Abstract
Green tea and black tea (BT) contain gallated [(−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin-3-gallate] and nongallated [(−)-epicatechin, (−)-epigallocatechin (EGC)] tea polyphenols (PP). During BT production, PP undergo oxidation and form larger polymers such as theaflavins (THE) and thearubigins, which contribute to the health benefit of BT. This article gives an overview of the role of chemical characteristics and endogenous metabolism of tea PP and their bioavailability in humans and describes attempts to increase their bioavailability. At pH close to neutral, EGCG and EGC form homo- and heterodimers generating hydrogen peroxide. To confirm the pH instability of EGCG, EGC, and THE in cell culture medium, their anti-proliferative activity was determined in the presence and absence of catalase. The antiproliferative activity in LNCaP prostate cancer cells was decreased when incubated with catalase prior to EGCG, EGC, and THE treatment. In addition, new findings demonstrated that the formation of methyl-EGC increased the stability at neutral pH compared with EGC. Approaches to increase the bioavailability of flavan-3-ols are reviewed, which include the administration of tea in combination with fruit juices, coadministration with piperine, and peracetylation of EGCG. Future intervention studies will need to focus on the bioactivity not only of green tea and BT PP but also of their metabolites and biotransformation products.
Introduction
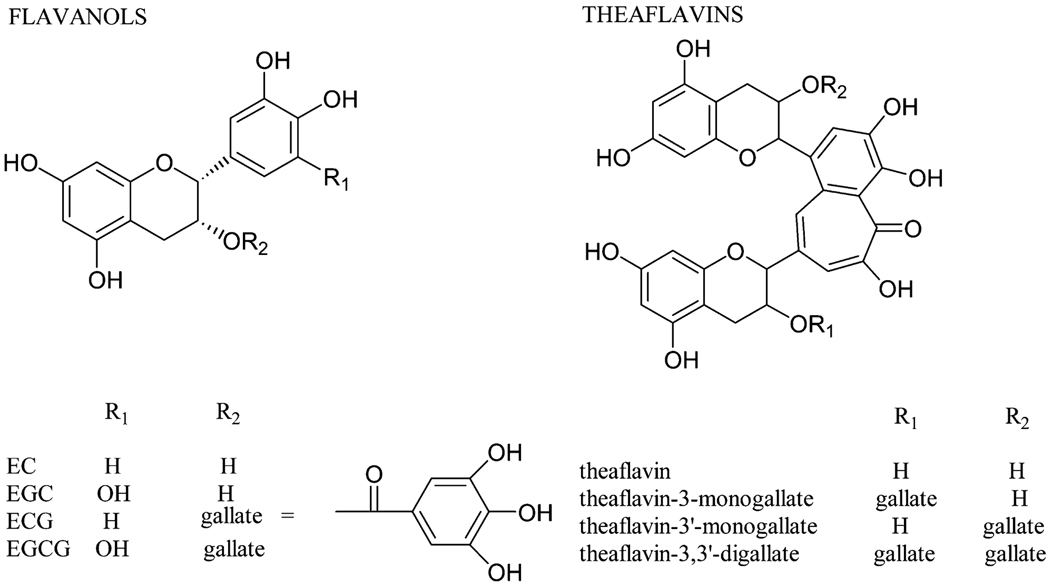
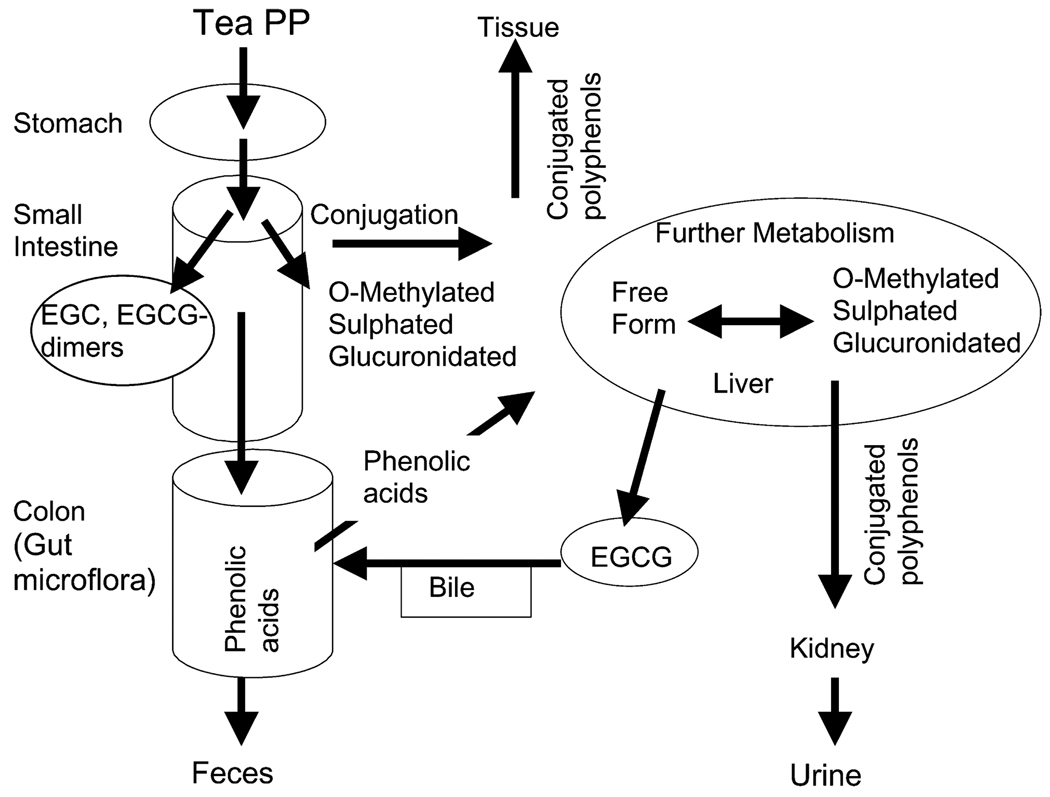
Tea is widely consumed throughout the world. Green tea (GT),4 white tea, oolong tea, and black tea (BT) are prepared by brewing leaves from the plant Camellia sinensis. The active phytochemicals in tea are flavonoids, which are chemically polyphenols (PP). The key differences among the various types of teas are in the degree to which natural fermentation is allowed to progress after harvesting. GT and white tea are steamed after harvest to stop the fermentation process. Fermentation is partially stopped in oolong tea and allowed to progress in BT. Tea flavonoids belong to the subgroup called flavan-3-ols. The 4 major flavan-3-ols occurring in nonfermented teas such as GT and white tea are (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin (EC), and (−)-epicatechin-3-gallate (ECG). Two of the tea flavan-3-ols (EGCG, ECG) contain a gallic acid moiety at position 3 on the C ring (Fig. 1). During the fermentation process for BT and oolong teas, tea PP are oxidized to form larger polymers called theaflavins (THE) and thearubigens (THR) (Fig. 