Abstract
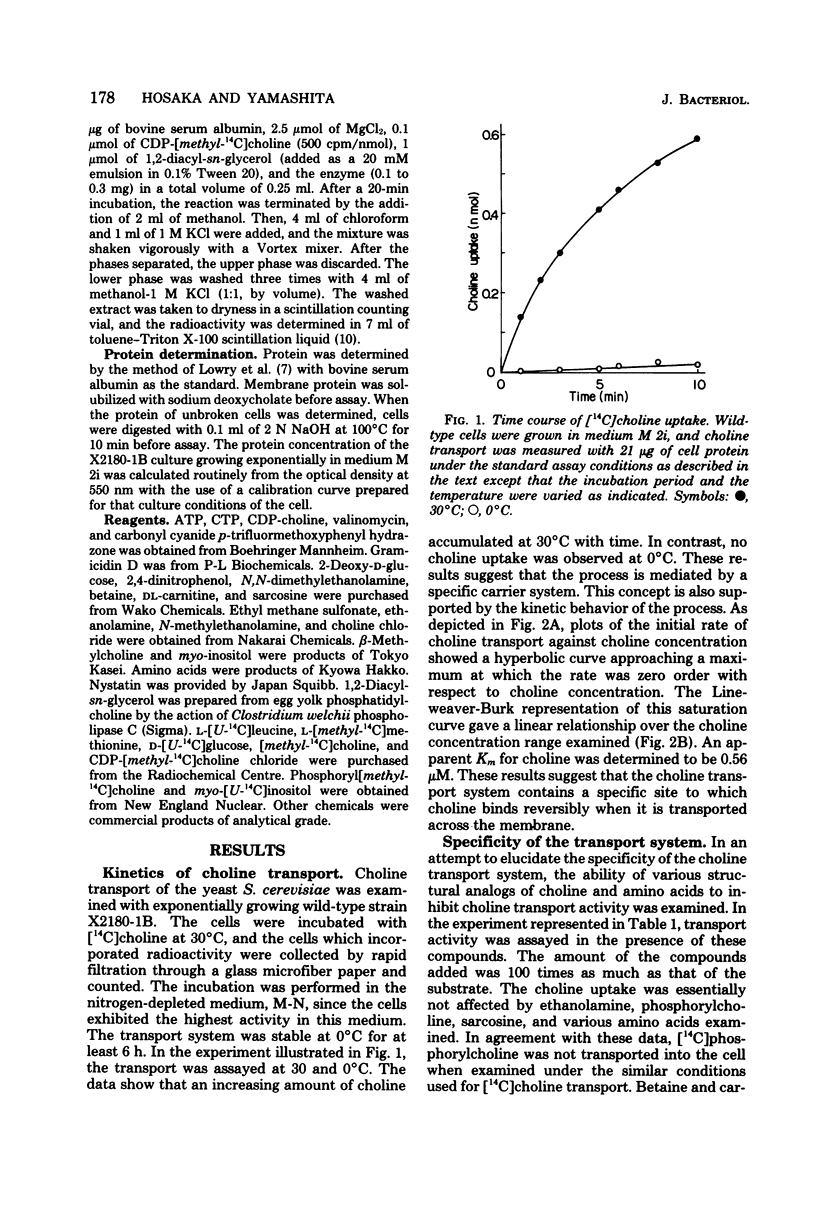
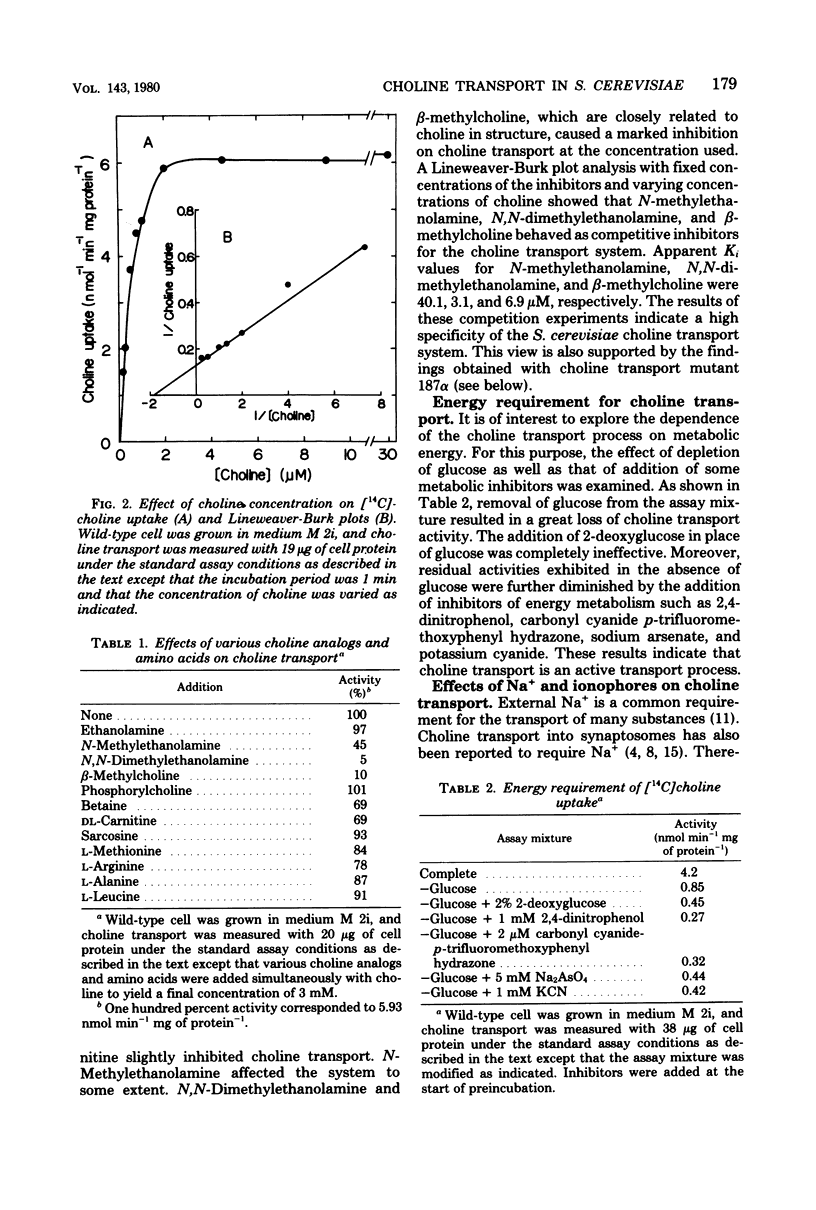
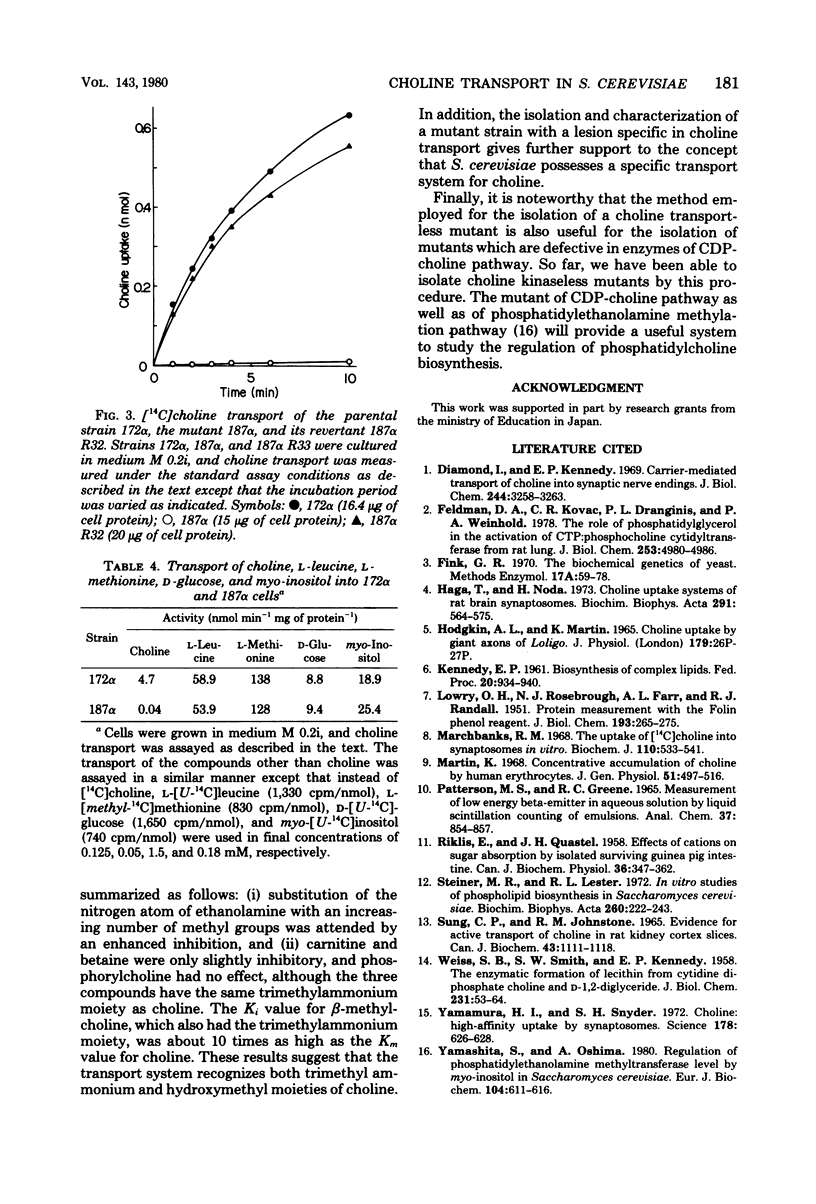
Choline transport of Saccharomyces cerevisiae was measured by the filtration method with the use of glass microfiber paper. The uptake was time and temperature dependent. The kinetics of choline transport showed Michaelis behavior; an appearent Km for choline was 0.56 microM. N-Methylethanolamine, N,N-dimethylethanolamine, and beta-methylcholine were competitive inhibitors of choline transport, with Ki values of 40.1, 3.1, and 6.9 microM, respectively. Ethanolamine, phosphorylcholine, and various amino acids examined had no effect. Choline transport required metabolic energy; removal of glucose resulted in a great loss of transport activity, and the remaining activity was abolished by 2,4-dinitrophenol, carbonyl cyanide p-trifluoromethoxyphenyl hydrazone, arsenate, and cyanide. External Na+ was not required, and the transport was not effected by ionophores, valinomycin, and gramicidin D. These results indicate that S. cerevisiae possess an active choline transport system mediated by a specific carrier. This view is further supported by the isolation and characterization of a choline transport