Abstract
Objective:
The pedunculopontine nucleus region (PPNR) is being investigated as a target for deep brain stimulation (DBS) in Parkinson disease (PD), particularly for gait and postural impairment. A greater understanding of how PPNR activities and oscillations are modulated with voluntary movements is crucial to the development of neuromodulation strategies.
Methods:
We studied 7 patients with PD who underwent DBS electrode implantations in the PPNR. PPNR local field potential and EEG were recorded while patients performed self-paced wrist and ankle movements.
Results:
Back-averaging of the PPNR recording showed movement-related potentials before electromyography onset. Frequency analysis showed 2 discrete movement-related frequency bands in the theta (6- to 10-Hz) and beta (14- to 30-Hz) ranges. The PPNR theta band showed greater event-related desynchronization with movements in the ON than in the OFF medication state and was coupled with the sensorimotor cortices in the ON state only. Beta event-related desynchronization was observed in the PPNR during the premovement and movement execution phases in the OFF state. In contrast, premovement PPNR beta event-related synchronization occurred in the ON state. Moreover, beta band coherence between the PPNR and the midline prefrontal region was observed during movement preparation in the ON but not the OFF state.
Conclusions:
Activities of PPNR change during movement preparation and execution in patients with PD. Dopaminergic medications modulate PPNR activities and promote the interactions between the cortex and PPNR. Beta oscillations may have different functions in the basal ganglia and PPNR, and may be prokinetic rather than antikinetic in the PPNR.
GLOSSARY
- AC
= anterior commissure;
- BG
= basal ganglia;
- cusum
= cumulative sum amplitude;
- BP
= Bereitschaftspotential;
- DBS
= deep brain stimulation;
- ERD
= event-related desynchronization;
- ERS
= event-related synchronization;
- GPi
= globus pallidus internus;
- LFP
= local field potential;
- MRP
= movement-related potential;
- PC
= posterior commissure;
- PD
= Parkinson disease;
- PPN
= pedunculopontine nucleus;
- PPNR
= pedunculopontine nucleus region;
- SMA
= supplementary motor area;
- STN
= subthalamic nucleus;
- VT
= ventral thalamus.
The pedunculopontine nucleus (PPN) plays an important role in movement control, particularly for the initiation and maintenance of gait.1 The PPN has 2 main divisions: the pars compacta contains cholinergic neurons, and the pars dissipata contains mainly glutamatergic cells.1 The PPN has extensive connections with the basal ganglia (BG). It receives projections from output structures of the BG, including the substantia nigra pars reticulata, globus pallidus internus (GPi), and subthalamic nucleus (STN) and in turn projects to the STN, substantia nigra pars compacta, and GPi.1–3 Neuroanatomical studies revealed that the human PPN has connections with the supplementary motor area (SMA), sensorimotor cortices, thalamus, cerebellum, and spinal cord.4,5 The PPN modulates limb movements and locomotion. PPN neurons increased their firing rate during voluntary movements.6,7 PPN stimulation elicited locomotion in cats,8 decerebrate rats,9 and monkeys with thalamic lesions.10 PPN inhibition suppressed limb movements11 and locomotion in animals.9,12
The PPN was recently introduced as target for deep brain stimulation (DBS) in patients with Parkinson disease (PD) who had drug-resistant gait and postural instability.13–15 Stimulation at 30 to 70 Hz was reported to produce optimal therapeutic benefits.7,13,15,16 These frequencies are lower than frequencies (130 to 180 Hz) used for STN or GPi DBS in PD.
The purpose of the present study was to examine human PPN activities and its interactions with the cortex during voluntary movements through recording of local field potentials (LFPs) from the PPN region (PPNR). We hypothesize that the PPNR displays different patterns of activities compared with those reported for the BG.
METHODS
Patients.
We studied 7 patients with PD (table 1) who had PPNR DBS electrodes unilaterally implanted. Four patients were studied in OFF and then ON dopaminergic medication states, 1 patient was studied in OFF state only, and 2 were studied in ON state only. See appendix e-1 on the Neurology® Web site at www.neurology.org for further details of the patients studied.
Table 1 Clinical details of patients studied
Standard protocol approvals and patient consent.
All patients provided written informed consent, and the study was approved by the University Health Network Research Ethics Board.
Paradigm and recordings.
The study was performed 3 to 5 days after electrode implantations. Sitting in a comfortable chair, the patients were instructed to make brisk, self-paced wrist extension or ankle dorsiflexion movements followed immediately by passive wrist flexion or ankle plantarflexion approximately once every 10 seconds. The movements were studied separately for the left and right sides for 10 to 15 minutes per session.
PPNR LFP from the DBS electrode and EEG at Fp1, Fz, Cz, C3, C4, CP3, and CP4 according to the International 10–20 System were recorded with linked ears reference. EMG was recorded to monitor wrist and ankle movements. Further details of the recording procedure are available in appendix e-1.
Data analysis.
Data were down sampled to a sampling rate of 1 kHz. DBS recordings were transformed into bipolar montage with the adjacent contacts (0–1, 1–2, 2–3). EEG electrodes were analyzed in monopolar montage for the movement-related potential (MRP) analysis. For frequency analyses, bipolar montages that represent different cortical topographies including midline (Cz–Fz) and ipsilateral or contralateral sensorimotor regions (C3–CP3/C4–CP4) were used. Laterality of the EEG bipolar montage was defined according to the side of the PPNR electrode. Epochs of 4 seconds before and 1 second after movement onsets were created for MRP analyses. For frequency analysis, we examined epochs of 4 seconds before and 3.5 seconds after movement onset. Epochs contaminated with eye or muscle movement artifacts were rejected.