1). In vivo and in vitro biological activities of tea flavan-3-ols include inhibition of carcinogenesis, protection from cardiovascular disease, enhanced loss of body fat, increase of bone density, protection from neurodegenerative diseases, and improvement in type 2 diabetes (1,2). However, the design and interpretation of epidemiological and human intervention studies have been limited by inadequate information on bioavailability and metabolism/biotransformation of tea flavan-3-ols. Only a small percentage of the flavan-3-ols are absorbed from the small intestine into the intestinal epithelial cell. Here they undergo conjugation to sulfated, glucuronidated, or methylated conjugates by phase II enzymes such as sulfotranferase, glucuronosyltransferase, and catechol-O-methyltransferase (Fig. 2). This process has been discussed in detail by Lambert et al. (3).
FIGURE 1.
Chemical structure of flavan-3-ols and THE.
FIGURE 2.
Metabolism of tea flavan-3-ols in the human body.
Here we discuss the following: 1) pH sensitivity and dimer formation of tea flavan-3-ols, 2) metabolism by intestinal microflora, 3) metabolism of tea flavan-3-ols by phase II enzymes in human pharmacokinetic studies, 4) tissue bioavailability of flavan-3-ols, and 5) new approaches to increase bioavailability based on new and already published data from our laboratory and others. In addition, new data demonstrating the increased pH stability of methyl-EGC compared with the parent compound EGC as well as the instability of EGCG, EGC, and THE in cell culture experiments are presented.
Materials and Methods
EGCG, EGC, EC, and ECG were purchased at Sigma-Aldrich. THE and THE monogallates and digallate were purchased from Wako Chemicals.
Cell culture conditions
A hormone-responsive human prostate cancer cell line (LNCaP) was obtained from American Type Culture Collection. LNCaP cells were cultured in RPMI 1640 medium from VWR Scientific supplemented with 10% fetal bovine serum (FBS), 105 U/L of penicillin, and 100 µg/L of streptomycin. Cells were grown at 37°C in a humidified atmosphere supplemented with 5% CO2 in air. The doubling time for LNCaP was 36 h. To test the antiproliferative activity of tea PP, cells were seeded in 96-well plates at a concentration of 5 × 107 cells/L, 100 µL/well, and cultured at 37°C for 24 h before treatment. Proliferation was determined after 48 h of incubation with 10, 20, and 40 µmol/L tea flavan-3-ol. Catalase treatment (final concentration of 104 U/L) was started 5 min before the addition of flavan-3-ols. Proliferation was measured using the CellTiter-Glo luminescent assay.
