Abstract
Summary
Background
Breast cancer in men is an uncommon disease. Nearly all cases of male breast cancer originate in the terminal ductulolobular unit, with exceedingly rare reports of lobular carcinoma in men. Invasive lobular cancer is found in no more than 1-2% of male breast cancer cases. Most of what is known about this disease is in the form of approximately 30 case reports in the literature.
Case Report
We report the case of a 52-year-old man who presented at our institution with a lump in his left breast. Ultrasound-guided biopsy revealed an invasive lobular cancer. The rare histological type was confirmed by the result of the histological examination of the mastectomy specimen. The treatment was completed by dose dense chemotherapy, radiation, and endocrine therapy.
Conclusion
Even though lobular structures are quite infrequent in the normal male, sporadic cases of invasive lobular breast cancer have been described. A short overview will be given in this case report.
Key Words: Male breast cancer, Lobular carcinoma, Rare histology
Abstract
Zusammenfassung
Hintergrund
Brustkrebs ist eine ungewöhnliche Erkrankung beim Mann. Nahezu alle Erkrankungen leiten sich histopathologisch von der terminalen duktulolobulären Einheit der Brustdrüse ab. Das invasiv lobuläre Karzinom ist äußerst selten und ist nur in 1-2% der Fälle beim Mammakarzinom des Mannes beschrieben. Die Erkenntnisse rühren von zirka 30 publizierten Kasuistiken her.
Fallbericht
In unserer Einrichtung stellte sich ein 52-jähriger Mann mit einem Tastbefund in der linken Brust vor. Die sonografisch gestützte Stanzbiopsie ergab das Vorliegen eines invasiv lobulären Mammakarzinoms. Der seltene histopathologische Befund wurde bei der Untersuchung des Mastektomiepräparates bestätigt. Die Behandlung des Patienten wurde vervollständigt durch eine dosisdichte Chemotherapie, Bestrahlung und endokrine Behandlung.
Schlussfolgerung
Obwohl lobuläre Strukturen in der Brustdrüse des Mannes seltener und wesentlich kleiner sind, ist das sporadische Auftreten des invasiv lobulären Mammakarzinoms beim Mann beschrieben worden. In diesem Artikel soll ein kurzer Überblick darüber gegeben werden.
Introduction
Male breast cancer represents approximately 1% of all invasive breast cancer cases and less than 1% of all malignancies in men [1]. It is a serious disease for those diagnosed, with a median overall survival of 7 years [2]. Breast cancer is more likely to be detected at an advanced stage in men than in women, which may contribute to the poorer survival seen in men. The overall 5-year survival rates are similar in men and women when adjusted for stage and for the expected survival rate of the population in the United States for race, gender, and age [1]. Most primary breast cancers (90-93.7%) in men are of invasive ductal histology [1, 3]. A small percentage of cases are ductal carcinoma in situ, and under 2% are of lobular histology [1, 4]. We present the case of a man diagnosed with locally advanced (stage IIIC) invasive lobular breast cancer.
Case Report
Clinical Presentation
A 52-year-old white man presented at our institution with a 3-month history of a lump associated with a burning sensation in his left breast. A mammogram showed an irregular 2-cm spiculated mass in the subare-olar left breast. Clinical evaluation was notable for a visible left nipple retraction and distortion, but no break in the epidermis and no nipple discharge. On exam, a 1.5-2.0-cm firm nodule was palpable in the immediate subareolar area, together with a firm 2-cm node in the left axilla. Ultrasound revealed a 2.1-cm solid breast mass suspicious for malignancy. Ultrasound-guided core biopsy of the breast mass revealed invasive lobular carcinoma, and needle aspiration of the axillary nodes yielded cytopathology consistent with nodal metastases. Medical history was notable for a 2-year history of hypertension, hypercholesterolemia, pathologic myopia, and choroidal neovascularization. Prescribed medications included lisinopril/hydrochlorothiazide, atorvastatin, and eye drops consisting of dorzolamide, timolol, and latanoprost. He had no history of liver disease or exposure to estrogens or estrogenic agents. His family history was negative for breast cancer or ovarian cancer.
