) 8. As shown in our study and by others knowledge of the brachial blood pressure is an imperfect estimate of central blood pressure levels 8 (Figure 3).
Studies comparing brachial pulse pressure compared with central pulse pressure using outcomes such as left ventricular mass, or the occurrence of cardiovascular target organ damage (carotid intima-media thickness, or death) have shown independent predictive value for central pulse pressure measurements 23 24 25 10 26. On the other hand, a recent meta-analysis of cardiovascular outcomes in longitudinal studies in which central hemodynamic measures were incorporated showed that whereas both central pulse pressure and the AIX predicted cardiovascular events and mortality, only the AIX did so independently of the brachial blood pressure 27. The kidney is particularly vulnerable to increased pulsatile stress as reviewed recently by Loutzenhiser 28.
Patients with CKD 1 29 and end stage renal disease have substantial cardiovascular risk 30 31. In a study of 349 subjects with CKD stages 4/5 a brachial pulse pressure of > 80 mmHg was an independent predictor of cardiovascular death or progression to ESRD requiring dialysis 32. In a non-CKD population, the Strong Heart Study, central pulse pressure measures were superior to brachial pulse pressure in predicting carotid intima-media thickness and cardiovascular outcomes 10. A second analysis of the Strong Heart Study data, including additional follow up time, derived a central pulse pressure of 50 mmHg or higher as a clinical meaningful threshold for increased cardiovascular target organ damage 17. For that reason we chose to show the proportion of our populations, stratified by kidney function, with a central pulse pressure above 50 mmHg recognizing our study is observational while the Strong Heart Study data were longitudinal (Figure 2). The recognition that reduced kidney function in CKD is related strongly to cardiovascular disease morbidity and mortality has stimulated an ongoing search for biologic markers beyond traditional Framingham risk factors since these explain some but not all of the extra cardiovascular burden in CKD 1 33.
Our study had several limitations. We were unable to successfully capture radial pulse waveforms in about 23% of our CKD participants and there were differences in patient characteristics between those with versus without successful waveforms. Importantly, though, the brachial pulse pressure was identical in both groups. Some laboratory values are only available at the baseline visit which occurred about 2 years before the first central pulse pressure measurement and these may have changed between baseline and the second year follow up visit. This caveat applies especially to glycosylated hemoglobin and serum PTH concentrations. Lastly, our participants take a number of antihypertensive medications which may affect CPP, although we did not find an independent effect of the number of antihypertensive agent classes taken.
Our findings in this large, diverse and unique CKD population show associations of age, gender, diabetes, heart rate, brachial systolic and pulse pressure with central pulse pressure. These are important associations in a group with such exceptional cardiovascular disease burden and are imperfectly represented by standard brachial pulse pressure determinations. The low resistance nature of the renal circulation predisposes the kidney to pressure-mediated damage 34. Brachial pulse pressure measures have been shown to predict both the development of CKD and the rate of loss of kidney function when CKD is already present. Knowledge of brachial pulse pressure is an imperfect predictor of central pulse pressures, thus, measures of central pulse pressure may be useful in patients with CKD to quantify their risk of cardiovascular disease and CKD progression. The role of central pulse pressure in predicting progressive kidney function loss as well as incident cardiovascular disease in established mild-to moderate CKD remains to be seen.
Perspectives
Our findings in this large, diverse and unique CKD population show associations of age, gender, diabetes, heart rate, brachial systolic and pulse pressure with central pulse pressure. These are important associations in a group with such exceptional cardiovascular disease burden and are imperfectly represented by standard brachial pulse pressure determinations. The low resistance nature of the renal circulation predisposes the kidney to pressure-mediated damage. Brachial pulse pressure measures have been shown to predict both the development of CKD and the rate of loss of kidney function when CKD is already present. Knowledge of brachial pulse pressure is an imperfect predictor of central pulse pressures, thus, measures of central pulse pressure may be useful in patients with CKD to quantify their risk of cardiovascular disease and CKD progression. The role of central pulse pressure in predicting progressive kidney function loss as well as incident cardiovascular disease in established mild-to moderate CKD remains to be seen. Longitudinal data from CRIC and other studies pursuing the value of central blood pressure measures will clarify further this important issue.
