Abstract
Trypanothione is a unique diglutathionyl-spermidine conjugate found in abundance in trypanosomes but not in other eukaryotes. Because trypanothione is a naturally occurring polyamine thiol reminiscent of the synthetic drug amifostine, it may be a useful protector against radiation and oxidative stress. For these reasons we hypothesized that trypanothione might serve as a radioprotective agent when produced in bacteria. To accomplish this objective, the trypanothione synthetase and reductase genes from T. cruzi were introduced into E. coli and their expression was verified by qPCR and immunoblotting. Trypanothione synthesis in bacteria, detected by HPLC, resulted in decreased intracellular levels of reactive oxygen species as determined by H2DCFDA oxidation. Moreover, E. coli genomic DNA was protected from radiation-induced DNA damage by 4.6-fold in the presence of trypanothione compared to control bacteria. Concordantly, the transgenic E. coli expressing trypanothione were 4.3-fold more resistant to killing by 137Cs γ radiation compared to E. coli devoid of trypanothione expression. Thus we have shown for the first time that E. coli can be genetically engineered to express the trypanothione biosynthetic pathway and produce trypanothione, which results in their radioresistance. These results warrant further research to explore the possibility of developing trypanothione as a novel radioprotective agent.
INTRODUCTION
Effective radioprotectors are needed for biomedical applications as well as for radiation disaster countermeasures. One approach to the discovery of novel radioprotectors is to identify and investigate the efficacy of potentially novel naturally occurring radioprotector molecules. One such molecule is trypanothione, a unique diglutathionyl-spermidine conjugate found in kinetoplastids within the suborder Trypanosomatida (1). Trypanosomes are extremely resistant to ionizing radiation and oxidative stress at least in part through the molecular physiology of the trypanothione molecule (2-4). Trypanothione is formed by the unification of two glutathione molecules with one spermidine molecule via the trypanosome specific enzyme trypanothione synthetase (TS). As a polyamine dithiol, trypanothione is reminiscent of man-made polyamine thiol radioprotectors such as amifostine. Polyamine cations catalyze the condensation of DNA, and when coupled with thiols they galvanize the DNA with reducing equivalents capable of detoxifying damaging free radicals and reactive species generated near the DNA (5). The availability of two vicinal thiol reducing equivalents per spermidine provides trypanothione with potentially better reducing potential than amifostine while maintaining the polyamine moiety to target these reducing equivalents to the vicinity of nucleic acids. These properties make trypanothione a superior radioprotector of DNA in solution compared to other thiol-containing molecules such as dithiothreitol, cysteine, cysteamine and glutathione (6). Trypanothione has been shown to be four times more effective than glutathione at protecting purified bacterial DNA from the harmful effects of ionizing radiation in vitro under anoxic conditions (6). However, whether trypanothione can serve as a radioprotector in a biologically intact organism other than trypanosomes has not been tested. To this end, we used a gene transfer strategy to establish the trypanothione biosynthesis pathway in E. coli and tested the effect of trypanothione accumulation on bacterial radiation biology and survival after exposure to ionizing radiation.
MATERIAL AND METHODS
Plasmids and Bacterial Growth
The T. cruzi expression plasmids encoding trypanothione synthetase, pET15b-TcTryS (Ampr), and trypanothione reductase, pBRT, were a generous gift from Dr. Alan Fairlamb (University of Dundee, Scotland) (7, 8). For simplicity, pET15b-TcTryS plasmid is referred to as pETA/TS in this paper. The TR gene was PCR amplified from the pBRT vector using the following PCR primers: TcRed forward: TTCCAGAAGAATCATGATGTC, and TcRed reverse: CTCTTTCCTTACAGAGATGCC. The TR expression vector pETK/TR (Kanr) was generated following the manufacturer’s conditions for cloning the TR PCR product into the pEK/LIC 30 bacterial expression vector (Novagen). All plasmid cloning and amplification was performed in the TOP10 E. coli strain (Invitrogen), and bacterial cultures were grown for 16 h at 37°C with shaking in Luria Broth supplemented with ampicillin or kanamycin, each at a final concentration of 100 μg/ml. All experimental procedures were performed in BL21 Star (DE3) E. coli (Invitrogen) transformed with both pETA/TS and pETK/TR in Luria Broth supplemented with both ampicillin and kanamycin. For studies of growth kinetics, BL21 E. coli was transformed with the empty vector pETA (Ampr) and pETK (Kanr) plasmids. To induce expression of recombinant TS and TR for HPLC analysis of trypanothione, Bl21 E. coli transformed with pETA/TS and pETK/TR were grown to an optical density (OD) at 600 nm of ~1.2; the culture was then cooled, induced with 0.5 mM isopropyl β-d-1-thiolgalactopyranoside (IPTG), and grown at 25°C at 100 rpm for 16 h. Alternatively, for nickel column purification and all irradiation experiments, transformed E. coli was induced in log phase with growth normalized to an OD of 0.6. Cultures were then induced with 0.25 mM IPTG for 3 h at 25°C at 100 rpm, after which the bacteria were collected for purification or irradiated.