For MRP analysis, at least 30 artifact-free epochs were averaged. MRP onset latencies and amplitudes were obtained from significant waveforms. Waveforms with amplitudes more than mean ± 3 SDs of the baseline (−4 to −3 seconds) and lasting more than 500 milliseconds before movement onset were considered significant MRPs. The MRP amplitude was the difference between maximum deflection of the MRP and the baseline. Paired t tests were used to examine the effects of locations (cortex vs PPNR), laterality (ipsilateral vs contralateral), and type of movement (wrist vs ankle) on MRP onset latencies and amplitudes in OFF and ON states.
For frequency analysis, the contact pair that represented PPNR LFP was chosen for each patient (table 1) according to postoperative MRI. One contact was most likely in the PPNR based on postoperative MRI (figure e-1B), and the adjacent contact was chosen based on highest movement-related changes in relative power. For patient 2, who did not have postoperative MRI, we analyzed the contact pair used for therapeutic DBS. Frequency bands sensitive to hand or ankle movements were identified by comparing the relative power between the baseline and movement periods. Power changes in these frequency bands were examined by calculating event-related desynchronization (ERD) and event-related synchronization (ERS). ERD/ERS waveforms greater than mean ± 3 SDs of the baseline with durations more than 1 second were considered significant. Cumulative sum amplitudes (cusum) of ERD/ERS waveforms were calculated to determine the magnitude of ERD/ERS.
Coherence was examined from 4 to 100 Hz between the PPNR contact pair and bipolar EEG that represented different cortical topographies. OFF and ON states were analyzed separately using the same epochs used for ERD/ERS. Permutation statistics were used to determine the significance level of EEG-PPNR coherence.
Phase coherence between EEG and PPNR was examined if significant coherence was found. The time delay between EEG and PPNR was determined. See appendix e-1 for further details of the data analysis.
Postoperative MRI and electrode localization.
The technique used to localize electrode contacts has been previously described.15,17 Briefly, postoperative axial 3-dimensional inversion recovery and T2-weighted images were transferred to a StealthStation workstation and merged using FrameLink4.1 software (Medtronic SNT, Minneapolis, MN). Coronal and sagittal planes were reconstructed based on axial images. The anterior and posterior commissures (AC, PC) were then targeted in the axial plane, and 3 additional points were plotted in the midline. Thereafter, images were reformatted parallel to the AC-PC plane and orthogonal to the midline. DBS electrodes were visualized in all 3 planes, and the location of each electrode was estimated. We considered the center of the sphere-shaped artifacts as the center of the contacts.18 We defined the PPNR in the anterolateral portion of the pontine/mesencephalic tegmentum that extended from 2 mm below the inferior colliculus to the transition between the inferior and superior colliculi, according to the Paxinos and Huang atlas.19
RESULTS
Movement-related potentials.
In both OFF and ON states, significant premovement potentials or Bereitschaftspotentials (BPs) preceding self-paced wrist or ankle movements were observed in all studied sides from EEG and from bipolar DBS contacts. We were not able to distinguish between different subcomponents of BP,20 probably due to the relatively small number of epochs recorded. Figure 1 shows an example of scalp and PPNR MRPs recorded from patient 1. Because the MRP onset latencies and amplitudes for ipsilateral and contralateral movements were approximately the same at the Cz electrode (−2.4 seconds, 22 μV) and the PPNR contacts (−1.9 seconds, 2 μV), the MRP onset latencies and amplitudes were combined for ipsilateral and contralateral movements to examine differences between OFF and ON states. At the Cz electrode, the BP onset latencies and amplitudes were −2.3 ± 0.3 seconds (mean ± SD; range −1.9 to −2.6 seconds) and 22.9 ± 3.6 μV in the OFF state and −2.3 ± 0.2 seconds (range −2 to −2.6 seconds) and 22.9 ± 1.7 μV in the ON state. At the PPNR, the BP onset latencies and amplitudes were −1.7 ± 0.2 seconds (range −1.3 to −2.1 seconds) and 2.4 ± 0.8 μV in the OFF state and −2.1 ± 0.2 seconds and 2.2 ± 0.3 μV in the ON state. No significant difference was found in the MRP onset latencies and amplitudes between OFF and ON states at the EEG and PPNR electrodes. Table e-1 summarizes the MRP onset latencies and amplitudes for the movements studied. Phase reversals between adjacent pairs of PPNR DBS contacts were observed for all movements in the patients studied (figure 1).
