Abstract
A 13-year-old, neutered male domestic cat presented with signs of weight loss, anemia, and hepatomegaly. Pathognomonic signs of porphyria were identified. Charcoal-like renal calculi and severe liver changes were observed, neither of which has been previously reported in association with feline porphyria.
Résumé
Porphyrie féline associée à l’anémie, à une maladie hépatique grave et aux calculs rénaux. Un chat mâle castré âgé de 13 ans a été présenté avec des signes de perte de poids, d’anémie et d’hépatomégalie. Des signes pathognomoniques de porphyrie ont été identifiés. Des calculs rénaux ressemblant à du charbon et des changements hépatiques graves ont été observés, ni l’un ni l’autre de ces symptômes n’avaient été antérieurement signalés avec la porphyrie féline.
(Traduit par Isabelle Vallières)
A 13-year-old, 4.4-kg, neutered male domestic shorthaired cat was referred to the Atlantic Veterinary College with signs of progressive weight loss, mild anemia, and a 1-week history of coughing. Hepatomegaly with irregular margins and renal mineralization was reported on radiographs performed by the referring veterinarian. The patient was an indoor cat in a multi-cat household, and he had not traveled outside of Atlantic Canada. His owners had acquired him at 6 mo of age, and there was no history of drug or toxin exposure. Pentosan polysulfate sodium injections (Catrophen Vet; Arthropharm Pharmaceuticals, Ottawa, Ontario) had been administered once every 5 wk for recurrent idiopathic cystitis. Over the previous year, 2.3 kg of weight loss had been reported. Mild anemia (hematocrit: 0.221 L/L) and mild thrombocytosis had been documented on a complete blood (cell) count (CBC) performed several days prior to referral.
Case description
The cat was moderately thin with a body condition score of 2 out of 5. Gingival mucous membranes were mildly pale. Yellow-brown discoloration of the dentition was noted with moderate tartar. A cough could be elicited upon tracheal palpation.
A CBC demonstrated moderate normochromic, non-regenerative anemia (hematocrit: 0.196 L/L) with Heinz bodies, anisocytosis, acanthocytes, and fragmented red cells. Mild hyperglycemia (6.9 mmol/L; reference range: 3.3 to 5.6 mmol/L), mild hypercholesterolemia (5.06 mmol/L; reference range: 2.00 to 4.00 mmol/L); a mild elevation of alanine transaminase (68 U/L; reference range: 13 to 55 U/L), and a very mild hypoproteinemia (66 g/L; reference range: 68 to 80 g/L) with a normal albumin to globulin ratio were observed on a chemistry profile. T4 levels, prothrombin time, and activated partial thromboplastin time were within normal limits. Urinalysis demonstrated relatively low density (specific gravity 1.020), an acidic pH (5.0), and excess erythrocytes (20 to 25 per high-powered field). Thoracic radiographs were unremarkable; however, faint mineral-dense opacities were noted over the plane of the kidneys. Multiple hyperechoic splenic masses of varying size with irregular margins were observed on abdominal ultrasound. Very little normal splenic parenchyma was identified. No significant changes were reported with respect to the liver. Mineralization was observed within both kidneys with hyperechoic debris within the urinary bladder. A urine culture yielded no bacteria.
Ultrasound-guided fine-needle aspirates of the liver and spleen were performed. Splenic aspirates had changes consistent with extramedullary hematopoieisis. Liver aspirates showed focal vacuolar hepatocyte degeneration with cytoplasmic green-black pigment granules and focal accumulations of brownish pigment granules. A Mycoplasma hemofelis polymerase chain reaction (PCR) test was negative.
The patient was returned to the veterinary college approximately 3 wk following the initial evaluation for further diagnostics and an exploratory laparotomy. A CBC, chemistry profile, and urinalysis were repeated. Results were similar when compared with previous data except that a mild elevation of alkaline phosphatase (38 U/L; reference range: 10 to 35 U/L) and mild bilirubinuria were detected. A bone marrow aspirate and biopsy were performed. Bone marrow cytology showed a very mild left shift of the myeloid cell line and the presence of black-green pigmented material suspected to be iron. No remarkable changes were noted on histopathological analysis of a bone marrow core sample.
A repeat abdominal ultrasound showed that the previously described hyperechoic nodules in the splenic tissue were still apparent and the appearance of mineral foci of the right kidney and bladder debris remained unchanged.
The spleen and liver were diffusely abnormal upon exploratory laparotomy; therefore, a splenectomy and liver biopsy were performed. Severe hepatic disease or hepatic neoplasia was suspected based on the gross appearance of the liver.