Measurement of TS and TR Expression in E. coli
Total bacterial RNA was extracted from pETA/TS and pETK/TR-or pETA and pETK-transformed E. coli using Trizol (Invitrogen) according to the manufacturer’s specifications. After UV spectro-photometric determination of bacterial RNA yield, 1 μg of RNA was treated with DNase I as outlined in the RQ1 RNase-Free DNase Kit (Promega). After DNase treatment, the bacterial RNA was reverse transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems). Polymerase chain reaction was performed by making a 25-μl mixture containing 4 ng of cDNA, 12.5 pmol of forward oligonucleotide primer, 12.5 pmol of the corresponding reverse oligonucleotide primer for TS, TR or E. coli 16S rRNA (9), and 2X Power SYBR Green PCR Master Mix (Applied Biosystems). Oligonucleotide primers used for these studies were as follows: TS forward: TGCCGCTGATTCACGAGAA, TS reverse: CCCGTTTGAAGTGAAGCGACT, TR forward: GATTTGGTTGTCATTGGCGC, TR reverse: TCGGAACGCAGCCAACATT, 16S357 forward: CTCCTACGGGAGGCAGCAG, and 16S519 reverse: GWATTACCGCGGCKGCTG. Reactions were thermocycled according to the following conditions: 50°C for 2 min, 95°C for 10 min, 40 cycles (95°C for15 s and 60°C for 1 min). Ten microliters of the resulting reaction was fractionated by electrophoresis on a 2% agarose and 1× TBE gel, stained with ethidium bromide, and photographed by UV shadowing using an Alpha Imager camera system (Alpha Innotech Corporation).
Nickel Purification Strategy and Immunoblotting
Histidine tagged trypanothione synthetase and trypanothione reductase recombinant protein was purified using the Ni Sepharose gravity flow column His GraviTrap (GE Healthcare) according to the manufacturer’s protocol. The resulting input, flow through, wash and eluate were run on a 4–12% SDS/PAGE gel (BioRad). The composition of the resulting eluate was then visualized by Coomassie Blue staining. The nickel purification strategy was validated by SDS-PAGE fractionation of the eluate followed by immunoblotting with a monoclonal 6X His antibody (Invitrogen).
Analysis of Trypanothione Metabolism in E. coli
Detection of trypanothione by high-performance liquid chromatography (HPLC) in these studies was adapted from protocols described previously for detecting trypanothione from S. cerevisiae extracts (7). Trypanothione standards (Bachem) were prepared at concentrations ranging from 100 to 100,000 fmol/20 μl in diethylene-triaminepentaacetic acid (DETAPAC). Bacterial homogenates were prepared in 80 μl DETAPAC buffer after rinsing with PBS. Samples and standards were reduced with an equal volume of 5.0 mM DTT (dithiothreitol) in DETAPAC and incubated for 30 min. Then 20 μl of the sample was added to 230 μl nanopure water followed by derivatization with 750 μl 0.5 mM Thioglo® 3 (Covalent Associates, Inc.) at pH 8.0. After a 5-min incubation period, the samples were acidified to pH 2.5 to ensure that they remained stable. The maleimide derivatives were filtered and injected onto a phenyl-silica reverse-phase column (0.46 cm × 25 cm) (Vydac 219 TP) (10) using a Shimadzu HPLC system (SIL-10A auto injector, LC-10AT liquid chromatograph, RF-10 Axl fluorescence detector, and SCL-10 Avp system controller) operating at a flow rate of 0.8 ml/min with an isocratic mobile phase (65% acetonitrile, 25% nanopure water, 0.05% acetic acid, 0.05% o-phosphoric acid, pH 2.5).