Histopathological Examination
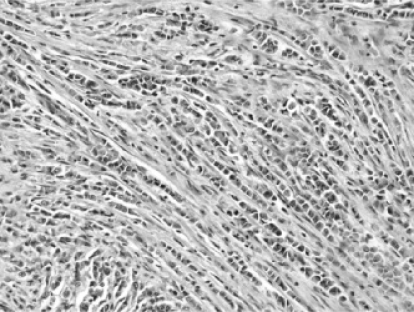
Modified radial mastectomy was performed and revealed a 2.4-cm mass located in the subareolar region. Histological examination showed a pure invasive lobular carcinoma (Elston-Ellis grade 2 of 3). The tumor was composed of a mixture of sheet-like architecture and an individual cell filing pattern (‘Indian filing’; fig. 1). Nests or tubules/glands were not present. The tumor cells were medium-sized, round, and discohesive with increased nuclear-to-cytoplasmic ratios and a mild amount of eosinophilic cytoplasm. Occasional plasmacytoid morphology was evident. The nuclei were moderately enlarged, round, and had slightly irregular chromatin distribution. The nuclear membranes were mostly smooth but displayed occasional irregular outlines. A small proportion of cells contained small chromocenters, but well-developed nucleoli were not present. Lymph-vascular space invasion was focally seen. The tumor invaded skin microscopically, but grossly evident skin nodules were not observed. Invasive carcinoma was present 1 mm from the deep surface of the specimen. The non-neoplastic breast did not contain usual duct hyperplasia, atypical ductal hyperplasia, ductal carcinoma in situ, atypical lobular hyperpla-sia, or lobular carcinoma in situ. Metastatic breast carcinoma involved 30 of 32 lymph nodes and showed extranodal extension. The lymph node metastases showed the same morphologic features as seen in the primary tumor. The pTNM stage was pT2N3MX. Immunohistochemical stains were performed on the tumor in the breast and showed strong expression of both estrogen receptors (ER) and progesterone receptors (PR) in >95% of the tumor cells and 2+ staining for HER-2/neu. Fluorescence in situ hybridization for HER-2/neu showed no amplification (HER-2/neu: D17Z1 ratio = 1.3).
Fig. 1.
H&E histological section of the breast tumor showing invasive lobular carcinoma. A classic individual cell filing pattern is present.
Treatment and Follow-up
Postoperatively, the patient was treated with 4 cycles of dose dense AC (doxorubicin at 60 mg/m2 with cyclophosphamide at 600 mg/m2 given every 2 weeks), followed by 4 cycles of dose dense paclitaxel (140 mg/m2 given every 2 weeks). After chemotherapy, he received radiation therapy to the left chest wall, axilla, and supraclavicular areas to a total of 6,000 cGy over 48 elapsed days. He began a 5-year course of tamoxifen 20 mg per day following radiotherapy. The patient was most recently seen 20 months after completion of chemotherapy with no evidence of disease on exam. The evaluation for a mutation of the breast cancer genes BRCA1 and BRCA2 showed a deleterious mutation in BRCA2.
Discussion
The majority of patients with male breast cancer presents with a palpable mass and/or visible nipple changes as did this patient [5]. Our patient had no obvious risk factors for breast cancer: no family history of breast cancer, no Ashkenazi ancestry, and no known exposure to estrogens or estrogenic agents, either exogenous or endogenous. Our patient has a mutation of the BRCA2 gene. Mutations of either the BRCA1 or the BRCA2 gene are thought to be found in 4-40% of men with breast cancer, with BRCA2 mutation being more frequent [5, 6]. The age at diagnosis of this patient was younger than the median age for men with breast cancer, which is in their 60s [3]. The usual surgical therapy for men with node positive disease, mastectomy with axillary lymph node dissection, was performed. Given the patient's gender and the histological appearance, metastatic prostatic adenocarcinoma could be considered in the differential diagnosis. However, histological features such as acini or glandular formation or prominent nucleoli, were not present, and the overall histological appearance was typical of lobular carcinoma of the breast. Variable expression of either ER or PR has been described in a subset of prostatic adenocarcinomas; however, the combined extent and intensity of expression of both ER and PR together as seen in this case, especially in the setting of the histological features, support the diagnosis of invasive lobular carcinoma [7, 8]. Systemic chemotherapy and endocrine therapy were given to this multinode-positive patient. Because of his high risk of chest wall recurrence, radiation was given to improve loco-regional control.
