Abstract
Objective:
To test the hypothesis that frequent cognitive activity predicts slower cognitive decline before dementia onset in Alzheimer disease (AD) and faster decline thereafter.
Methods:
As part of a longitudinal cohort study, older residents of a geographically defined population were assessed at 3-year intervals with brief cognitive performance tests from which a composite measure of global cognition was derived. After each wave of testing, a subset was sampled for clinical evaluation. The present analyses are based on 1,157 participants. They were free of dementia at study enrollment at which time they rated frequency of participation in common cognitively stimulating activities from which a previously validated summary measure was derived. They were sampled for clinical evaluation a mean of 5.6 years after enrollment and subsequently followed a mean of 5.7 years with brief cognitive performance testing at 3-year intervals.
Results:
On clinical evaluation, 614 people had no cognitive impairment, 395 had mild cognitive impairment, and 148 had AD. During follow-up, the annual rate of global cognitive decline in persons without cognitive impairment was reduced by 52% (estimate = 0.029, SE = 0.010, p = 0.003) for each additional point on the cognitive activity scale. In the mild cognitive impairment group, cognitive decline rate was unrelated to cognitive activity (estimate = −0.019, SE = 0.018, p = 0.300). In AD, the mean rate of decline per year increased by 42% (estimate = 0.075, SE = 0.021, p < 0.001) for each point on the cognitive activity scale.
Conclusion:
Mentally stimulating activity in old age appears to compress the cognitive morbidity associated with AD by slowing cognitive decline before dementia onset and hastening it thereafter.
GLOSSARY
- AD
= Alzheimer disease;
- MCI
= mild cognitive impairment.
More frequent cognitive activity has been associated with reduced risk of cognitive decline and dementia1–11 but not with the neurodegenerative lesions associated with dementia.11 This suggests that cognitive experiences may somehow protect cognitive systems from incipient neurodegeneration. A critical test of this hypothesis is the fate of cognitively active people when they develop dementia and activity's protective effect is apparently lost or greatly diminished.12,13 If cognitive activity before dementia onset were truly protective, higher activity would be associated with a relatively greater pathologic burden at time of diagnosis and therefore with more rapid cognitive decline thereafter.14 This result would imply that cognitive activity substantially compresses the cognitive morbidity of Alzheimer disease (AD) by associations with delayed dementia onset and then more rapid dementia progression, thereby reducing the proportion of the lifespan spent in a state of dementia.
We investigated these ideas in a community population of older people. They rated frequency of participation in 7 cognitive activities. At 3-year intervals thereafter, samples of residents underwent uniform clinical evaluation and were classified as having no cognitive impairment, mild cognitive impairment (MCI), or AD. Following clinical classification, brief cognitive performance tests were administrated at 3-year intervals. We constructed mixed-effects models to characterize subsequent rate of change in cognitive function within each diagnostic group and to test the hypotheses that higher level of cognitive activity would be associated with slower cognitive decline prior to dementia onset in AD and with more rapid decline thereafter.
METHODS
Participants.
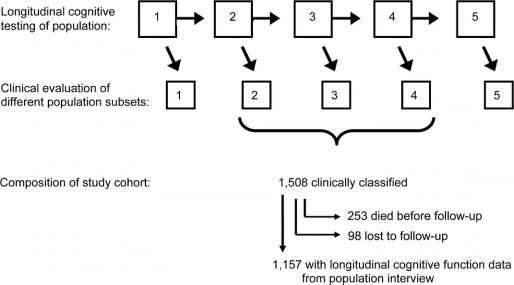
Subjects are from the Chicago Health and Aging Project, an ongoing longitudinal study of risk factors for AD conducted in 4 adjacent neighborhoods in Chicago.15 After a census of the study area beginning in 1993, all residents aged 65 years or older were invited to participate in an in-home interview. A stratified random sample of interviewees was asked to undergo a detailed clinical evaluation. Approximately 3 years later, the entire population was re-interviewed and a stratified random sample of those judged dementia-free in the first wave of data collection had a clinical evaluation. The interview of the full population and clinical evaluation of a previously dementia-free subset has been repeated at 3-year intervals, with the fifth wave of data collection currently in progress, as depicted in the top 2 rows of figure 1. The clinical evaluation was designed to support clinical classification of MCI, dementia, and AD. It includes a structured medical history, complete neurologic examination, administration of a battery of 18 cognitive performance tests, and laboratory testing, as previously described.8,16 A boarded neuropsychologist reviewed the cognitive test data and rated impairment in 5 cognitive domains. Based on these data and an in-person examination, a physician diagnosed dementia and AD. Dementia criteria required a history of cognitive decline and evidence of impairment in at least 2 areas of cognitive function, 1 of which must be memory to meet criteria for AD.17 As previously described,18 the neuropsychologist and physicians were provided with algorithmic ratings of cognitive impairment, dementia, and AD to help enhance uniformity in clinical decision-making.