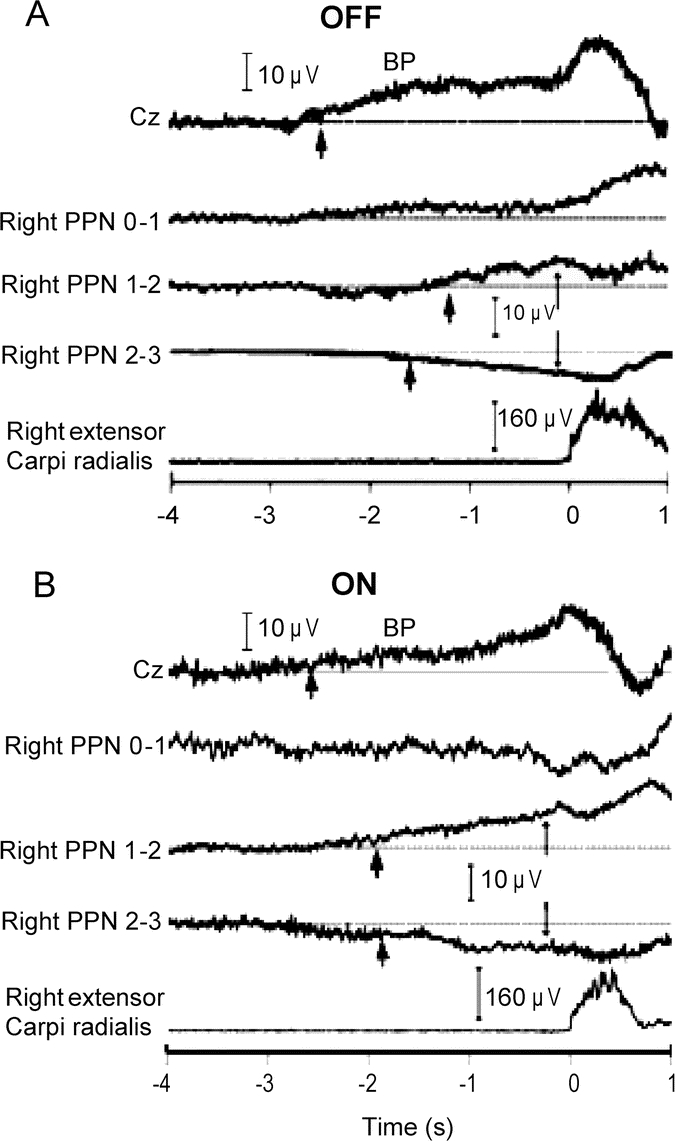
Figure 1 Examples for scalp and bipolar PPNR MRP recordings
Recordings from self-paced ipsilateral wrist extension movements from patient 1 in OFF (A) and ON (B) states. Cz is the vertex scalp electrode, and 0–1, 1–2, and 2–3 represent bipolar montage of the quadripolar deep brain stimulation (DBS) electrodes at the pedunculopontine nucleus region (PPNR). The lowest traces show the rectified and averaged EMG activity of the right extensor carpi radialis muscle (A and B). The horizontal lines represent the baselines. The scalp recording shows a slow, negative movement-related potential (MRP) (Bereitschaftspotential [BP]). The thick arrows point to MRP onsets. The thin arrows signify phase reversals between adjacent DBS contact pairs. The BP onset latencies (approximately −2.5 seconds) for the scalp electrodes were similar between OFF and ON medication states. For the PPNR electrodes, the BP onset occurred at approximately −2 seconds in the OFF and ON states. PPN = pedunculopontine nucleus.
Event-related desynchronization and synchronization.
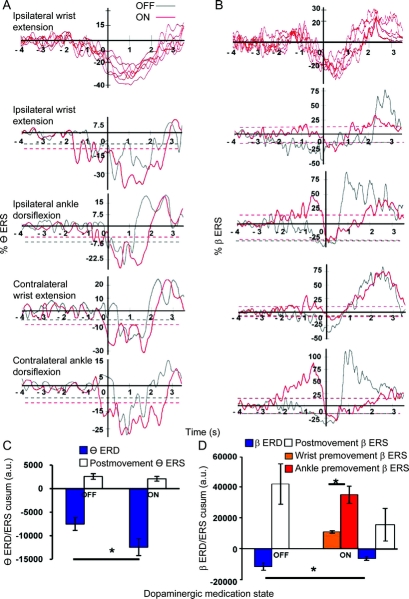
Two frequency bands, theta (6–10 Hz) and beta (14–30 Hz), were found to exhibit changes with movements and dopaminergic medication states (figure 2). The theta band was reduced during movement only in the ON state. The theta and beta ERD/ERS were similar with different movements but showed conspicuous differences between OFF and ON states.
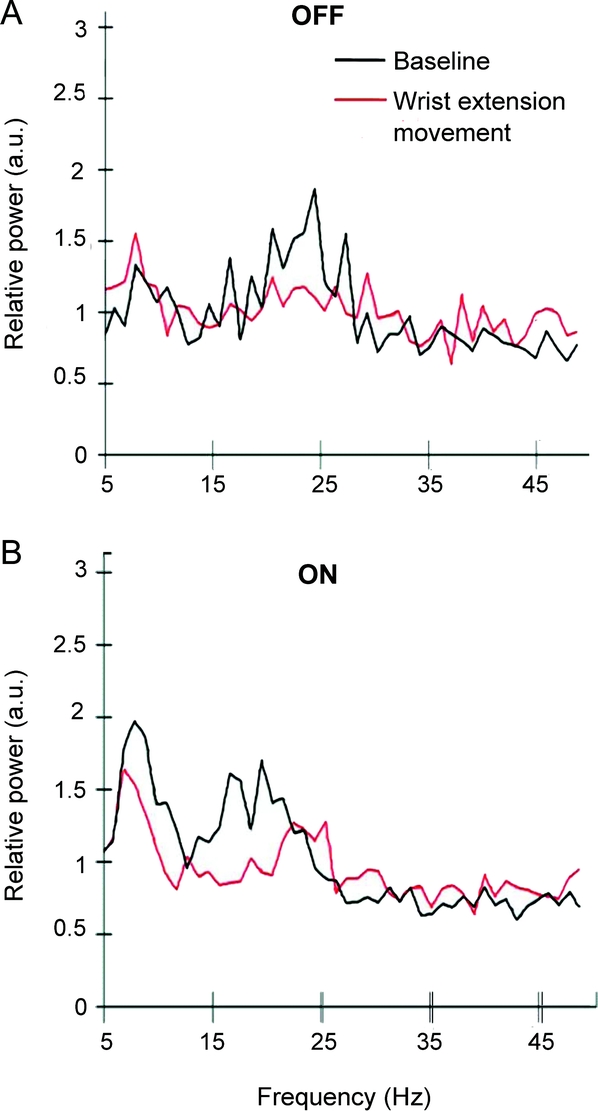
Figure 2 Grand average of relative power spectra of the PPNR during baseline and contralateral wrist extension movement in the OFF (n = 5) and ON (n = 6) medication states from 5 to 50 Hz
The baseline period comprised recordings from 4 to 3 seconds before movement onset and movement period from 0 to 1 seconds after movement onset. The power spectra were computed from an average of approximately 40 epochs from each patient. There were 2 prominent frequency bands in the pedunculopontine nucleus region (PPNR): the theta band, approximately 6 to 10 Hz, and the beta band, approximately 14 to 30 Hz. a.u. = arbitrary units.