Methyl-EGC synthesis
Methyl-EGC was synthesized following the procedure by Donovan et al. (4). A mixture of EGC, potassium carbonate, and methyl iodide in acetone was mixed in a sonication bath. The progress of the reaction was monitored by HPLC. The major product of the reaction was purified by semipreparative HPLC.
Exposure to pH 7
A mixture of EGC, EC, EGCG, and ECG was diluted to a final concentration of ~30 µmol/L with sodium phosphate buffer, 0.05 mmol/L, pH 7. An aliquot was injected into the HPLC every 55 min. A mixture of THE, THE-3′-G, THE-3″-G, and THE-3′3″-digallate as well as a mixture of methyl-EGC and EGC were treated the same way. The HPLC consisted of a 1050 Agilent system with a Shimadzu UV/VIS detector at 260 nm, an Alltima guard column (7.5 mm × 4.6 mm), and a C18 RP Alltima column. The column was eluted with a gradient from 100% mobile phase A (75 mmol/L citric acid, 25 mmol/L ammonium acetate, pH 2.7) to 60% mobile phase B (mobile phase A:acetonitrile 50:50) as described by Henning et al. (5).
Statistical analysis
GraphPad PRISM statistical analysis software package version 4 (GraphPad Software) was used for statistical analyses. Data are expressed as means ± SD. The antiproliferative activity of LNCaP cells was compared when they were grown with or without catalase for EGCG, ECG, and THE at each concentration using Student’s t-test. Differences were considered significant at P < 0.05.
Results
pH Sensitivity
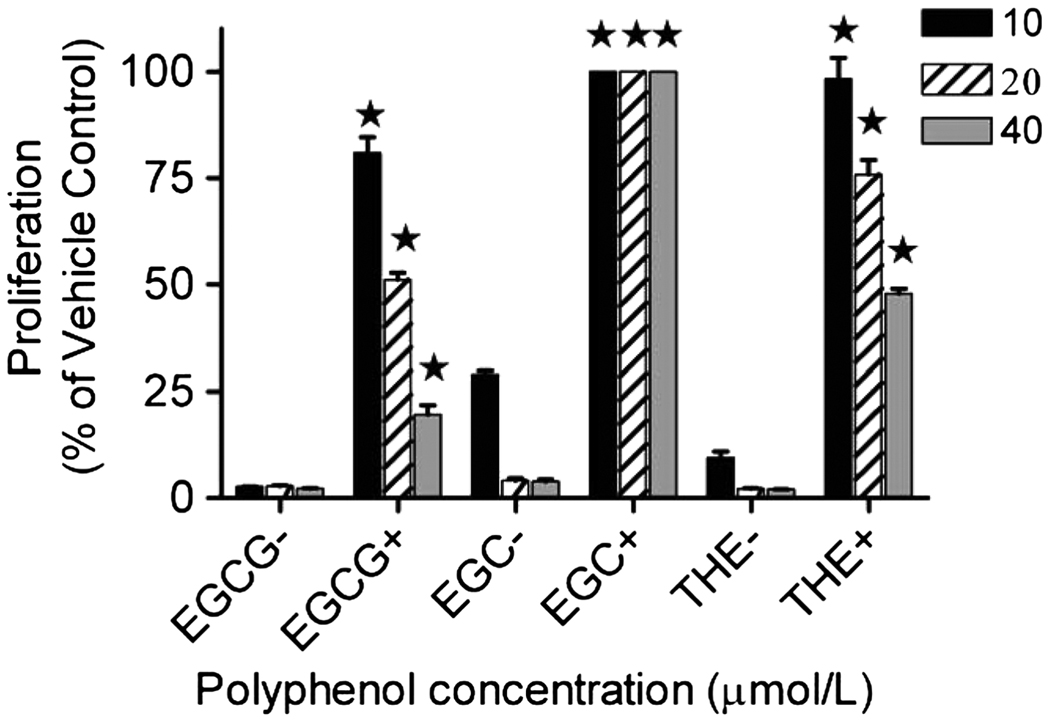
The stability of tea flavan-3-ols, THE, and methyl-EGC was tested at pH 7. EGCG and EGC were degraded most rapidly with only 50% remaining after 2–3 h (Fig. 3 A). EC and ECG were stable for 8 h and decreased by 10 and 25%, respectively, at 17 h. The pH stability increased in the following sequence: EGCG < EGC < THE3′G < THE3G < THE < THE33′GG < ECG < EC. The pH instability most likely affects the absorption of the tea flavan-3-ols in the small intestine (pH 7.2). However, conjugated forms of flavan-3-ols may be more stable at pH 7. This was confirmed in our experiment demonstrating that methylated EGC was stable for >16 h (Fig. 3 B). The pH sensitivity leading to dimer and hydrogen peroxide (H2O2) formation at alkaline pH also interferes with cell culture experiments, which are frequently used for mechanistic studies of flavon-3-ols. Because cell cultures are maintained in medium with pH close to 7, tea flavan-3-ols will not be stable. Under these conditions, the formation of H2O2 contributes to the antiproliferative activity and exaggerates the biological effect. For example, when LNCaP prostate cancer cells were incubated with EGCG (10–40 µmol/L) alone, cell growth was inhibited completely (Fig. 4). However, when LNCaP cells were preincubated with 104 U/L of catalase before the administration of EGCG, cell proliferation was inhibited by 20%, 50%, and 70% using 10, 20, and 40 µmol/L of EGCG, respectively. A similar effect was observed for EGC and THE (Fig. 4). When LNCaP cells were preincubated with 104 U/L of catalase before the administration of EGC or THE, the antiproliferative effect was decreased significantly (Fig. 4).