Supplementary Material
Acknowledgements
Marshall Joffe, MD, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Support:
These studies were supported by NIH/NIDDK grants including R01-DK-067390, U01-DK-060984, and NIH/NCRR grants UL1-RR024134, UL1 RR-025005, M01 RR-16500, UL1 RR-024989, M01 RR-000042, UL1 RR-024986, UL1RR029879, M01 RR-05096, UL1 RR-024131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The Corresponding author (RRT) received funding from the NIH/NIDDK. There is no other COI to declare.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Ix JH, Katz R, Kestenbaum B, Fried LF, Kramer H, Stehman-Breen C, Shlipak MG. Association of mild to moderate kidney dysfunction and coronary calcification. J Am Soc Nephrol. 2008;19:579–585. doi: 10.1681/ASN.2007070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astor BC, Hallan SI, Miller ER, III, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 4.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Ham LL, Hostetter T, Hsu C-y, Jamerson K, Joffe MP, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, III, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 6.Gosse P, Coulon P, Papaioannou G, Litalien J, Lemetayer P. Long-term decline in renal function is linked to initial pulse pressure in the essential hypertensive. J Hypertension. 2009;27:1303–1308. doi: 10.1097/HJH.0b013e32832a5ab3. [DOI] [PubMed] [Google Scholar]
- 7.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinsin I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 8.McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, Cockcroft JR, Wilkinson IB. Central Pressure: Variability and Impact of Cardiovascular Risk Factors. The Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51:1476–1482. doi: 10.1161/HYPERTENSIONAHA.107.105445. [DOI] [PubMed] [Google Scholar]
- 9.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson IB, Prasad K, Hall IR, Thomas A, MacCallum H, Webb DJ, Frenneaux MP, COckcroft JR. Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol. 2002;39:1005–1011. doi: 10.1016/s0735-1097(02)01723-0. [DOI] [PubMed] [Google Scholar]
- 12.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morganstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 14.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O'Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend RR, Weir M, Wright JT. Hypertension Awareness, Treatment, and Control in Adults with Chronic Kidney Disease: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2009;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Rosner B. Regression and correlation methods. In: Rosner B, editor. Fundamentals of Biostatistics. 6th ed. Belmont,CA: Thomson; 2006. pp. 464–556. [Google Scholar]
- 17.Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol. 2009;54:1730–1734. doi: 10.1016/j.jacc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benetos A, Thomas F, Joly L, Blacher J, Pannier B, Labat C, Salvi P, Smulyan H, Safar ME. Pulse Pressure Amplification A Mechanical Biomarker of Cardiovascular Risk. J Am Coll Cardiol. 2010;55:1032–1037. doi: 10.1016/j.jacc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 20.Filipovsky J, Svobodova V, Pecen L. Reproducibility of radial pulse wave analysis in healthy subjects. J Hypertens. 2000;18:1033–1040. doi: 10.1097/00004872-200018080-00007. [DOI] [PubMed] [Google Scholar]
- 21.Savage MT, Ferro CJ, Pinder SJ, Tomson CR. Reproducibility of derived central arterial waveforms in patients with chronic renal failure. Clin Sci (Lond) 2002;103:59–65. doi: 10.1042/cs1030059. [DOI] [PubMed] [Google Scholar]
- 22.Wimmer NJ, Townsend RR, Joffee MM, Lash JP, Go AS. CRIC Study Investigators. Correlation between pulse wave velocity and other measures of arterial stiffness in chronic kidney disease. Clin Nephrol. 2007;68:133–143. [PubMed] [Google Scholar]
- 23.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 24.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26:2657–2563. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- 25.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 26.Pini R, Cavallini MC, Palmieri V, Marchionni N, DiBari M, Devereaux RB, Masotti G, Roman MJ. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol. 2008;51:2432–2439. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 27.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:G1–G7. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 28.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Go AS, Lo JC. Epidemiology of non-dialysis-requiring chronic kidney disease and cardiovascular disease. Curr Opin Nephrol Hypertens. 2006;15:296–302. doi: 10.1097/01.mnh.0000222698.30207.aa. [DOI] [PubMed] [Google Scholar]
- 30.Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci. 2003;325:163–167. doi: 10.1097/00000441-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 31.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, DeMeester J, Wetzels JF, Rosendaal FR, Dekker FW. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–1789. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee D, Brincat S, Gregson H, Contreras G, Streather C, Oliveira D, Nelson S. Pulse pressure and inhibition of renin-angiotensin system in chronic kidney disease. Nephrol Dial Transplant. 2006;21:975–978. doi: 10.1093/ndt/gfi345. [DOI] [PubMed] [Google Scholar]
- 33.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;87:S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 34.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.