The factors leading to an individual case of male breast cancer are often unknown. Numerous etiologies have been elucidated, such as genetic mutations, especially BRCA2, and conditions associated with an imbalance between estrogen and testosterone. Certain medications, such as cimetidine [9, 10], are hypothesized to increase the risk for male breast cancer via an estrogenic mechanism. Testicular infection, injury, or maldescent resulting in a relative deficiency of testosterone have been proposed as possible predisposing factors [11]. Some environmental factors, such as exposure to heavy industry toxins, have been implicated in the development of male breast cancer [12]. Recently, a case of male breast cancer following allogeneic bone marrow transplantation was described [13]. Invasive lobular cancer is a very rare histopathological type of male breast cancer, as lobular and acinar structures are infrequent in the normal male breast [14]. Lobular cancer makes up a smaller percentage of breast cancer cases in men (1-2%) than in women (10-15%). The first case of invasive lobular carcinoma in a male patient without known estrogen exposure was described in 1989 [15], and meanwhile more than 30 cases have been reported in the literature. From the reports in the literature, there do not appear to be common elements in the cases of lobular carcinoma of the male breast that are distinctly different from the far more common cases of ductal carcinoma. Goss et al. [4] described 1.9% of male breast cancer presenting as invasive lobular in 229 patients. Evaluation of 2,537 male breast cancer cases from the SEER database between 1973 and 1998 found 1.5% of invasive lobular carcinomas [16]. The highest percentage, 4.2%, was noted by Nahleh et al. [2] who analyzed the Veterans Affairs Central Cancer registry with 612 male breast cancer patients. None of these studies were able to review the original pathology for confirmation of the lobular histology. There is no information in the literature regarding optimal treatment of lobular carcinoma of the male breast. There are no randomized clinical trials specifically for men with breast cancer of any histology, and for most existing breast cancer trials men are not eligible. Clinical knowledge of male breast cancers overall derives mostly from singleinstitution retrospective studies and from database studies. Our understanding of male breast cancer, and in particular its rarer subtypes, is limited. We present here a case of locally advanced lobular carcinoma of the male breast.
References
- 1.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 2.Nahleh ZA, Srikantiah R, Safa M, Jazieh AR, Muhleman A, Komrokji R. Male breast cancer in the veterans affairs population: a comparative analysis. Cancer. 2007;109:1471–1477. doi: 10.1002/cncr.22589. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Ayantunde AA, Rampaul R, Robertson JF. Male breast cancer: a review of clinical management. Breast Cancer Res Treat. 2007;103:11–21. doi: 10.1007/s10549-006-9356-z. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Reid C, Pintilie M, Lim R, Miller N. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955-1996. Cancer. 1999;85:629–639. doi: 10.1002/(sici)1097-0142(19990201)85:3<629::aid-cncr13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Rudlowski C. Male breast cancer. Breast Care. 2008;3:183–189. doi: 10.1159/000136825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerber B. Breast cancer in special situations. Breast Care. 2008;3:161–162. doi: 10.1159/000138038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobisch A, Hittmair A, Daxenbichler G, Wille S, Radmayr C, Hobisch-Hagen P, Bartsch G, Klocker H, Culig Z. Metastatic lesions from prostate cancer do not express oestrogen and progesterone receptors. J Pathol. 1997;182:356–361. doi: 10.1002/(SICI)1096-9896(199707)182:3<356::AID-PATH863>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Hiramatsu M, Maehara I, Orikasa S, Sasano H. Im-munolocalization of oestrogen and progesterone receptors in prostatic hyperplasia and carcinoma. Histopathology. 1996;28:163–168. doi: 10.1046/j.1365-2559.1996.280326.x. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of breast cancer in men with liver cirrhosis. Am J Gastroenterol. 1998;93:231–233. doi: 10.1111/j.1572-0241.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- 10.San Miguel P, Sancho M, Enriquez JL, Fernandez J, Gonzalez-Palacios F. Lobular carcinoma of the male breast associated with the use of cimetidine. Virchows Arch. 1997;430:261–263. doi: 10.1007/BF01324811. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DB, Jimenez LM, McTiernan A, Rosenblatt K, Stalsberg H, Stemhagen A, Thompson WD, Curnen MG, Satariano W, Austin DF, et al. Breast cancer in men: risk factors with hormonal implications. Am J Epidemiol. 1992;135:734–748. doi: 10.1093/oxfordjournals.aje.a116360. [DOI] [PubMed] [Google Scholar]
- 12.Cocco P, Figgs L, Dosemeci M, Hayes R, Linet MS, Hsing AW. Case-control study of occupational exposures and male breast cancer. Occup Environ Med. 1998;55:599–604. doi: 10.1136/oem.55.9.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe T, Luu T, Shen J, Bhatia S, Shibata S, Stein A, Somlo G. Male breast cancer 15 years after allo-geneic hematopoietic cell transplantation including total body irradiation for recurrent acute lymphob-lastic leukemia. Onkologie. 2008;31:266–269. doi: 10.1159/000121395. [DOI] [PubMed] [Google Scholar]
- 14.Ouriel K, Lotze MT, Hinshaw JR. Prognostic factors of carcinoma of the male breast. Surg Gynecol Obstet. 1984;159:373–376. [PubMed] [Google Scholar]
- 15.Nance KV, Reddick RL. In situ and infiltrating lobular carcinoma of the male breast. Hum Pathol. 1989;20:1220–1222. doi: 10.1016/s0046-8177(89)80017-6. [DOI] [PubMed] [Google Scholar]
- 16.Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137:678–687. doi: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]