FIGURE 3.
Stability of (A) tea flavan-3-ols and THE and (B) methyl-EGC and EGC incubated at pH 7 for 0–16 h. Values are means ± SD; n = 2.
FIGURE 4.
Growth-inhibitory effect of EGCG, EGC, and a THE mix incubated with and without catalase on human prostate cancer cells (LNCaP). Values are means ± SD; n = 3.
Discussion
Bioavailability of tea PP
The bioavailability of tea PP is relatively low (6). Possible reasons are their degradation and dimerization at neutral pH as well as the fact that tea PP are subject to active efflux by multidrug resistance-associated proteins (MRP) at the apical surface of the intestine (3). Conditions promoting autoxidation and degradation are present in some foods, digestive juices in the small intestine, plasma, bile, and cell culture medium. Among the tea PP tested, EGCG and EGC were the least stable, and EC and ECG the most stable, with the THEs showing intermediate stability at pH 7 (Fig. 3). The same differential stability of flavan-3-ols at neutral pH was also found by Neilson et al. (7), who investigated in further detail the oxidative processes taking place when EGCG and ECG are exposed to neutral pH during digestive conditions simulating the stomach (pH 2) and small intestine (pH 7.2). Intestinal losses were 64–88% for EGCG and EGC, 54–55% for ECG, and 7.6–10.3% for EC at concentrations from 25 to 250 µmol/L (7). At the same time, homo- and heterodimer formation was observed. With concomitant formation of H2O2, EGCG formed theasinensin A and D and P-2 dimers. Several other minor dimers were detected (7). The H2O2 formation from tea PP in cell culture experiments contributes to the antiproliferative activity and exaggerates the biological effect (Fig. 4). This effect has also been previously reported by Yang et al. (8). The extent of the H2O2 effect depends on the type of cell culture medium. To avoid the H2O2 effect, flavan-3-ol testing should be performed only in the presence of catalase to convert H2O2 to water and oxygen (9).
In vitro colonic digestion with human microflora
Tea PP, which are not absorbed in the small intestine or effluxed back to the intestine, are transported to the colon. Here the intestinal microflora plays an important role in the degradation of PP to phenolic acids. Evidence of this process has been demonstrated in in vitro experiments simulating the colonic digestion (10). For example, when GT or BT extracts were infused in a colon simulator with constant pH, anaerobic condition, a medium similar to human colon content, and a standardized pooled stool sample, the lumen concentration after GT and BT extract infusion showed that EGC was present in the highest concentration (11). This was expected for GT because EGC was present in the highest concentration in the GT extract. However, the BT extract contained only a small amount of EGC. It appears that EGC was formed in the colon simulator, possibly from the degradation of larger tea polymers such as THR (11). In addition, the gas chromatograph/mass spectrometry analysis of the lumen content revealed that phenolic acids such as 3-methoxy-4-hydroxyphenylacetic acid, 4-hydroxyphenyl acetic acid, 3,4-dihydroxyphenylacetic acid, and 3-(3-hydroxyphenyl) propionic acid were formed (11). Types and concentrations of phenolic acids formed after the incubation of GT and BT extracts were very similar (11). A human intervention study by Mulder et al. (12) confirmed that phenolic acids formed by BT and GT PP are absorbed, metabolized, and excreted in the urine. They demonstrated that the administration of 6 g of BT or GT solids resulted in an increase in the excretion of hippuric acid into the urine. Hippuric acid can be formed in the human liver from benzoic acid and glycine. Benzoic acid in turn can be formed in the colon by colonic bacteria from flavan-3-ol via valerolactone and phenylpropionic acid (12). Mulder et al. (12) performed a calculation to determine whether all urinary hippuric acid was formed from the simple dietary PP such as gallic acid, EC, EGC, EGCG, ECG, and THE. They determined that the total amount of free PP in the BT could not fully account for the increased urinary hippuric acid. Consequently, they suggested that the complex THR must have contributed to hippuric acid (12). The same effect had been demonstrated earlier in the BT intervention study by Clifford et al. (13). Mulder et al. (12) also determined the total content of PP using the Folin-Ciocalteu assay. BT solids contained 9.08 mmol gallic acid equivalents in the daily dose of 6 g tea solids compared with 13.3 mmol for GT. This was associated with a urinary hippuric acid increase of 1.86 mmol (20%) and 2.33 mmol (18%) for BT and GT, respectively (12). Therefore, GT and BT consumption had comparable effects on urinary hippuric acid excretion. Another study by Halliwell and colleagues (14) emphasized the magnitude of the content of formed aromatic and phenolic acids (932 µmol/L) in fecal water compared with the concentration of parent flavonoids (2.7 µmol/L). However, it is not known to what degree phenolic acids contribute to the bioactivity of tea. Some of the phenolic acids have been demonstrated to have antiproliferative activity. For example, 3,4-dihydroxyphenylacetic acid inhibited the proliferation of HCT 116 colon tumor cells with an IC50 of ~75 µmol/L but did not inhibit growth of normal colon cells (IEC 6) (11).