Figure 3A summarizes the theta ERD/ERS findings. The theta ERD/ERS waveforms were consistent among patients (figure 3A, top). For the grand averages of all movement in the OFF state, theta ERD had a mean onset latency of 0.07 seconds (0 = EMG onset, positive values after and negative values before EMG onset), a duration of 2.4 seconds, and a cusum of 7,486. In the ON state, the mean theta ERD onset was −0.8 seconds, the duration was 3.8 seconds, and the cusum was 12,426. Paired t tests revealed that theta ERD exhibited earlier onset (t = 5.8, p = 0.01), longer duration (t = −3.9, p = 0.04), and larger cusum (t = −4.4, p = 0.04) (figure 3C) in the ON state than in the OFF state. No theta ERS was observed in movements studied.
Figure 3 Grand average of PPNR theta (6–10 Hz) and beta (14–30 Hz) ERD/ERS and cusum amplitude from 5 patients in the OFF state and 6 patients in the ON state
(A and B) Event-related desynchronization (ERD)/event-related synchronization (ERS) waveforms from pedunculopontine nucleus region (PPNR) theta (A) and beta (B) bands. The abscissa denotes time in seconds, where 0 represents movement onset, and the ordinate denotes ERS percentage with respect to the mean value of the reference interval of −4 to −3 seconds. Positive values represent ERS, and negative values represent ERD. An ERD/ERS waveform is significant if the maximum ERD/ERS value exceeds 3 SDs (dotted line) from the reference intervals and has a duration of more than 1 second. (A) Theta ERD waveforms are significant in both OFF and ON states. (B) Significant premovement beta ERS waveforms present only in the ON state. Significant beta ERD waveforms are found in both OFF and ON states in the premovement and movement execution periods. The top figures in A and B show individual theta and beta ERD/ERS waveforms from 6 patients during ipsilateral wrist extension movements in the ON state, showing that the waveforms were highly consistent among patients. (C and D) The means represent the average cumulative sum amplitude (cusum) of theta (C) or beta (D) ERD/ERS during all movements. Positive values represent ERS and negative values represent ERD. (C) Cusum of PPNR theta ERD was significantly higher in the ON state than in the OFF state during movements. (D) Cusum of PPNR beta ERD was significantly lower in the ON state than in the OFF state during movements. Premovement beta ERS was significantly higher during ankle movements than during wrist movements in the ON state. * p < 0.05. a.u. = arbitrary units.
Figure 3B summarizes the beta ERD/ERS findings. The beta ERD/ERS waveforms were consistent among patients (figure 3B, top). In the OFF state, significant ERD was observed in all movements in the premovement period with an average onset latency of −1.4 seconds. The beta ERD continued into movement execution and ended at 1.1 seconds, followed by postmovement ERS. In contrast, in the ON state, significant premovement beta ERS was detected with an average onset latency of −1.8 seconds, followed by premovement beta ERD with average onset latency of −0.12 seconds. The beta ERD continued into movement execution and ended at 1.1 seconds, followed by postmovement ERS. The average duration and cusum of ERD during premovement and movement execution periods were 2.4 seconds and 11,644 in the OFF state and 1.2 seconds and 6,238 in the ON state. The average duration and cusum for premovement ERS in the ON state were 1.5 seconds and 23,131. The total beta ERD cusum was smaller in the ON state than in the OFF state (t = 3.1, p = 0.04; figure 3D). Premovement beta ERS cusum during ankle movements (mean = 35,262) was larger than wrist movements (mean = 11,010) (t = 3.9, p = 0.03; figure 3D).
Coherence and phase coherence between the cortex and the PPNR.
In the OFF state, coherence between EEG and PPNR was not observed in any movement or average of all movements with 550 trials. In the ON state, theta band coherences were found between the ipsilateral or contralateral sensorimotor regions and the PPNR but not between the PPNR and the midline region. Beta band coherences were found between the midline, ipsilateral, or contralateral sensorimotor regions and the PPNR for all movements studied in the ON state (figure 4). Theta band coherence between the PPNR and the ipsilateral (figure 4, A–D) or contralateral sensorimotor regions (figure 4, E and F) were present in both the premovement and movement periods and displayed an intermittent pattern. Prominent attenuations in theta coherence were observed from approximately 0.8 to 0.2 seconds before onset of self-paced movements (figure 4, A–F). Beta band coherence was present only in the premovement period from approximately −2.5 to 0 seconds but was absent in the movement execution period (figure 4). Permutation statistics showed that beta band coupling between the ipsilateral sensorimotor region (figure 4, B and D) and the PPNR was more robust than the contralateral connection (figure 4F).