Growth Analysis of Trypanothione-Expressing E. coli after Exposure to Ionizing Radiation
Cultures of pETA/TS and pETK/TR- or pETA and pETK-transformed BL21 E. coli were normalized to 0.6 OD and induced for 3 h with 0.25 mM IPTG and then received sham exposure or 600 Gy 137Cs γ rays at a dose rate of 29.9 Gy/min (J. L. Shepherd Irradiator). All cultures were then diluted with Luria Broth to an OD of 0.6 and cultured at 37°C at 200 rpm. The OD was then monitored every 5 h. Clonogenic cell survival after irradiation was determined in the presence or absence of IPTG and radiation. The E. coli was serially diluted in LB broth and plated at 10−3, 10−4, 10−5 and 10−6 on Luria Broth agar plates containing kanamycin and ampicillin. After overnight incubation at 37°C, surviving bacterial colonies were counted. The surviving fraction was calculated by dividing the number of colonies surviving irradiation in the presence or absence of IPTG cultures by their respective sham-irradiated counterparts.
Assessment of Bacterial Oxidative Stress
Oxidative stress was measured in sham-irradiated or irradiated pETA/TS and pETK/TR-transformed BL21 E. coli, grown as described above in the presence and absence of IPTG, by measuring fluorescence of the oxidation sensitive H2DCFDA probe [5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate; Molecular Probes]. Briefly, irradiated E. coli were pelleted by centrifugation and washed in PBS and were then incubated with 20 μM H2DCFDA or the oxidation-insensitive DCFDA probe [5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate; Molecular Probes] in PBS for 30 min in the dark. The E. coli were again washed and then pelleted and lysed for 20 min with BugBuster reagent (Novagen). The fluorescence of the bacterial cell lysate was read on a Spectrafluor Plus fluorescence plate reader (TECAN) with an excitation wavelength of 450 nm and an emission wavelength of 550 nm. Mean fluorescence was normalized to the protein concentration determined by the Bradford assay (Bio-Rad). Bacteria treated with 1 μM hydrogen peroxide for 30 min prior to measuring H2DCFDA fluorescence were used as a positive control.
Evaluation of DNA Damage in Trypanothione-Expressing E. coli
E. coli transformed with pETA/TS and pETK/TR were cultured with or without IPTG, then irradiated with 0 or 600 Gy 137Cs γ rays as described above. Two hours after irradiation, DNA was extracted using DNeasy Tissue Kit (Qiagen). DNA was dephosphorylated with calf intestine alkaline phosphatase (CIAP, Promega), then repurified using a Qiaquick kit (Qiagen). Bacteriophage T4 polynucleotide kinase (PNK) was then used to end-label 1 μg of dephosphorylated DNA from pETA/TS and pETK/TR transformed E. coli with [γ-32P]ATP (3000 Ci/mmol) (Perkin/Elmer). The reaction was carried out at 37°C for 10 min, then stopped by adding 0.5 M EDTA and heating to 78°C for 1 min (5′ End-Labeling System, Promega). The labeled DNA was then purified over a Sephadex G-50 spin column (Roche). The radiolabeled DNA was then spotted on Whatman circular filters with scintillation fluid and counted on a scintillation counter (LS6500 Beckman). The results are presented as counts per minute (cpm) per μg of DNA.
RESULTS
To determine whether expression of the trypanosome metabolite trypanothione (Fig. 1A) has a radioprotective effect on E. coli, we first engineered bacterial strains expressing the TS and TR genes. Expression of TS and TR was detected in pETA/TS and pETK/TR-transformed bacterial strains but not in E. coli transformed with pETA and pETK (Fig. 1B). TS and TR protein expression was verified after nickel column chromatography purification of histidine-tagged recombinant proteins from bacterial lysates. Fractionation of the nickel affinity purified bacterial eluates by SDS-PAGE followed by either Coomassie staining (Fig. 1C) or immunoblotting (Fig. 1D) demonstrated that pETA/TS and pETK/TR-transformed bacterial strains expressed two proteins corresponding to the molecular weights of T. cruzi TS (74 kDa) and TR (50 kDa) (7, 11). Consequently, transformation and selection for pETA/TS and pETK/TR replication in E. coli resulted in bacterial expression of T. cruzi TS and TR.
FIG. 1.

Generation of transgenic E. coli expressing the trypanothione biosynthesis pathway. Panel A: Chemical structure of the reduced form of the Trypanosome metabolite trypanothione [drawn with the aid of MarvinSketch, version 5.3.02 (ChemAxon)]. Panel B: Total RNA isolated from E. coli transformed with both pETA/TS and pETK/TR was reverse-transcribed and subjected to PCR using primers specific for the TS and TR genes and the E. coli 16S rRNA. PCR products were size fractionated on a 2% agarose gel and stained with ethidium bromide. Panel C: pETA/TS and pETK/TR-transformed bacterial cell lysates were sonicated and recombinant His-tagged trypanothione synthetase and reductase proteins were purified by nickel affinity chromatography. The resulting eluate was fractionated by SDS-PAGE, stained with Coomassie blue, and compared to the input, unbound and wash fractions. Panel D: The nickel purification strategy was also validated by immunoblotting of the recombinant His-tagged proteins after SDS-PAGE fractionation of the eluate.