Figure 1 Composition of study cohort sampled from community population
Persons who did not meet dementia criteria but were impaired in at least 1 cognitive domain were classified as MCI. These MCI criteria have previously been associated with mortality,18,19 cognitive decline,18,20 and postmortem evidence of neurodegeneration.21–23
As shown in figure 1, the current analyses are based on individuals who completed a clinical evaluation in the second, third, or fourth wave of data collection. We excluded clinical evaluations from the first wave to restrict the study to incident AD and from the ongoing fifth wave because of insufficient time for follow-up. Of 2,176 individuals sampled for the second, third, or fourth clinical evaluations, 1,521 (69.9%) participated, and 1,508 (69.3%) of the evaluations yielded sufficient data to support clinical classification (946 from second wave clinical evaluation, 297 from third, 265 from fourth). There were 253 deaths before follow-up (164 from second wave clinical evaluation, 44 from third, 45 from fourth). Of the 1,255 survivors, 98 (7.8%) were lost to follow-up (71 from second wave clinical evaluation, 7 from third, 20 from fourth) and 1,157 (92.2%) had follow-up cognitive data (711 from second wave clinical evaluation, 246 from third, 200 from fourth). They had a mean of 2.7 valid global cognitive scores per individual, a mean age of 78.9 (SD = 5.5) at the time of clinical classification, and a mean of 13.0 (SD = 3.6) years of schooling; 63.2% were women; 50.2% were African American.
Standard protocol approvals, registrations, and patient consents.
The Chicago Health and Aging Project was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all participants.
Assessment of cognitive activity.
At the initial population interview, subjects rated frequency of participation in 7 activities on a 5-point scale as every day or about every day (5), several times a week (4), several times a month (3), several times a year (2), or once a year or less (1). The activities, chosen because information processing plays a central role, were viewing television; listening to radio; reading newspapers; reading magazines; reading books; playing games like cards, checkers, crosswords, or other puzzles; and going to a museum. Ratings were averaged to form a composite measure of frequency of participation in cognitively stimulating activities.24 Higher scores have been associated with decreased rate of cognitive decline and risk of developing dementia in this8,9 and other10 longitudinal cohort studies.
Assessment of cognitive function.
Each population interview included 4 brief cognitive performance tests: immediate and delayed recall of 12 ideas contained in the East Boston Story25,26; Symbol Digit Modalities Test,27 a measure of perceptual speed; and the Mini-Mental State Examination, a 30-point mental status test. Because all 4 tests loaded on a single factor in a previous factor analysis,24 we used a composite of the tests in analyses. Raw scores on each test were converted to z scores, using the population mean and SD at the initial interview, and the z scores were averaged to yield the composite measure of global cognition.9,24,28
Data analysis.
We used mixed-effects models29 to characterize rate of cognitive change within diagnostic groups and to test whether premorbid level of cognitive activity modified these rates. In this growth curve approach, individual paths of change in cognitive function are assumed to follow the mean trajectory in the population except for random effects that cause baseline level of cognitive function to be higher or lower and rate of change to be faster or slower. These 2 random effects are assumed to follow a bivariate normal distribution. The model also estimates deviations of the observed measurements from each person's overall smooth trajectory. These deviations are assumed to be independent and comparably distributed normal errors with a common unknown variance. The assumptions were examined graphically and analytically and found to be adequately met.
Those without cognitive impairment served as a reference group that was contrasted with MCI and AD groups. The primary model had 7 key terms of interest: time (in years since the population interview aligned with the diagnostic evaluation), cognitive activity, cognitive activity × time, cognitive activity × MCI, cognitive activity × MCI × time, cognitive activity × AD, and cognitive activity × AD × time. The term for time indicates the mean change per year in the reference group. The terms for cognitive activity and its interaction with time test the relation of cognitive activity to baseline level of cognitive function and annual rate of cognitive change in the reference group. The interactions of cognitive activity with MCI and MCI × time and with AD and AD × time indicate the difference from the reference group in cognitive activity's associations with baseline level of cognitive function and annual rate of change. Terms were included for MCI and AD and their interactions with time to account for between-group differences in baseline level of cognition and rate of cognitive change. The model also included terms for age at diagnosis, sex, race, education, and their interactions with time so that the effects of the variables of interest were conditional on the fixed effects of these demographic variables on baseline level of cognitive function and rate of cognitive change.