Based on these observations, we have proposed that phenolic acids formed by colonic microflora from tea are present in sufficient concentration to potentially contribute to colon cancer chemoprevention. More clinical studies are needed to determine the degree to which these phenolic acids are absorbed into the circulation and exhibit systemic chemopreventive effects.
Human pharmacokinetic studies
The absorption process of flavan-3-ols in the small intestine has been mainly investigated in in vitro experiments using Caco-2 intestinal cells (15,16). These experiments demonstrated that EGCG can be taken up by passive diffusion as well as by carrier-mediated transport such as a monocarboxylate transporter and MRP (3). Once absorbed into the small intestinal epithelial cell, flavan-3-ols undergo methylation, glucuronidation, and sulfation. The conjugated metabolites are transported into the circulation by MRP transport (3). However, MRP also has been shown to efflux flavan-3-ols to the apical side back into the intestinal lumen. This process contributes to the limited absorption of flavan-3-ols (3). Conjugated flavan-3-ols are further metabolized in the liver and finally excreted in the urine. EGCG has been demonstrated to be excreted mainly through the enterohepatic circulation into the intestine (17). Several studies have confirmed that gallated flavan-3-ols such as EGCG and ECG mainly occur in plasma and urine in the free form, whereas the majority of nongallated flavan-3-ols (EC, EGC) occur in the conjugated form (18–20). However, further human studies are needed to investigate the status of conjugation at target tissues.
Several pharmacokinetics (PK) studies have been performed by us and other investigators (5,18,20–23). All PK studies found that flavan-3-ols are absorbed and eliminated rapidly with a peak concentration reached at 1.3–1.6 h and excretion between 0 and 8 h. The earliest PK studies were performed by Chow et al. (20) and Lee et al. (21) using the GT extract polyphenon E or pure EGCG. Chow et al. determined that the administration of increasing concentrations of EGCG led to a linear increase in plasma concentration and that there was no difference in EGCG absorption whether it was administered alone versus in the form of polyphenon E, a GT extract (19). They also demonstrated that the administration of 800 mg of EGCG was well tolerated (19). Lee et al. found methyl-EGC at concentrations higher than EGC in plasma and urine (18). However, in another phase II human intervention trial by Wang et al. (24), no methyl-EGC was found either in plasma or urine after consumption of 1000 mg of GT extract daily for 3 mo. Other metabolites such as ring-fission metabolites (−)-5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4) and (−)-5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (M6) were found in significant amounts in urine and plasma (18). M4 and M6 are intermediate metabolites in the conversion of flavan-3-ols to phenolic acids (18).
In this study, following the administration of decaffeinated GT, maximum plasma concentration was 0.13%, 0.53%, and 0.7% of the ingested dose of EGCG, EGC, and EC and 0.1% for EGCG administered alone (18). More recent studies by Chow et al. (19) compared the bioavailability of polyphenon E when consumed after overnight fasting to the fed state. The study revealed that the maximum plasma concentration of EGCG after administration of 800 mg of polyphenon E was 5-fold higher when consumed after an overnight fast compared with the fed state (19). By contrast to EGCG the maximum concentration of total (but not free form) of nongallated flavan-3-ols (EC and EGC) was decreased in the fasting compared with the fed state. Because nongallated flavan-3-ols occur in plasma mainly in the conjugated form, conjugation is likely to be limited in the fasting state. It has been postulated by the authors that fasting can acutely deplete precursors for the glucuronidation reaction (25).
In general, PK studies have demonstrated a strong intersubject variability in the PK of flavan-3-ols (20,22). This is an important factor in designing tea intervention studies and calculating the statistical power necessary to demonstrate activity.