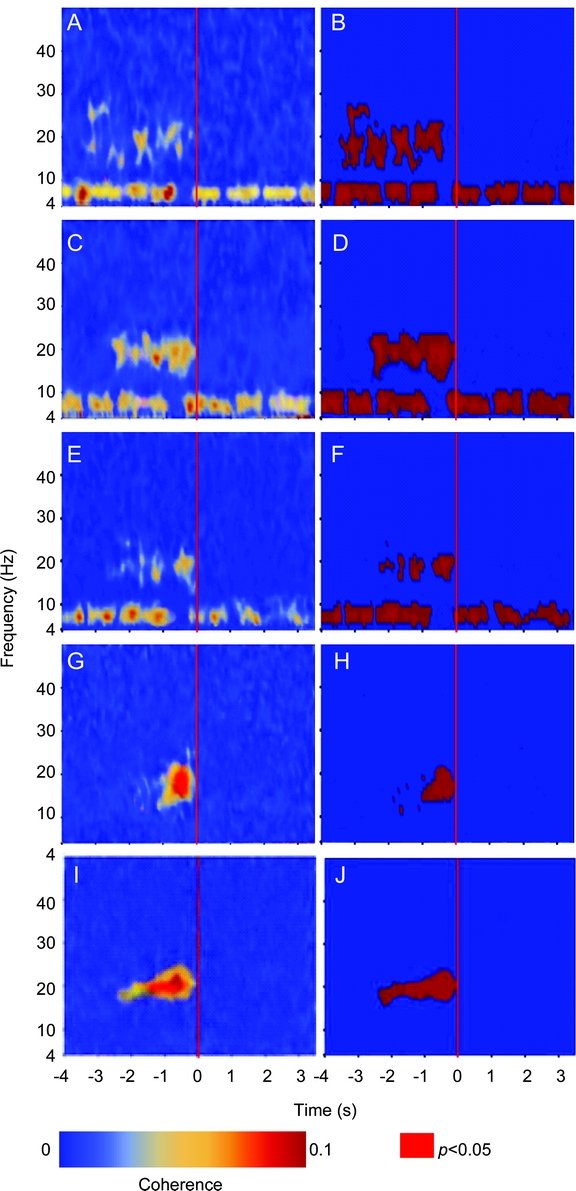
Figure 4 Temporal representation of the grand average coherences (left) and the corresponding permutation tests (right) between the cortices and the PPNR
The data were collected from 6 patients in the ON state. Coherence between the ipsilateral sensorimotor region and the pedunculopontine nucleus region (PPNR) during ipsilateral wrist extension movements (A and B); coherence between the ipsilateral sensorimotor region and the PPNR during ipsilateral ankle dorsiflexion movements (C and D); coherence between the contralateral sensorimotor region and the PPNR during ipsilateral ankle dorsiflexion movements (E and F); coherence between scalp EEG electrodes represent the midline prefrontal region (Cz–Fz) and the PPNR during ipsilateral wrist extension movements (G and H) and ipsilateral ankle dorsiflexion movements (I and J). The abscissa denotes time in seconds, where the red marker at time 0 represents movement onset, and the ordinate denotes frequency (4–50 Hz). Red areas in B, D, F, H, and J indicate p < 0.05 based on permutation tests. In A, C, and E, coherence in the theta frequency range (6–10 Hz) was present intermittently from the start to the end of the epoch and diminished for approximately 0.6 seconds before movement onset. Coherence in the beta frequency range (15–25 Hz) was present during the premovement period from approximately −2.5 to 0 seconds and was most conspicuous in the ipsilateral sensorimotor region (A to D) during ipsilateral ankle movements (C and D). In G and I, coherence in the beta frequency range (15–25 Hz) was present before movement onset. Coherence between the midline and the PPNR in the beta band was more prominent before ankle movements (J) than before wrist movements (H). Beta coherence between the PPNR and the cortex was stronger in the midline than in the sensorimotor cortical regions (G and A; I and C).
Beta coherences between the PPNR and the midline prefrontal region that likely represented the SMA were observed in all movements from 14 to 23 Hz, with a peak at approximately 20 Hz (figure 4, G–J). The coupling between midline and PPNR was also found predominantly in the premovement stage, and the durations of coupling were longer during ankle movements (figure 4, I and J) than during wrist movements (figure 4, G and H).
Phase coherence analyses revealed bidirectional theta band relations between the ipsilateral sensorimotor regions and the PPNR. The PPNR lagged the ipsilateral sensorimotor region by 3.4 ± 0.4 milliseconds in the premovement period from −3 to −1 seconds but led the ipsilateral sensorimotor region by 2.2 ± 0.6 milliseconds in the movement execution period from 0 to 1 second (figure e-2, A and B). For the beta band, the PPNR lagged the midline region by 10.5 ± 4 milliseconds (figure e-2C) in the premovement period. No other phase coherence was observed in the theta and beta bands.
DISCUSSION
The present study examined the interactions between the human PPNR and the cortices during both arm and leg movements. We found that voluntary movements changed PPNR activities and functional interactions between the cortices and the PPNR. In addition, dopaminergic medications modulated PPNR activities and their interactions with the cortices during planning and execution of voluntary movements.