The final model estimates were weighted to account for the stratified random sampling. Variance estimation was based on jackknife repeated replication.30
To adjust for the temporal interval from the initial population interview (when cognitive activity was assessed) to the date of clinical classification, we repeated the initial model with terms for time from cognitive activity assessment to diagnostic evaluation and its interaction with time following the diagnosis. To assess the effect of education, we repeated the analysis without terms for education and its interaction with time. To assess the effect of attrition, we constructed a model to predict being excluded from longitudinal analyses with terms for age, sex, race, education, diagnosis, and cognitive activity. We then developed weights based on the inverse of the predicted probabilities from this model, multiplied these by the sampling weights, and then repeated the original analysis with the new weights.31,32
RESULTS
Cognitive activity participation.
As part of the initial population interview, all subjects rated frequency of participation in 7 cognitively stimulating activities from which a previously established composite score was derived. In the 1,157 subjects eligible for analyses, scores ranged from 1.43 to 4.71, with higher values indicating more frequent activity (unweighted mean = 3.34 [SD = 0.59]; weighted mean = 3.34 [SE = 0.03]). In crude analyses upweighted to the population, more frequent activity was associated with younger age (r = −0.09, p = 0.023), more education (r = 0.44, p < 0.001), and being white (weighted mean of 3.13 [SE = 0.04] in African Americans vs 3.58 [SE = 0.03] in white participants; F1,100 = 80.1, p < 0.001). The activity levels of men and women did not differ (weighted mean of 3.28 [SE = 0.04] in men vs 3.37 [SE = 0.03] in women; F1,100 = 3.6, p = 0.059).
Cognitive activity and cognitive decline.
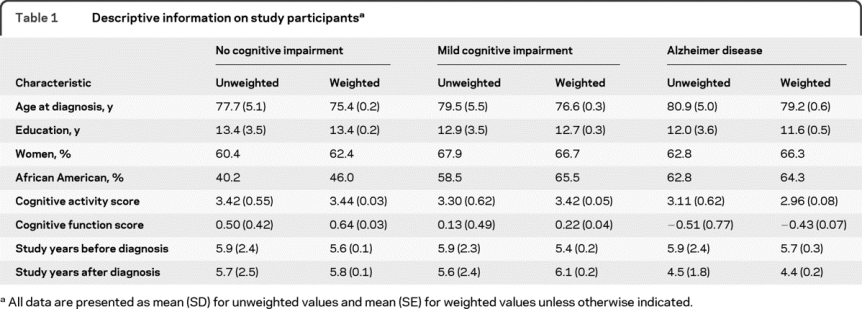
On clinical evaluation, 614 individuals had no cognitive impairment, 395 had MCI, and 148 had AD. As shown in table 1, those with AD were older, less educated, and more likely to be African American than those without AD, and they had a lower premorbid level of cognitive activity.
Table 1 Descriptive information on study participants
We used mixed-effects models to characterize change in cognitive function in persons in each diagnostic subgroup and to test the relation of premorbid cognitive activity to change in each subgroup. The outcome is a composite measure of global cognition based on 4 tests administered at 3-year intervals as part of the population interview. For these analyses, we used cognitive data from the wave when the clinical evaluation took place and all subsequent population interviews (mean = 2.7 assessments per person, SD = 0.8, range = 2–4). At the initial assessment point, global cognitive scores ranged from −3.08 to 1.48 (unweighted mean = 0.25 [SD = 0.60]; weighted mean = 0.42 [SE = 0.03]), with higher scores denoting better performance.