Our studies have confirmed the limited bioavailability of tea flavan-3-ols. Calculating the amount of flavan-3-ols present in plasma and urine expressed as percentage of intake have demonstrated that the nongallated forms were present at a higher percentage of intake compared with the gallated forms (5). For example, after the administration of 1 large dose of either GT, BT or a GT supplement to healthy volunteers, plasma EGC and EC content was 0.26–0.75% of intake compared with EGCG and ECG with 0.07–0.2%. The same effect was observed for urine (5). This confirmed findings by Lee et al. mentioned earlier in this article (18). For our study, subjects arrived fasting and consumed a low-flavonoid breakfast before consuming the large dose of tea or tea supplement. Therefore, conjugation was not affected by fasting conditions, as demonstrated by Chow et al. (19) and described earlier in this article.
An important question in regard to tea bioavailability is whether flavan-3-ols are present in tissues in the human body. This was confirmed in another GT and BT intervention study, which found gallated and nongallated flavan-3-ols in human prostate after 1 wk of daily consumption of 5 cups of tea (23). EGC was found in the highest concentration after GT and BT intervention (23). In the same study, serum was collected before and after the tea intervention. Serum was used to replace FBS in an ex-vivo LNCaP prostate cancer cell culture experiment to determine the effect on proliferation. The serum was collected after an overnight fast. Because of the rapid excretion of tea flavan-3-ols, serum concentration was below the detection limit. Nevertheless postserum replacement of FBS in cell culture showed an inhibition of cell growth compared with preserum use in the same system (23). We postulated that this effect was caused by serum flavan-3-ol metabolites with longer half-lives than flavan-3-ols (23).
Approaches to increase bioavailability
Several approaches have been tested to increase the bioavailability of flavan-3-ols. Green et al. (26) determined whether a combination with common food additives or different fruit juices and GT flavan-3-ol solution could enhance the stability of flavan-3-ols during an in vitro simulation of stomach and small intestinal digestion. Without additives, <20% of total flavan-3-ols were recovered after digestion. Mixing tea flavan-3-ols with 50% bovine, soy, and rice milk, respectively increased total flavan-3-ol (EC, EGC, EGCG, ECG) recovery to 52, 55, and 69% after digestion. The recovery of flavan-3-ols after the addition of 30 mg of ascorbic acid showed differential effects on EGC, EGCG, EC, and ECG (74, 54, 82, and 45%) (26). The addition of up to 50% of fruit juice (grapefruit, orange, lemon, or lime) also improved the recovery of EGC (81–98%), EGCG (56–76%), EC (86–95%), and ECG (30–55%). A different approach to increase the stability and bioavailability was used by Lambert et al. (27) through the synthesis of peracetylated EGCG (AcEGCG). In vitro AcEGCG was rapidly converted to EGCG in HCT116 human colon adenocarcinoma cells (27). Treatment of HCT116 cells led to a 2.8- to 30-fold greater intracellular concentration of EGCG as compared with treatment with EGCG at different time points (1, 2, 5, or 24 h). EGCG could be detected within 5 min following the in vitro incubation of AcEGCG with mouse plasma or mouse microsomes at 37°C. Two metabolites were formed (27). Oral EGCG bioavailability was increased in CF-1 mice if EGCG followed intragastric administration of AcEGCG, mainly as a result of a delay in excretion (27). Another approach to increase bioavailability is to inhibit conjugation by concomitant administration of piperine, an alkaloid derived from black pepper. It has been reported that coadministration of piperine and curcumin led to an increase of bioavailability of curcumin in humans (28). Lambert et al. (29) confirmed that piperine inhibited glucuronidation activity. The intragastric coadministration of 164 µmol/kg EGCG and 70 µmol/kg piperine to CF-1 male mice increased plasma EGCG concentration, determined as area under the curve, 2-fold compared with mice treated with EGCG alone. However, the ratio of total to conjugated EGCG was not changed. This may be because piperine inhibited EGCG glucuronidation only in small intestinal microsomes and not in the hepatic microsomes (29). Therefore, once absorbed, EGCG can undergo glucuronidation in the liver.
In summary, these approaches to increase the bioavailability of flavan-3-ols are encouraging. However, further studies will be needed to demonstrate their utililty in human clinical applications.
Other articles in this supplement include references (30–39).