After demonstrating that transgenic TS and TR expression could be established in E. coli, we next confirmed that trypanothione was generated in this system. Using a method described previously for detecting trypanothione production in S. cerevisiae (7), a HPLC detection method was adapted using the fluorescent maleimide sulfhydryl derivatizing reagent Thioglo® 3 (10). The robustness of the HPLC method for detection of trypanothione was demonstrated by using a commercially available trypanothione preparation to generate a standard curve that revealed that detection of trypanothione by HPLC was linear within a range of 5 nM to 5 μM (Fig. 2A). Trypanothione synthesis was next detected in pETA/TS and pETK/TR-transformed E. coli bacterial cell lysates by HPLC. Trypanothione had an average peak retention time of 14.53 to 16.15 min (Fig. 2B), and after comparing the sample peak area to the areas with given trypanothione concentrations in the standard curve, the intracellular concentration of trypanothione in pETA/TS and pETK/TR-transformed bacteria was determined to be 74 μmol per mg bacterial protein. Clearly, therefore, stable expression of DNA encoding T. cruzi TS and TR in bacteria is sufficient to generate detectable levels of trypanothione in E. coli. Furthermore, despite the fact that TR expression was more favorable than TS expression under our experimental conditions, the level of TS expression achieved was sufficient to confer accumulation of trypanothione in pETA/TS and pETK/TR-transformed bacteria (Fig. 1C, D).
FIG. 2.
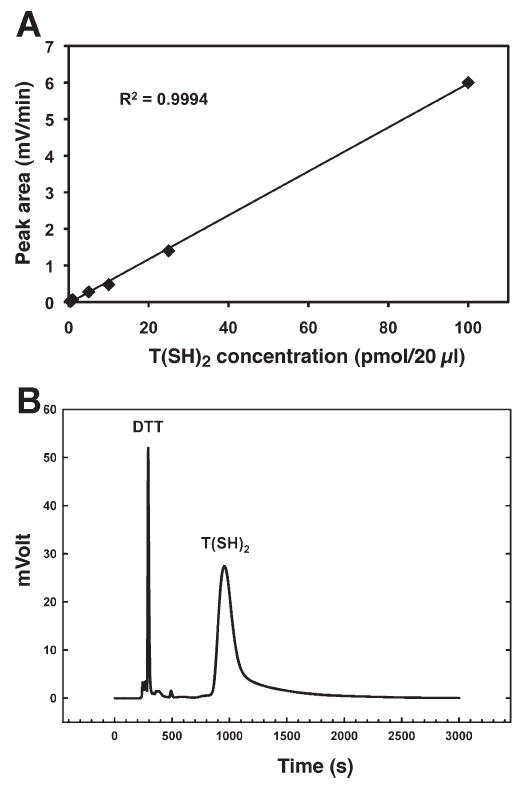
Determination of trypanothione levels in transgenic E. coli. Panel A: A standard curve for quantification of trypanothione by HPLC. Samples were reduced, derivatized and filtered prior to HPLC analysis using a phenyl-silica reverse-phase column with a Shimadzu HPLC system. Fluorescence was detected in the mobile phase at a flow rate of 0.8 ml/min. A standard curve with the equation y = 0.06x − 0.0295 was determined by linear regression, with trypanothione having an average retention time of 14.53–16.15 min. Panel B: Trypanothione levels in pETA/TS and pETK/TR-transformed bacterial cell homogenates were measured by HPLC and quantified by comparison to the standard curve (panel A). The DTT and trypanothione peaks are indicated.
To determine whether trypanothione expression affected bacterial response to ionizing radiation, we assessed survival of E. coli after irradiation in two ways. First, we assessed the growth delay in liquid cultures of E. coli after exposure to 600 Gy of ionizing radiation. All irradiated bacteria demonstrated a growth delay compared to unirradiated bacteria; however, the delay was considerably longer in cells lacking trypanothione (~9 h) than in cells with trypanothione (~4 h) (Fig. 3A). Interestingly, the postirradiation growth kinetics of non-IPTG-treated E. coli transformed with pETA/TS and pETK/TR was intermediate to the growth kinetics of irradiated non-IPTG-treated E. coli transformed with the pETA and pETK vector controls and irradiated, IPTG-treated pETA/TS and pETK/TR-transformed E. coli. This is likely due to basal non-IPTG induced trypanothione expression from the transgene constructs. Second, the clonogenic survival of trypanothione-expressing bacteria exposed to ionizing radiation was also examined. The surviving fraction of IPTG-treated pETA/TS and pETK/TR-transformed E. coli increased 4.6-fold compared to the survival of E. coli transformed with pETA and pETK alone (Fig. 3B, C). Therefore, bacterial survival in response to radiation can be enhanced through the expression of the trypanosome specific metabolite trypanothione.