In those without cognitive impairment on clinical evaluation, the global cognitive score subsequently declined a mean of 0.056 unit per year, as shown by the term for time in model A of table 2 (table e-1 on the Neurology® Web site at www.neurology.org shows an expanded version of the table, and table e-2 provides model results before upweighting). Each additional point on the cognitive activity scale in the no cognitive impairment subgroup was associated with a 0.116-unit increase in baseline cognitive score and a 0.029-unit decrease, or about 52% (0.029/0.056), in annual rate of global cognitive decline, as shown by the terms for cognitive activity and cognitive activity × time. In the MCI subgroup, however, cognitive activity was not related to level of global cognition at study baseline or subsequent rate of cognitive decline. In AD, cognitive activity was not related to baseline level of cognition, but it was related to change, with each point on the premorbid cognitive activity scale associated with a 0.075-unit increase, or about 42% (0.075/0.180), in annual rate of global cognitive decline.
Table 2 Relation of premorbid cognitive activity to rate of change in cognitive function in persons with no cognitive impairment, MCI, or AD
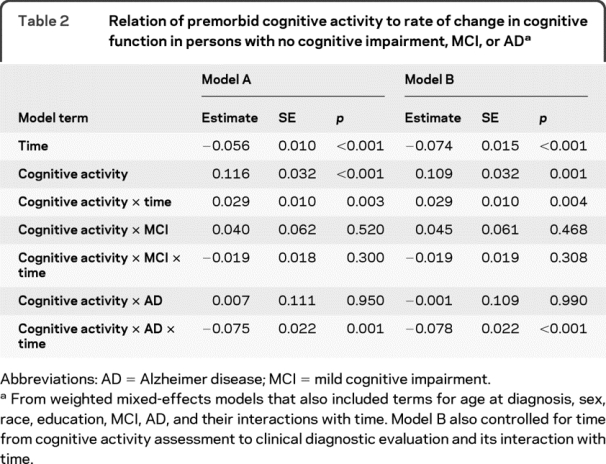
Because cognitive activity was assessed from 1.5 to 12.4 years before clinical classification took place and this could have affected results, we repeated the analysis with terms added for the duration of this interval and its interaction with time after diagnosis. As shown in table 2 (model B), the association of cognitive activity with cognitive decline was unchanged. Results were also comparable when terms for education and its interaction with time were dropped from the model (estimate for cognitive activity × time = 0.028, SE = 0.010, p = 0.005; estimate for cognitive activity × AD × time = −0.078, SE = 0.022, p < 0.001).
Follow-up cognitive data were missing in 351 persons (253 died, 98 lost to follow-up) and they had lower cognitive activity (mean of 3.23 [SD = 0.58] vs 3.34 [SD = 0.59]; t[1,506] = 3.0, p = 0.003) and baseline cognitive function (mean of −0.06 [SD = 0.72] vs 0.25 [SD = 0.60]; t[509.2] = 7.1, p < 0.001) than participants with follow-up data. To examine whether these missing data affected results, we repeated the analysis with weighting to account for the probability of being missing,31,32 and the key interactions involving cognitive activity and time were essentially unchanged.
Figure 2 shows the predicted 6-year paths of change in cognitive function for individuals with low (10th percentile, dotted line) vs high (90th percentile, solid line) levels of cognitive activity in the no cognitive impairment (black lines), MCI (blue lines), and AD (red lines) groups. The figure suggests differences in the relation of late-life cognitive activity to cognitive decline at different points along the spectrum from intact cognitive functioning to AD. High level of cognitive activity was associated with reduced cognitive decline in asymptomatic individuals, unrelated to cognitive decline in mildly symptomatic persons, and associated with accelerated decline after dementia onset in AD.
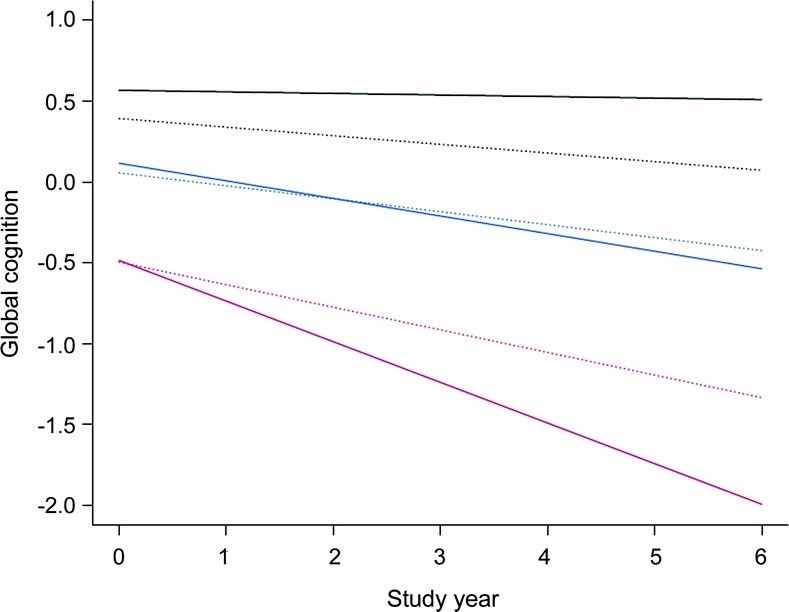
Figure 2 Relation of premorbid cognitive activity to cognitive decline in diagnostic groups
Predicted 6-year paths of change in cognitive function associated with a low level of premorbid cognitive activity (score 2.6, 10th percentile, dotted line) vs a high level (score = 4.0, 90th percentile, solid line) in persons with no cognitive impairment (black line), mild cognitive impairment (blue line), or Alzheimer disease (red line), adjusted for age, sex, race, and education.