Footnotes
Published in a supplement to The Journal of Nutrition. Presented at the conference “Fourth International Scientific Symposium on Tea and Human Health,” held in Washington, DC at the U.S. Department of Agriculture on September 18, 2007. The conference was organized by the Tea Council of the U.S.A., and was cosponsored by the American Cancer Society, the American College of Nutrition, the American Medical Women’s Association, the American Society for Nutrition, and the Linus Pauling Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Tea Council of the U.S.A. or the cosponsoring organizations. Supplement coordinators for the supplement publication were Lenore Arab, University of California, Los Angeles, CA and Jeffrey Blumberg, Tufts University, Boston, MA. Supplement coordinator disclosure: L. Arab and J. Blumberg received honorarium and travel support from the Tea Council of the U.S.A. for cochairing the Fourth International Scientific Symposium on Tea and Human Health and for editorial services provided for this supplement publication; they also serve as members of the Scientific Advisory Panel of the Tea Council of the U.S.A.
Supported by NIH Grants No. NIH/NCI RO3 CA91163, 1RO1 CA116242.
Author disclosures: S. M. Henning received an honorarium and travel support from the Tea Council of the U.S.A. for speaking at the Fourth International Scientific Symposium on Tea and Human Health and for preparing this manuscript for publication; J. J. Choo and D. Heber, no conflicts of interest.
Abbreviations used: AcEGCG, peracetylated EGCG; BT, black tea; CG, catechin gallate; EC, (−)-epicatechin; ECG, (−)-epicatechin-3-gallate; EGC, (−)-epigallocatechin; EGCG, (−)-epigallocatechin-3-gallate; FBS, fetal bovine serum; GT, green tea; MRP, multidrug resistance-associated protein; PK, pharmacokinetics; PP, polyphenol; THE, theaflavin; THR, thearubigin.
Literature Cited
- 1.Khan N, Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81:519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Lambert JD, Sang S, Lu AY, Yang CS. Metabolism of dietary polyphenols and possible interactions with drugs. Curr Drug Metab. 2007;8:499–507. doi: 10.2174/138920007780866870. [DOI] [PubMed] [Google Scholar]
- 4.Donovan JL, Luthria DL, Stremple P, Waterhouse AL. Analysis of (+)-catechin, (−)-epicatechin and their 3𲀲- and 4′-O-methylated analogs. A comparison of sensitive methods. J Chromatogr B Biomed Sci Appl. 1999;726:277–283. doi: 10.1016/s0378-4347(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 5.Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang H, Go VL, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am J Clin Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- 6.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38:771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 7.Neilson AP, Hopf AS, Cooper BR, Pereira MA, Bomser JA, Ferruzzi MG. Catechin degradation with concurrent formation of homo- and heterocatechin dimers during in vitro digestion. J Agric Food Chem. 2007;55:8941–8949. doi: 10.1021/jf071645m. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ. Tea and tea polyphenols in cancer prevention. J Nutr. 2000;130:472S–478S. doi: 10.1093/jn/130.2.472S. [DOI] [PubMed] [Google Scholar]
- 9.Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS. 2007;115:81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x. [DOI] [PubMed] [Google Scholar]
- 10.Henning SM, Krul C, Seeram NP, Niu Y, Liu Y, Heber D. Colonic metabolism of flavanols from green and black tea studied using an in vitro large intestinal model. FASEB J. 2005;19:A415. [Google Scholar]
- 11.Gao K, Xu A, Krul C, Venema K, Liu Y, Niu Y, Lu J, Bensoussan L, Seeram NP, et al. Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity. J Nutr. 2006;136:52–57. doi: 10.1093/jn/136.1.52. [DOI] [PubMed] [Google Scholar]
- 12.Mulder TP, Rietveld AG, Van Amelsvoort JM. Consumption of both black tea and green tea results in an increase in the excretion of hippuric acid into urine. Am J Clin Nutr. 2005;81:256S–260S. doi: 10.1093/ajcn/81.1.256S. [DOI] [PubMed] [Google Scholar]
- 13.Clifford MN, Copeland EL, Bloxsidge JP, Mitchell LA. Hippuric acid as a major excretion product associated with black tea consumption. Xenobiotica. 2000;30:317–326. doi: 10.1080/004982500237703. [DOI] [PubMed] [Google Scholar]
- 14.Jenner AM, Rafter J, Halliwell B. Human fecal water content of phenolics: the extent of colonic exposure to aromatic compounds. Free Radic Biol Med. 2005;38:763–772. doi: 10.1016/j.freeradbiomed.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Vaidyanathan JB, Walle T. Cellular uptake and efflux of the tea flavonoid (−) epicatechin-3-gallate in the human intestinal cell line Caco-2. J Pharmacol Exp Ther. 2003;307:745–752. doi: 10.1124/jpet.103.054296. [DOI] [PubMed] [Google Scholar]