mutant. The choline transport activity in this mutant was very low, whereas the transport of L-leucine, L-methionine, D-glucose, and myo-inositol was normal. Together with the choline transport mutant, mutants defective in choline kinase were also isolated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diamond I., Kennedy E. P. Carrier-mediated transport of choline into synaptic nerve endings. J Biol Chem. 1969 Jun 25;244(12):3258–3263. [PubMed] [Google Scholar]
- Feldman D. A., Kovac C. R., Dranginis P. L., Weinhold P. A. The role of phosphatidylglycerol in the activation of CTP:phosphocholine cytidylyltransferase from rat lung. J Biol Chem. 1978 Jul 25;253(14):4980–4986. [PubMed] [Google Scholar]
- Haga T., Noda H. Choline uptake systems of rat brain synaptosomes. Biochim Biophys Acta. 1973 Jan 26;291(2):564–575. doi: 10.1016/0005-2736(73)90508-7. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marchbanks R. M. The uptake of [14C] choline into synaptosomes in vitro. Biochem J. 1968 Dec;110(3):533–541. doi: 10.1042/bj1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. Concentrative accumulation of choline by human erythrocytes. J Gen Physiol. 1968 Apr;51(4):497–516. doi: 10.1085/jgp.51.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- RIKLIS E., QUASTEL J. H. Effects of cations on sugar absorption by isolated surviving guinea pig intestine. Can J Biochem Physiol. 1958 Mar;36(3):347–362. [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Suga N. Analysis of frequency-modulated sounds by auditory neurones of echo-locating bats. J Physiol. 1965 Jul;179(1):26–53. doi: 10.1113/jphysiol.1965.sp007648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung C. P., Johnstone R. M. Evidence for active transport of choline in rat kidney cortex slices. Can J Biochem. 1965 Jul;43(7):1111–1118. doi: 10.1139/o65-124. [DOI] [PubMed] [Google Scholar]
- WEISS S. B., SMITH S. W., KENNEDY E. P. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958 Mar;231(1):53–64. [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Choline: high-affinity uptake by rat brain synaptosomes. Science. 1972 Nov 10;178(4061):626–628. doi: 10.1126/science.178.4061.626. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Oshima A. Regulation of phosphatidylethanolamine methyltransferase level by myo-inositol in Saccaromyces cerevisiae. Eur J Biochem. 1980 Mar;104(2):611–616. doi: 10.1111/j.1432-1033.1980.tb04465.x. [DOI] [PubMed] [Google Scholar]