DISCUSSION
More than 1,100 older community residents without dementia rated frequency of participation in cognitively stimulating activities. After a mean of about 6 years, they underwent clinical evaluation for MCI and AD and then were followed with brief cognitive performance tests for another 5 to 6 years. More frequent cognitive activity was related to slower cognitive decline in those without cognitive impairment and more rapid cognitive decline in AD, with no effect in MCI. The results suggest that late-life cognitive activity compresses the cognitive morbidity of AD by delaying its onset and by hastening cognitive decline after dementia onset.
In prior research, higher level of cognitive activity has been associated with reduced cognitive decline in persons without dementia.2,9–11 However, its association with cognitive decline within no cognitive impairment or MCI subgroups has not been examined. The present results suggest that the association of frequent cognitive activity with reduced cognitive decline is primarily due to its association with the initial development of cognitive impairment rather than with slowing the progression of cognitive impairment thereafter. Further, few studies have examined the relation of premorbid cognitive activity to cognitive decline in AD and results have been mixed. One study found, like the present one, that higher premorbid activity predicted more rapid cognitive decline, but the cohort was selected and premorbid activity was assessed by retrospective informant report.12 The other study, like the present one, used a population-based sample and self-report of cognitive activity prior to dementia onset, but findings were mainly negative. Higher level of cognitive activity was not related to more rapid cognitive decline following dementia onset though there was some evidence of the association when cognitive function assessments prior to dementia onset were used in analyses.13 The present results show the correlation of premorbid cognitive activity with cognitive decline in AD to be statistically robust and comparable in magnitude, but opposite in direction, to cognitive activity's well-established association with change in cognitive function prior to dementia onset.
These findings suggest that cognitive activity somehow enhances the brain's ability to maintain relatively normal function despite the accumulation of a mild to moderate neuropathologic burden, perhaps due to activity-dependent changes in the function and structure of neural systems underlying cognitive functioning.33,34 Any protection provided by cognitive activity must be limited, however, because cognitively active people do develop dementia. If cognitive activity does somehow allow the brain to tolerate more pathologic changes, those with high premorbid cognitive activity are likely to have a higher pathologic burden than those with low premorbid activity at the time of dementia onset and therefore to experience a more rapidly progressive dementia course. In effect, these results suggest that the benefit of delaying the initial appearance of cognitive impairment comes at the cost of more rapid dementia progression.
Because AD gradually evolves over many years, some factors that predict dementia, such as impaired olfaction,35 are really early signs of the disease. Although a reverse causality hypothesis can account for cognitive activity's association with risk of dementia, it cannot easily explain the correlation between premorbid cognitive activity and cognitive decline in AD observed here or the apparent lack of association between cognitive activity and the neuropathologic lesions underlying AD.11
These observational data suggest that interventions designed to enhance cognitive plasticity36 may prove beneficial in compressing the cognitive morbidity of AD. In this regard, narrow interventions targeting executive control processes37,38 and multimodal interventions that engage older persons in challenging pursuits such as taking acting classes39 or working in an elementary school40 seem particularly promising. The present results suggest that cognitive enrichment interventions may need to be initiated before the development of cognitive impairment, possibly because many persons with MCI already have substantial levels of AD pathology.21–23
This study has several strengths. Participants were sampled from a defined population and represent a broad spectrum of cognitive function from no impairment to frank dementia, suggesting the results are generalizable. Clinical classification was based on a uniform evaluation and widely used criteria, and cognitive function and cognitive activity were assessed with previously established psychometrically sound measures, enhancing our ability to model cognitive activity's association with cognitive decline in each diagnostic subgroup.