FIG. 3.

Trypanothione production confers radioresistance to E. coli. Panel A: E. coli transformed with both pETA/TS and pETK/TR were sham-irradiated (dashed lines) or γ-irradiated (solid lines) with 600 Gy 137Cs. Fresh LB broth with antibiotics was inoculated with equal numbers of treated bacteria, trypanothione expression was induced by addition of IPTG (squares), and growth was compared to empty vector-transformed E. coli or transformed but not IPTG-treated E. coli by measuring the optical density (OD at 600 nm). Panel B: E. coli transformed with both pETA/TS and pETK/TR were grown to 0.6 OD in liquid LB cultures, and then cultures were treated with 0.25 mM IPTG and allowed to grow for 3 h at 25°C. Samples were then γ-irradiated with 600 Gy 137Cs and serially diluted and plated on LB agar plates containing kanamycin and ampicillin. After overnight incubation at 37°C visible colonies were counted. Panel C: Average surviving fractions of bacteria irradiated in the presence and absence of trypanothione; error bars are standard deviations for three plates from a representative experiment.
Finally, we examined the biological ramifications of trypanothione expression on the physiology of bacterial cells. Because of the inherent reducing potential of trypanothione, the effect of trypanothione expression on the bacterial redox status was examined first. Bacterial trypanothione expression completely prevented the increase in reactive oxygen species observed after exposure to ionizing radiation (Fig. 4A) as determined by the oxidation-sensitive probe H2DCF-DA. In addition, the baseline oxidation of the H2DCF-DA probe was also decreased compared to E. coli that did not express trypanothione, suggesting that trypanothione production attenuates the basal pro-oxidant state of E. coli. There was no difference in the fluorescence of the oxidation-insensitive probe DCF-DA, indicating the specificity of the probe to alterations in bacterial redox state (data not shown). Next we measured the frequency of radiation-induced DNA lesions by examining the integrity of the E. coli chromosome in trypanothione-expressing bacteria. Taking advantage of the fact that E. coli genomes are circular in nature, we developed a polynucleotide kinase radiolabeling assay to assess the relative frequency of DNA strand breaks. As expected, bacteria exposed to ionizing radiation increased the incorporation of 32P label into bacterial genomic DNA (Fig. 4B), indicating a greater number of DNA strand breaks than in unirradiated bacteria. Expression of trypanothione inhibited bacterial DNA damage after ionizing radiation and decreased the frequency of E. coli chromosome breakage by ~4.3-fold in irradiated bacteria expressing trypanothione compared to control E. coli (Fig. 4B). Remarkably, irradiated trypanothione-expressing bacteria displayed 2.5-fold fewer DNA lesions than unirradiated E. coli (Fig. 4B). Taken together, these results demonstrate that trypanothione expression acts as an effective radioprotector with respect to both suppression of DNA damage and accumulation of reactive oxygen species.
FIG. 4.
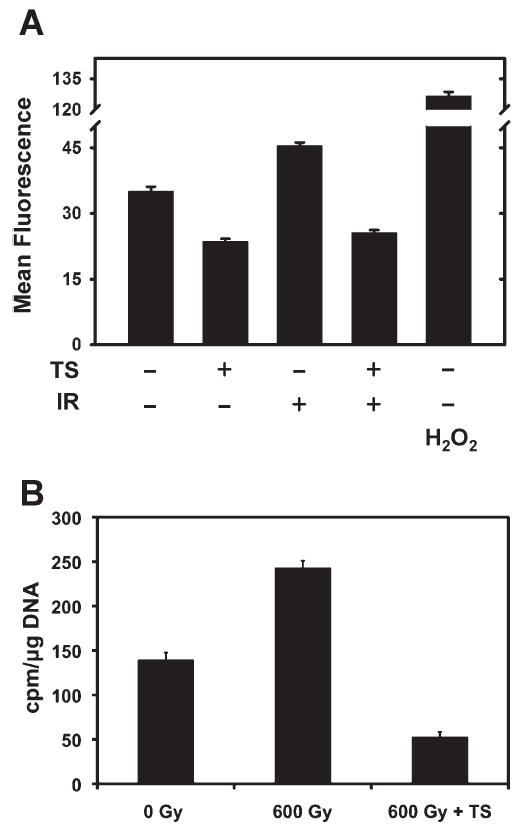
Trypanothione protects E. coli from radiation-induced reactive oxygen species accumulation and DNA damage. pETA/TS and pETK/TR-transformed E. coli were grown to 0.6 OD in liquid LB cultures and then treated with 0.25 mM IPTG. Panel A: One hour after γ irradiation, bacteria were incubated with 20 mM H2DCF-DA for 30 min at 37°C in the dark and the fluorescence of the oxidative sensitive probe was measured. Bacteria treated with 1 μM H2O2 were used as a pro-oxidant positive control. Fluorescence intensity is shown as the average measurement of duplicate determinations from a representative experiment; error bars represent the standard deviations. Panel B: DNA was extracted from pETA/TS and pETK/TR-transformed E. coli 2 h after γ irradiation and quantified by UV spectrophotometry. Strand breaks in circular bacterial genomic DNA were enzymatically end-labeled with T4 PNK in the presence of [γ-32P]ATP. γ-32P incorporation into end-labeled DNA was measured using a scintillation counter. Data are averages of duplicate determinations from a representative experiment; error bars are standard deviations.