The PPNR MRP onset latencies of approximately −2 seconds in the ON state were similar to MRP onset latencies recorded from the STN in the ON state and the ventral thalamus (VT) (approximately −2.1 seconds).21,22 We found phase reversals between adjacent PPNR contact pairs (figure 1), suggesting that PPNR MRP represents focal activities instead of far field potentials. The findings of MRP in the STN, the VT, and the PPNR are consistent with the hypothesis that subcortical nuclei may contribute to the cortical MRP23 and the PPNR is part of the subcortical circuits involved in movement preparation.
Frequency analysis revealed modulations of PPNR theta and beta frequencies that were similar with upper or lower limb movements and between the ipsilateral and contralateral sides. The strength of coherence between EEG and PPNR LFP was approximately 0.1, similar to previous studies of coherences between EEG and STN or GPi LFP.24,25 Because PPNR coherence with the cortex was found only in the ON state, dopaminergic medications promoted the interactions between the cortex and the PPNR in patients with PD. This is consistent with findings that dopaminergic medications restore the deficient activation of SMA and related motor circuits in PD.26,27
We found that PPNR theta ERD during movement preparation and execution was more prominent in the ON state than in the OFF state (figure 3, A and C), suggesting that dopaminergic medications promoted suppression of theta rhythm during voluntary movements. Coherences between the PPNR and the sensorimotor regions in the theta band were stronger between the ipsilateral than the contralateral sensorimotor region (figure 4, C and E), consistent with a diffusion tractography study showing strong connections between the human PPNR and the ipsilateral sensorimotor cortex.4
The ipsilateral sensorimotor region led the PPNR in the premovement period (figure e-2A) but lagged the PPNR in the movement execution period (figure e-2B), suggesting that the ipsilateral sensorimotor cortex may drive the PPNR during movement preparation and receive sensory feedback through the PPNR during movement execution. Animal studies supported the idea that the PPNR is involved with sensory feedback, especially the selection of relevant sensory information for motor programming and execution.3 Theta band coherences were observed between the ipsilateral sensorimotor cortex and the VT in both humans and animals28,29 and the brainstem trigeminal sensory nucleus in rats.29 These theta coherences were thought to link different neuronal populations for the continuous monitoring of sensory information in the environment.28,29 The human PPNR theta band may be part of cortical brainstem thalamocortical system that monitors continuous stream of sensory information.
Beta oscillation is a prominent feature of human parkinsonism.30,31 Excessive beta oscillations in the BG30,32–34 and in the cortices may lead to abnormal cortical motor output responsible for parkinsonian motor symptoms.32,35 Levodopa as well as voluntary movements decreased beta oscillations in the STN and GPi.32,36,37 STN or GPi high-frequency DBS (>100 Hz) as well as dopaminergic medications may disrupt the abnormal beta synchronization in the basal ganglia, resulting in improvement of motor symptoms.35
Premovement beta oscillation in the PPNR decreased in the OFF state but increased in the ON state (figure 3B). Premovement beta ERS has not been observed in the BG or the cortex, suggesting that beta oscillation may have different functional significances in the BG (STN and GPi) and the PPNR. There was stronger activation of PPNR beta frequency before ankle movements than before wrist movements (figure 3, B and D), and PPNR beta coherence with the cortices was also more robust before ankle movements than before wrist movements (figure 4, A–D and G–J). This may indicate that the PPNR is more involved with lower than with upper extremity movements, consistent with studies showing that PPNR DBS normalized lower limb spinal reflexes38 and improved falls in patients with advanced PD.15
The occurrence of beta coherence between the cortices and PPNR before movement onset is opposite to the findings in the VT in tremor patients, where there was beta band coherence between the midline EEG and VT in the resting period, but it was reduced approximately 1 second before movement onset. Also, beta coherence between the midline EEG and STN decreased rather than increased just before movement onset in patients with PD.39,40 These differences in beta coherence may indicate that SMA modulates the PPNR, the VT, and the BG during movement preparation through distinct pathways.
Beta coherence between the midline prefrontal region and the PPNR was only found in the ON state (figure 4, G–J), suggesting that dopaminergic medications may facilitate cortical excitatory drive from the SMA to the PPNR (figure e-2C), consistent with findings in human neuroimaging studies regarding the facilitatory effects of dopaminergic medications and PPNR DBS on the SMA-related motor circuits.5,26,27 The premovement beta excitatory drive from the SMA to the PPNR may be involved with formulation of motor program for movement executions. Thus, the beta rhythm in the human PPNR may be prokinetic rather than antikinetic. This may explain the general findings that PPNR DBS at lower frequencies was more effective than at high frequencies (>100 Hz) for the alleviation of motor symptoms in patients with PD.7,15,16 PPNR DBS at beta frequency (approximately 30 Hz) was reported to alleviate rigidity and gait dysfunctions, whereas PPNR DBS at gamma frequencies (50 to 70 Hz) was found to decrease falls.15
The PPNR beta band coherence was present just before movements (figure 4, G and J), coincident in time with the beta ERS (figure 3B) and the brief attenuation of PPNR theta band coherence (figure 4, A–F). The different findings for PPNR theta and beta rhythms suggest that they may have different functions associated with 2 different PPNR cortical circuits. The sensory circuits may act through theta oscillations between the PPNR and the sensorimotor cortices, which are active continuously and switch off before movement execution. The motor circuit is then activated and may act through the PPNR beta oscillations driven through the SMA for mediating motor-related function. See appendix e-1 for discussion of the limitations of the study.
Activities of the PPNR change during movement preparation and execution in patients with PD. PPNR theta and beta oscillatory activities are modulated by voluntary movements and dopaminergic medications. Dopaminergic medications promote interactions between the cortices and the PPNR. Compared with oscillations in the BG, the human PPNR oscillations may be modulated differently by voluntary movements and dopaminergic medications and may have different functional significances.