- 16.Vaidyanathan JB, Walle T. Transport and metabolism of the tea flavonoid (−)-epicatechin by the human intestinal cell line Caco-2. Pharm Res. 2001;18:1420–1425. doi: 10.1023/a:1012200805593. [DOI] [PubMed] [Google Scholar]
- 17.Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–354. [PubMed] [Google Scholar]
- 18.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 19.Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 20.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–58. [PubMed] [Google Scholar]
- 21.Lee MJ, Wang ZY, Li H, Chen L, Sun Y, Gobbo S, Balentine DA, Yang CS. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev. 1995;4:393–399. [PubMed] [Google Scholar]
- 22.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea poly-phenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- 23.Henning SM, Aronson W, Niu Y, Conde F, Lee NH, Seeram NP, Lee RP, Lu J, Harris DM, et al. Tea polyphenols and theaflavins are found in prostate tissue of humans and mice after green and black tea consumption. J Nutr. 2006;136:1839–1843. doi: 10.1093/jn/136.7.1839. [DOI] [PubMed] [Google Scholar]
- 24.Wang JS, Luo H, Wang P, Tang L, Yu J, Huang T, Cox S, Gao W. Validation of green tea polyphenol biomarkers in a phase II human intervention trial. Food Chem Toxicol. 2008;46:232–240. doi: 10.1016/j.fct.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price VF, Jollow DJ. Mechanism of decreased acetaminophen glucuronidation in the fasted rat. Biochem Pharmacol. 1988;37:1067–1075. doi: 10.1016/0006-2952(88)90512-6. [DOI] [PubMed] [Google Scholar]
- 26.Green RJ, Murphy AS, Schulz B, Watkins BA, Ferruzzi MG. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol Nutr Food Res. 2007;51:1152–1162. doi: 10.1002/mnfr.200700086. [DOI] [PubMed] [Google Scholar]
- 27.Lambert JD, Sang S, Hong J, Kwon SJ, Lee MJ, Ho CT, Yang CS. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab Dispos. 2006;34:2111–2116. doi: 10.1124/dmd.106.011460. [DOI] [PubMed] [Google Scholar]
- 28.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 29.Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS. Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J Nutr. 2004;134:1948–1952. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 30.Arab L, Blumberg JB. Introduction to the Proceedings of the Fourth International Scientific Symposium on Tea and Human Health. J Nutr. 2008;138:1526S–1528S. doi: 10.1093/jn/138.8.1526S. [DOI] [PubMed] [Google Scholar]
- 31.Auger C, Mullen W, Hara Y, Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J Nutr. 2008;138:1535S–1542S. doi: 10.1093/jn/138.8.1535S. [DOI] [PubMed] [Google Scholar]
- 32.Song WO, Chun OK. Tea is the major source of flavan-3-ol and flavonol in the U.S. diet. J Nutr. 2008;138:1543S–1547S. doi: 10.1093/jn/138.8.1543S. [DOI] [PubMed] [Google Scholar]
- 33.Kuriyama S. The relation between green tea consumption and cardiovascular disease as evidenced by epidemiological studies. J Nutr. 2008;138:1548S–1553S. doi: 10.1093/jn/138.8.1548S. [DOI] [PubMed] [Google Scholar]
- 34.Grassi D, Aggio A, Onori L, Croce G, Tiberti S, Ferri C, Ferri L, Desideri G. Tea, flavonoids, and NO-mediated vascular reactivity. J Nutr. 2008;138:1554S–1560S. doi: 10.1093/jn/138.8.1554S. [DOI] [PubMed] [Google Scholar]
- 35.Arts ICW. A review of the epidemiological evidence on tea, flavonoids, and lung cancer. J Nutr. 2008;138:1561S–1566S. doi: 10.1093/jn/138.8.1561S. [DOI] [PubMed] [Google Scholar]
- 36.Hakim IA, Chow HHS, Harris RB. Green tea consumption is associated with decreased DNA damage among GSTM1 positive smokers regardless of their hOGG1 genotype. J Nutr. 2008;138:1567S–1571S. doi: 10.1093/jn/138.8.1567S. [DOI] [PubMed] [Google Scholar]
- 37.Kelly SP, Gomez-Ramirez M, Montesi JL, Foxe JJ. L-Theanine and caffeine in combination affect human cognition as evidenced by oscillatory alpha-band activity and attention task performance. J Nutr. 2008;138:1572S–1577S. doi: 10.1093/jn/138.8.1572S. [DOI] [PubMed] [Google Scholar]
- 38.Mandel SA, Amit T, Kalfon L, Reznichenko L, Youdim MBH. Targeting multiple neurodegenerative diseases etiologies with multimodal-acting green tea catechins. J Nutr. 2008;138:1578S–1583S. doi: 10.1093/jn/138.8.1578S. [DOI] [PubMed] [Google Scholar]
- 39.Stote KS, Baer DJ. Tea consumption may improve biomarkers of insulin sensitivity and risk factors for diabetes. J Nutr. 2008;138:1584S–1588S. doi: 10.1093/jn/138.8.1584S. [DOI] [PubMed] [Google Scholar]