DISCUSSION
Trypanosomes have long been known to be one of the most radioresistant eukaryotic organisms. It requires 100 Gy of X rays to render the T. cruzi strain noninfectious and 600 Gy of X rays to destroy the T. gambiense parasite (2, 3). The radioresistance of trypanosomes has been explained in part by the induction of Rad51 after exposure of T. cruzi to γ rays. Rad51 is a key component of double-strand DNA repair, and as such T. cruzi strains engineered to overexpress Rad51 overcome the genotoxicity associated with ionizing radiation faster than wild-type T. cruzi strains (12). The importance of trypanothione redox biology on trypanosome radioresistance can be inferred from the fact that TR-null trypanosomes are more susceptible to hydrogen peroxide-mediated killing than wild-type parasites (4). Despite the fact that trypanothione is the cornerstone of thiol-mediated redox reactions in Trypanosomatids, the need for trypanothione for the radioresistant properties of trypanosomes has never been tested. Testing the role of trypanothione in parasite radioresistance has proven difficult, because the TS enzyme is necessary for the viability of Trypanosomatids (13, 14). In spite of this, the ability to manipulate a conditionally null TS parasite has recently been developed through the generation of an engineered T. brucei strain in which TS deletion can be induced (15). This system might be used to determine whether intrinsic trypanosome radioresistance depends on trypanothione synthesis.
The use of thiol-containing molecules as cellular radioprotectors has been investigated extensively. Glutathione is relevant as a radioprotector because it is present at millimolar levels in human cells. Glutathione supplementation can render normal cells radioresistant (16, 17), and glutathione depletion increases cellular radiosensitivity (18, 19). Intravenous injection of cysteine into rats increases survival after X irradiation (20). Similarly, intraperitoneal injection of N-acetyl cysteine (NAC) protects mice from the effects of X radiation through antioxidant mechanisms such as maintenance of glutathione levels and decreased accumulation of oxidant markers of radiation-induced stress such as malondialdehyde and 8-hydroxy-deoxyguanosine (21). Amifostine treatment has been shown to decrease oxidative stress in human patients with bone marrow failure after total-body irradiation during the course of bone marrow transplantation, and intraperitoneal amifostine administration has been shown to increase antioxidant enzyme expression in normal mouse tissue (22, 23). Therefore, the ability of trypanothione to reduce intracellular oxidative stress is consistent with the characteristic of other thiol-containing radioprotector molecules. The di-thiol nature of trypanothione provides a unique aspect to its redox biochemistry compared to mono-thiol-containing radioprotector molecules (5). In the case of trypanothione, the fast reaction of one thiyl radical with the vicinal –SH will yield the disulfide radical anion of trypanothione, which can reduce molecular oxygen to yield the trypanothione disulfide and superoxide radical that can be readily decomposed by superoxide dismutases. This unique characteristic of trypanothione has been shown previously by demonstrating that trypanothione protects the biological transformative capacity of purified bacterial DNA in vitro from ionizing radiation more effectively than glutathione, spermidine or a combination of the two (6). Last, while the studies presented here focused on the direct removal of radiation-induced oxidative stress by trypanothione in E. coli, our findings do not rule out the possible indirect contribution of trypanothione expression on radioprotection. Such mechanisms might include facilitating DNA mismatch repair chemistry, since it has been shown that thiol-containing biomolecules can contribute to DNA mismatch repair pathways (24).