AUTHOR CONTRIBUTIONS
Statistical analysis was completed by E.W. Tsang.
ACKNOWLEDGMENT
The authors thank Mr. Utpal Saha for assisting in data recording, and Dr. Moran Weinberger and Dr. Karsten Hoechstetter for their helpful comments.
DISCLOSURE
Mr. Tsang receives research support from the Canada Graduate Scholarship Doctoral Award (CIHR). Dr. Hamani serves as a consultant for and has received speaker honoraria from St. Jude Medical, Inc.; and receives research support from the National Alliance for Research on Schizophrenia and Depression. Dr. Moro serves as a consultant for and has received speaker honoraria from Medtronic, Inc.; and receives research support from Medtronic, Inc., CIHR-AF-BMBF, Sick Kids Foundation (and Institute of Human Development, Child and Youth Health), and CurePSP. Ms. Mazzella reports no disclosures. Ms. Poon has received funding for travel from Medtronic, Inc. Dr. Lozano serves on scientific advisory boards for Johnson & Johnson, Codman & Shurtleff, Inc., Ceregene, Neurologix, Inc., and Functional Neuroscience, Inc.; serves as Deputy Editor of Brain Stimulation and on the editorial boards of the Journal of Neurosurgery, Neurosurgery, Movement Disorders, World Neurosurgery, Neurological Research Stereotactic and Functional Neurosurgery, Operative Neurosurgery, NeuroRx, Surgical Neurology, and Parkinsonism and Related Disorders; holds patents re: Methods of treating depression, mood disorders and anxiety using neuromodulation; Method of treating mood disorders and/or anxiety disorders by brain stimulation; Brain stimulation lead used for lesioning; and Method of treating movement disorders by electrical stimulation and/or drug infusion of the pedunculopontine nucleus; receives royalties from the publication of Surgical Treatment of Parkinson's Disease and Other Movement Disorders (Humana Press, 2002) and Textbook of Stereotactic and Functional Neurosurgery, 2nd ed. (Springer, 2009); has received honoraria and research support from Medtronic, Inc. and St. Jude Medical, Inc.; holds the Canada Research Chair in Neurosciences; and serves as a consultant for Medtronic, Inc., St. Jude Medical, Inc., Boston Scientific, Amgen, Ely Lilly and Company, Bristol-Myers Squibb, Elekta, Bayer Schering Pharma, and Schering-Plough Corp. Dr. Chen has served on scientific advisory boards for Medtronic, Inc., Teva Pharmaceutical Industries Ltd., Allergan, Inc., Novartis, and Biovail Corporation; has received funding for travel and speaker honoraria from Merz Pharmaceuticals, LLC and Allergan Inc.; serves/has served on the editorial boards of Clinical Neurophysiology, Muscle and Nerve, the Journal of Motor Behavior, the Canadian Journal of Neurological Sciences, Neural Plasticity, and Neurology; receives/has received research support from Medtronic Inc, CIHR, the Michael J. Fox Foundation for Parkinson's Research, and the Dystonia Medical Research Foundation; and has provided expert testimony and affidavit in welding-related litigation.
Supplementary Material
Address correspondence and reprint requests to Dr. Robert Chen, Toronto Western Hospital, McLaughlin Pavilion, 7th Floor, Room 411, 399 Bathurst St., Toronto, Ontario M5T 2S8, Canada robert.chen@uhn.on.ca
Editorial, page 944
Supplemental data at www.neurology.org
e-Pub ahead of print on August 11, 2010, at www.neurology.org.
Study funding: Supported by the Canadian Institutes of Health Research (CIHR) MOP15128. Eric W. Tsang is supported by a CIHR Canada Graduate Scholarship Doctoral Award, Robert Chen is supported by a CIHR–Industry Partnered Investigator Award, and Andres M. Lozano is supported by the Canada Research Chair in Neurosciences.
Disclosure: Author disclosures are provided at the end of the article.
Received November 6, 2009. Accepted in final form March 9, 2010.