Study limitations should also be noted. Differences between diagnostic subgroups could have affected results. The composite measures of cognitive function and cognitive activity do not allow determination of whether results vary across domains of cognitive function, as suggested by some prior research,2,10,11 or whether some activities are more important than others. In addition, with a mean of 2 to 3 observations per individual, we were not well-positioned to capture nonlinear change in cognitive function within diagnostic groups.
ACKNOWLEDGMENT
The authors thank the residents of Morgan Park, Washington Heights, and Beverly who participated in the study; Ann Marie Lane for community development and oversight of project coordination; Michelle Bos, Holly Hadden, Flavio LaMorticella, and Jennifer Tarpey for coordination of the study; Todd Beck for analytic programming; and the staff of the Rush Institute for Healthy Aging. The statistical analyses were performed by Todd Beck, MS, under the supervision of Drs. Hebert and Mendes de Leon.
DISCLOSURE
Dr. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition and Psychology and Aging and receives research support from the NIH/NIA (R01AG024871 [PI], P30AG10161 [Co-I], R01AG11101 [Co-I], R01AG15819 [Co-I], R01AG021972 [Co-I], U24AG026395 [Co-I], R01AG017917 [Co-I], R01AG009966 [Co-I], and U01AG016979 [Co-I]) and the NIH/NIEHS (ES10902 [Co-I]). Dr. Barnes serves on the editorial board of the Journal of Aging & Health and receives research support from the NIH (R01AG022018 [PI], R01NR009543 [neuropsychologist], P30AG010161 [Co-I], R01ES010902 [Co-I], R01AG031553 [neuropsychologist], and R01AG032247 [Co-I]) and the Alzheimer's Association. Dr. Aggarwal has served on a scientific advisory board for Pfizer Inc. and receives research support from the NIH (R01AG022018 [Co-I], P30AG010161 [core leader], R01AG011101 [neurologist], R01AG009966 [neurologist], R01HL084209 [Co-I], R01AG032247 [Co-I]) and the Alzheimer's Association. Dr. Boyle receives research support from the NIH (R01AG034374 [PI] and R01AG033678 [PI]) and the Illinois Department of Public Health. Dr. Hebert receives research support from the NIH (R01NR010211 [PI], R01AG009966 [biostatistician], R03AG029652 [PI], and R01AG030544 [biostatistician]). Dr. Mendes de Leon served as Associate Editor of the Journals of Gerontology Social Sciences and serves on the editorial boards of Psychosomatic Medicine, the Journal of Aging & Health, the International Journal of Behavioral Medicine, and the Archives of Internal Medicine and receives/has received research support from the NIH (R01AG021972 [Co-I], R01HL084209 [coinvestigator], R01AG011101 [coinvestigator], R01ES010902 [PI], R01AG032247 [PI], and R01 AG022018 [coinvestigator]). Dr. Evans has served on a Data Monitoring Committee for Eli Lilly and Company and receives research support from the NIH (R01AG11101 [PI], R01AG09966 [PI], R01AG030146 [PI], P30AG10161 [Co-I], R01AG021972 [Co-I], R01ES010902 [Co-I], NR009543 [Co-I], R01HL084209 [Co-I], and R01AG12505l [Co-I]).
Supplementary Material
Address correspondence and reprint requests to Dr. Robert S. Wilson, Rush Alzheimer's Disease Center, Rush University Medical Center, 600 South Paulina Avenue, Suite 1038, Chicago, IL 60612 rwilson@rush.edu
Supplemental data at www.neurology.org
e-Pub ahead of print on September 1, 2010, at www.neurology.org.
Study funding: Supported by NIH (NIA R01AG11101, NIA P30AG10161, and NIEHS R01ES10902).
Disclosure: Author disclosures are provided at the end of the article.
Received November 12, 2009. Accepted in final form May 12, 2010.