Despite the evidence that supports the potential use of trypanothione as a cellular radioprotector, several questions still exist regarding the clinical use of this polyamine di-thiol. First, the use of trypanothione as a tissue radioprotector is dependent upon its toxicity and its ability to provide less radioprotection in cancerous tissue than in normal tissues. Differential radioprotection of normal tissue by amifostine is thought to occur because diffusion into and use of amifostine in normal tissue is more favorable than in tumors (25, 26). It remains to be seen what effect if any trypanothione treatment will have on normal human cells.
It remains to be seen whether trypanothion protects DNA from the oxidative stress associated with ionizing radiation in mammalian cells. Mammalian genomes are inherently more complex than bacterial genomes in that they are dramatically larger, are packaged within an ordered nucleosome structure, and are contained within a dedicated cellular organelle. Of these characteristics, only the size of mammalian genomes differs significantly from Trypanosomatids (27), raising the question as to whether trypanothione could be synthesized to the extent necessary to shield a large metazoan genome.
Last, delivery of the TS and TR genes to human cells is also of concern when considering the efficacy of using trypanothione as a cellular radioprotector. While the pitfalls associated with gene therapy have been documented extensively, the question remains as to whether exogenously added trypanothione is taken up by human cells. Gamma-glutamyl transpeptidase indirectly increases intracellular glutathione levels by transporting the breakdown products of glutathione across the plasma membrane, but direct transport of glutathione across the plasma membrane has not been described (18). Given that trypanothione is different from glutathione because trypanothione has an overall net positive charge, it may be illogical to assume that trypanothione cannot translocate across the plasma membrane like other small molecular weight antioxidants, including NAC, vitamin C and polyphenols. Finally, if trypanothione supplementation should fail due to issues involving cellular trypanothione uptake, it would be worthwhile to determine whether modifications on the carboxy terminus of trypanothione, such as esterification, would facilitate efficacious trypanothione delivery in vivo (28). Taken together, our results support further investigation into the potential use of trypanothione as a radioprotector of normal tissue for biomedical applications and radiation countermeasures.
Acknowledgments
We would like to thank the Radiation and Free Radical Research Core at the University of Iowa in the Holden Comprehensive Cancer Center for support with experiments involving ionizing radiation and HPLC detection methodology for trypanothione (NIH P30 CA086862). RR is an International Research Scholar of the Howard Hughes Medical Institute. We are also greatly indebted to Drs. Marcelo Comini, Leopold Flohé and Lucia Piacenza for helpful discussions regarding Trypanosome biology.
References
- 1.Fairlamb AH, Blackburn P, Ulrich P, Chait BT, Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985;227:1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- 2.Halberstaedter L. The effect of X rays on trypanosomes. Br J Radiol. 1938;11:267–270. [Google Scholar]
- 3.Emmett J. Effect of X-radiation on Trypanosoma cruzi. J Parasitol. 1950;36:45–47. [PubMed] [Google Scholar]
- 4.Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol Microbiol. 2000;35:542–552. doi: 10.1046/j.1365-2958.2000.01721.x. [DOI] [PubMed] [Google Scholar]
- 5.Zheng S, Newton GL, Gonick G, Fahey RC, Ward JF. Radioprotection of DNA by thiols: relationship between the net charge on a thiol and its ability to protect DNA. Radiat Res. 1988;114:11–27. [PubMed] [Google Scholar]
- 6.Awad S, Henderson GB, Cerami A, Held KD. Effects of trypanothione on the biological activity of irradiated transforming DNA. Int J Radiat Biol. 1992;62:401–407. doi: 10.1080/09553009214552281. [DOI] [PubMed] [Google Scholar]
- 7.Oza SL, Tetaud E, Ariyanayagam MR, Warnon SS, Fairlamb AH. A single enzyme catalyses formation of Trypanothione from glutathione and spermidine in Trypanosoma cruzi. J Biol Chem. 2002;277:35853–35861. doi: 10.1074/jbc.M204403200. [DOI] [PubMed] [Google Scholar]
- 8.Borges A, Cunningham ML, Tovar J, Fairlamb AH. Site-directed mutagenesis of the redox-active cysteines of Trypanosoma cruzi trypanothione reductase. Eur J Biochem. 1995;228:745–752. doi: 10.1111/j.1432-1033.1995.tb20319.x. [DOI] [PubMed] [Google Scholar]