REFERENCES
- 1.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain 2000;123:1767–1783. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Rill E. The pedunculopontine nucleus. Prog Neurobiol 1991;36:363–389. [DOI] [PubMed] [Google Scholar]
- 3.Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci 2006;248:234–250. [DOI] [PubMed] [Google Scholar]
- 4.Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H. Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. Neuroimage 2007;37:694–705. [DOI] [PubMed] [Google Scholar]
- 5.Ballanger B, Lozano AM, Moro E, et al. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: a [(15)O] H2O PET study. Hum Brain Mapp 2009;30:3901–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumura M, Watanabe K, Ohye C. Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci Res 1997;28:155–165. [DOI] [PubMed] [Google Scholar]
- 7.Weinberger M, Hamani C, Hutchison WD, Moro E, Lozano AM, Dostrovsky JO. Pedunculopontine nucleus microelectrode recordings in movement disorder patients. Exp Brain Res 2008;188:165–174. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Rill E, Skinner RD. The mesencephalic locomotor region, I: activation of a medullary projection site. Brain Res 1987;411:1–12. [DOI] [PubMed] [Google Scholar]
- 9.Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD, Garcia-Rill E. Medioventral medulla-induced locomotion. Brain Res Bull 1990;24:509–516. [DOI] [PubMed] [Google Scholar]
- 10.Eidelberg E, Walden JG, Nguyen LH. Locomotor control in macaque monkeys. Brain 1981;104:647–663. [DOI] [PubMed] [Google Scholar]
- 11.Conde H, Dormont JF, Farin D. The role of the pedunculopontine tegmental nucleus in relation to conditioned motor performance in the cat, II: effects of reversible inactivation by intracerebral microinjections. Exp Brain Res 1998;121:411–418. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res 1985;330:43–54. [DOI] [PubMed] [Google Scholar]
- 13.Mazzone P, Lozano A, Stanzione P, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson's disease. Neuroreport 2005;16:1877–1881. [DOI] [PubMed] [Google Scholar]
- 14.Stefani A, Lozano AM, Peppe A, et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson's disease. Brain 2007;130:1596–1607. [DOI] [PubMed] [Google Scholar]
- 15.Moro E, Hamani C, Poon YY, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain 2010;133:215–224. [DOI] [PubMed] [Google Scholar]
- 16.Pereira EA, Muthusamy KA, De Pennington N, Joint CA, Aziz TZ. Deep brain stimulation of the pedunculopontine nucleus in Parkinson's disease: preliminary experience at Oxford. Br J Neurosurg 2008;22:S41–S44. [DOI] [PubMed] [Google Scholar]
- 17.Hamani C, Moro E, Zadikoff C, Poon YY, Lozano AM. Location of active contacts in patients with primary dystonia treated with globus pallidus deep brain stimulation. Neurosurgery 2008;62:217–223. [DOI] [PubMed] [Google Scholar]
- 18.Pollo C, Villemure JG, Vingerhoets F, Ghika J, Maeder P, Meuli R. Magnetic resonance artifact induced by the electrode Activa 3389: an in vitro and in vivo study. Acta Neurochir 2004;146:161–164. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Huang X. Atlas of the Human Brainstem. San Diego, CA: Academic Press; 1995. [Google Scholar]
- 20.Barrett G, Shibasaki H, Neshige R. Cortical potentials preceding voluntary movement: evidence for three periods of preparation in man. Electroencephalogr Clin Neurophysiol 1986;63:327–339. [DOI] [PubMed] [Google Scholar]
- 21.Paradiso G, Saint-Cyr JA, Lozano AM, Lang AE, Chen R. Involvement of the human subthalamic nucleus in movement preparation. Neurology 2003;61:1538–1545. [DOI] [PubMed] [Google Scholar]
- 22.Paradiso G, Cunic D, Saint-Cyr JA, et al. Involvement of human thalamus in the preparation of self-paced movement. Brain 2004;127:2717–2731. [DOI] [PubMed] [Google Scholar]
- 23.Rektor I. Scalp-recorded Bereitschaftspotential is the result of the activity of cortical and subcortical generators: a hypothesis. Clin Neurophysiol 2002;113:1998–2005. [DOI] [PubMed] [Google Scholar]
- 24.Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson's disease. Cereb Cortex 2006;16:64–75. [DOI] [PubMed] [Google Scholar]
- 25.Sharott A, Grosse P, Kuhn AA, et al. Is the synchronization between pallidal and muscle activity in primary dystonia due to peripheral afferance or a motor drive? Brain 2008;131:473–484. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins IH, Fernandez W, Playford ED, et al. Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann Neurol 1992;32:749–757. [DOI] [PubMed] [Google Scholar]
- 27.Rascol O, Sabatini U, Chollet F, et al. Supplementary and primary sensory motor area activity in Parkinson's disease: regional cerebral blood flow changes during finger movements and effects of apomorphine. Arch Neurol 1992;49:144–148. [DOI] [PubMed] [Google Scholar]
- 28.Marsden JF, Ashby P, Limousin-Dowsey P, Rothwell JC, Brown P. Coherence between cerebellar thalamus, cortex and muscle in man: cerebellar thalamus interactions. Brain 2000;123:1459–1470. [DOI] [PubMed] [Google Scholar]
- 29.Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science 1995;268:1353–1358. [DOI] [PubMed] [Google Scholar]
- 30.Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson's disease. Brain 2002;125:1196–1209. [DOI] [PubMed] [Google Scholar]
- 31.Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson's disease. Cereb Cortex 2006;16:64–75. [DOI] [PubMed] [Google Scholar]
- 32.Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J Neurosci 2001;21:1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberger M, Mahant N, Hutchison WD, et al. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J Neurophysiol 2006;96:3248–3256. [DOI] [PubMed] [Google Scholar]
- 34.Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson's disease. Cereb Cortex 2006;16:64–75. [DOI] [PubMed] [Google Scholar]
- 35.Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol 2007;17:656–664. [DOI] [PubMed] [Google Scholar]
- 36.Androulidakis AG, Kuhn AA, Chen CC, et al. Dopaminergic therapy promotes lateralized motor activity in the subthalamic area in Parkinson's disease. Brain 2007;130:457–468. [DOI] [PubMed] [Google Scholar]
- 37.Brucke C, Kempf F, Kupsch A, et al. Movement-related synchronization of gamma activity is lateralized in patients with dystonia. Eur J Neurosci 2008;27:2322–2329. [DOI] [PubMed] [Google Scholar]
- 38.Pierantozzi M, Palmieri MG, Galati S, et al. Pedunculopontine nucleus deep brain stimulation changes spinal cord excitability in Parkinson's disease patients. J Neural Transm 2008;115:731–735. [DOI] [PubMed] [Google Scholar]
- 39.Cassidy M, Mazzone P, Oliviero A, et al. Movement-related changes in synchronization in the human basal ganglia. Brain 2002;125:1235–1246. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn AA, Doyle L, Pogosyan A, et al. Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson's disease. Brain 2006;129:695–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.