REFERENCES
- 1.Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer's disease? A prospective study of Swedish twins. J Gerontol Psychol Sci 2003;58B:P249–P255. [DOI] [PubMed] [Google Scholar]
- 2.Hultsch D, Hertzog C, Small B, Dixon R. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 1999;14:245–263. [DOI] [PubMed] [Google Scholar]
- 3.Scarmeas N, Levy G, Tang M-X, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology 2001;57:2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006;66:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003;348:2508–2516. [DOI] [PubMed] [Google Scholar]
- 6.Wang H-X, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen Project. Am J Epidemiol 2002;155:1081–1087. [DOI] [PubMed] [Google Scholar]
- 7.Wang JYJ, Zhou DHD, Li J, et al. Leisure activities and risk of cognitive impairment: the Chongqing aging study. Neurology 2006;66:911–913. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002;59:1910–1914. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology 2003;61:812–816. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer's disease. JAMA 2002;287:742–748. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. The relation of cognitive activity to risk of developing Alzheimer's disease. Neurology 2007;69:1911–1920. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RS, Bennett DA, Gilley DW, Beckett LA, Barnes LL, Evans DA. Premorbid reading activity and patterns of cognitive decline in Alzheimer disease. Arch Neurol 2000;57:1718–1723. [DOI] [PubMed] [Google Scholar]
- 13.Helzner EP, Scarmeas N, Cosentino S, Portet F, Stern Y. Leisure activity and cognitive decline in incident Alzheimer disease. Arch Neurol 2007;64:1749–1754. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Bennett DA. Cognitive activity and risk of Alzheimer's disease. Curr Dir Psychol Sci 2003;12:87–91. [Google Scholar]
- 15.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project. J Alzheimer Dis 2003;5:349–355. [DOI] [PubMed] [Google Scholar]
- 16.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 2002;287:3230–3237. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59:198–205. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and AD. Arch Neurol 2009;66:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson RS, Aggarwal NT, Barnes LL, Mendes de Leon CF, Hebert LE, Evans DA. Cognitive decline in incident Alzheimer's disease in a community population. Neurology 2010;74:951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer's disease pathology and cerebral infraction. Neurology 2005;64:834–841. [DOI] [PubMed] [Google Scholar]
- 22.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol 2006;63:38–46. [DOI] [PubMed] [Google Scholar]
- 23.Peterson RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006;63:655–672. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol Psychol Sci 1999;54B:P155–P160. [DOI] [PubMed] [Google Scholar]
- 25.Albert M, Smith L, Scherr P, Taylor J, Evans D, Funkenstein H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci 1991;57:167–178. [DOI] [PubMed] [Google Scholar]
- 26.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 2002;17:179–193. [PubMed] [Google Scholar]
- 27.Smith A. Symbol Digit Modalities Test Manual–Revised. Los Angeles, CA: Western Psychological; 1984. [Google Scholar]
- 28.Wilson RS, Herbert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology 2009;72:460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird N, Ware J. Random-effects models for longitudinal data. Biometrics 1982;38:963–974. [PubMed] [Google Scholar]
- 30.Bienias JL, Kott PS, Beck TL, Evans DA. Incorporating multiple observations into logistic regression models of incident disease. Proceedings of the Annual Meeting of the American Statistical Association, Section on Survey Research Methods, 2767–2774 [CD-ROM], 2005. Alexandria, VA: American Statistical Association; 2005. [Google Scholar]
- 31.Rao RS, Sigurdson AJ, Doody MM, Graubard BI. An application of a weighting method to adjust for nonresponse in standardized incidence ratio analysis of cohort studies. Ann Epidemiol 2005;15:129–136. [DOI] [PubMed] [Google Scholar]
- 32.Kalton G. Handling wave nonresponse in panel surveys. J Official Stat 1986;2:303–314. [Google Scholar]
- 33.Draganski B, Gaser C, Kempermann G, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 2006;26:6314–6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 2000;97:4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry 2007;64:802–808. [DOI] [PubMed] [Google Scholar]
- 36.Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development. Can the functional capacity of older adults be preserved and enhanced? Psychol Sci Public Int 2009;9:1–65. [DOI] [PubMed] [Google Scholar]
- 37.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Nat Acad Sci USA 2008;105:6829–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basak C, Boot WR, Voss MW, Kramer AF. Can training in a real-time strategy video game attenuate cognitive decline in order adults? Psychol Aging 2008;23:765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noice H, Noice T. An arts intervention for older adults living in subsidized retirement homes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2009;16:56–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fried LP, Carlson MC, Freedman M, et al. A social model for health promotion for an aging population: initial evidence on the Experience Corps model. J Urban Health Bull NY Acad Med 2004;81:64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.