- 9.Stackebrandt E, Goodfellow M. Nucleic Acid Techniques in Bacterial Systematics. Wiley; Chichester and New York: 1991. [Google Scholar]
- 10.Steenkamp DJ. Simple methods for the detection and quantification of thiols from Crithidia fasciculata and for the isolation of trypanothione. Biochem J. 1993;292:295–301. doi: 10.1042/bj2920295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauth-Siegel RL, Enders B, Henderson GB, Fairlamb AH, Schirmer RH. Trypanothione reductase from Trypanosoma cruzi. Purification and characterization of the crystalline enzyme. Eur J Biochem. 1987;164:123–128. doi: 10.1111/j.1432-1033.1987.tb11002.x. [DOI] [PubMed] [Google Scholar]
- 12.Regis-da-Silva CG, Freitas JM, Passos-Silva DG, Furtado C, Augusto-Pinto L, Pereira MT, DaRocha WD, Franco GR, Macedo AM, Machado CR. Characterization of the Trypanosoma cruzi Rad51 gene and its role in recombination events associated with the parasite resistance to ionizing radiation. Mol Biochem Parasitol. 2006;149:191–200. doi: 10.1016/j.molbiopara.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Comini MA, Guerrero SA, Haile S, Menge U, Lunsdorf H, Flohe L. Validation of Trypanosoma brucei trypanothione synthetase as drug target. Free Radic Biol Med. 2004;36:1289–1302. doi: 10.1016/j.freeradbiomed.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Ariyanayagam MR, Oza SL, Guther ML, Fairlamb AH. Phenotypic analysis of trypanothione synthetase knockdown in the African trypanosome. Biochem J. 2005;391:425–432. doi: 10.1042/BJ20050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyllie S, Oza SL, Patterson S, Spinks D, Thompson S, Fairlamb AH. Dissecting the essentiality of the bifunctional trypanothione synthetase-amidase in Trypanosoma brucei using chemical and genetic methods. Mol Microbiol. 2009;74:529–540. doi: 10.1111/j.1365-2958.2009.06761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo A, Mitchell JB. Radiation response of Chinese hamster cells after elevation of intracellular glutathione levels. Int J Radiat Oncol Biol Phys. 1984;10:1243–1247. doi: 10.1016/0360-3016(84)90326-2. [DOI] [PubMed] [Google Scholar]
- 17.Wellner VP, Anderson ME, Puri RN, Jensen GL, Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci USA. 1984;81:4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen GL, Meister A. Radioprotection of human lymphoid cells by exogenously supplied glutathione is mediated by gamma-glutamyl transpeptidase. Proc Natl Acad Sci USA. 1983;80:4714–4717. doi: 10.1073/pnas.80.15.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dethmers JK, Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci USA. 1981;78:7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patt HM, Tyree EB, Straube RL, Smith DE. Cysteine protection against X irradiation. Science. 1949;110:213–214. doi: 10.1126/science.110.2852.213. [DOI] [PubMed] [Google Scholar]
- 21.Neal R, Matthews RH, Lutz P, Ercal N. Antioxidant role of N-acetyl cysteine isomers following high dose irradiation. Free Radic Biol Med. 2003;34:689–695. doi: 10.1016/s0891-5849(02)01372-2. [DOI] [PubMed] [Google Scholar]
- 22.Grdina DJ, Murley JS, Kataoka Y, Baker KL, Kunnavakkam R, Coleman MC, Spitz DR. Amifostine induces antioxidant enzymatic activities in normal tissues and a transplantable tumor that can affect radiation response. Int J Radiat Oncol Biol Phys. 2009;73:886–896. doi: 10.1016/j.ijrobp.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Facorro G, Sarrasague MM, Torti H, Hager A, Avalos JS, Foncuberta M, Kusminsky G. Oxidative study of patients with total body irradiation: effects of amifostine treatment. Bone Marrow Transplant. 2004;33:793–798. doi: 10.1038/sj.bmt.1704427. [DOI] [PubMed] [Google Scholar]
- 24.Lai GM, Ozols RF, Young RC, Hamilton TC. Effect of glutathione on DNA repair in cisplatin-resistant human ovarian cancer cell lines. J Natl Cancer Inst. 1989;81:535–539. doi: 10.1093/jnci/81.7.535. [DOI] [PubMed] [Google Scholar]
- 25.Yuhas JM. Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res. 1980;40:1519–1524. [PubMed] [Google Scholar]
- 26.Lindegaard JC, Grau C. Has the outlook improved for amifostine as a clinical radioprotector? Radiother Oncol. 2000;57:113–118. doi: 10.1016/s0167-8140(00)00235-8. [DOI] [PubMed] [Google Scholar]
- 27.El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran AN, Ghedin E, Worthey EA, Delcher AL, Andersson B. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- 28.Fleisher D, Bong R, Stewart BH. Improved oral drug delivery: Solubility limitations overcome by the use of prodrugs. Adv Drug Deliv Rev. 1996;19:115–130. [Google Scholar]