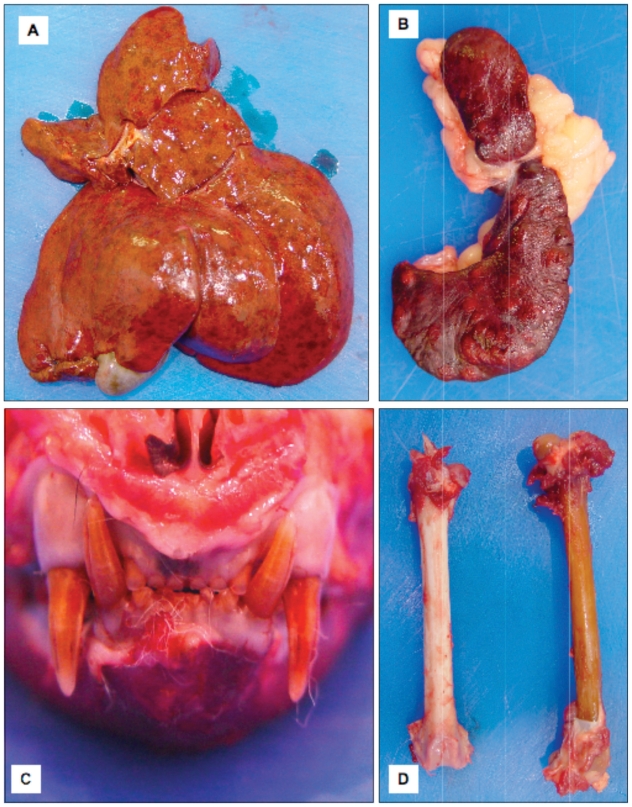
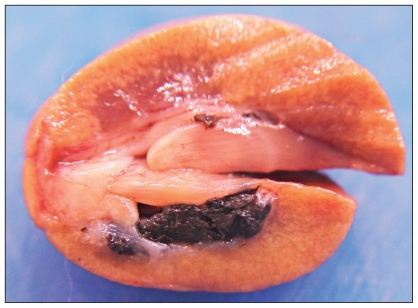
The patient’s owners elected for intra-operative euthanasia, after which a postmortem examination was conducted. A slight decrease in muscle mass with abundant fat stores was observed on postmortem examination. Numerous, well-delineated areas of red discoloration [< 5 mm in diameter (ID)] were scattered throughout the liver (Figure 1A). Numerous white nodules (2 to 5 mm ID) were observed throughout the splenic parenchyma (Figure 1B). Several small white nodular foci were distributed throughout the pancreas. Renal calculi consisting of aggregates of firm brown-black material were observed within the renal pelvis of both kidneys (Figure 2). All bones and teeth had diffuse brown discoloration (Figure 1 C, D). Bright orange fluorescence of the bones, teeth, and urine was observed under a handheld ultraviolet (UV) lamp (“Blak-Ray” UVL-22; UltraViolet Products, San Gabriel, California, USA). The marrow in the metaphyseal and diaphyseal regions of the long bones was diffusely red. The brown discoloration of the bones and teeth in association with bright orange fluorescence of the bones, teeth, and urine with UV light was considered to be pathognomonic for porphyria.
Figure 1.
A — The patient’s liver showing numerous areas of red discoloration. B — The spleen with numerous white nodules throughout the parenchyma. C — The patient’s skull showing brown discoloration of the dentition. D — Brown discoloration of the affected patient’s femur (right) compared to a femur from a cat that was not affected with porphyria (left).
Figure 2.
Sagittal section of a kidney with renal pelvic calculi consisting of aggregates of firm brown-black material.
Histopathological analysis of the liver identified severe vacuolar hepatopathy with marked nodular hyperplasia and hepatocellular loss. Many hepatocytes contained abundant stores of variable sized granules of dark brown-orange cytoplasmic pigment. This pigment did not stain positive for iron, copper, lipofuscin, or bile. Changes noted within the spleen were consistent with myelolipomas and extramedullary hematopoiesis. Sections of marrow from metaphyseal and diaphyseal regions had hematopoietic hyperplasia of both the erythroid and myeloid tissues.
Histopathologic examination of the renal calculi revealed aggregates of small globular to amorphous bright red-orange material. Further testing to isolate specific porphyrins and to measure enzyme function and concentration was not performed.
Discussion
Porphyria is a group of inherited or acquired disorders resulting in decreased function of specific enzymes within the heme biosynthetic pathway (1–3). Several inherited and acquired forms of porphyria have been reported in animals. Cases have been documented in cattle, pigs, sheep, cats, dogs, mice, and rats (4–10). Although the majority of the reports described in domestic animals involve congenital or inherited forms of porphyria, there have also been reports of drug-induced disease in mice, rats, and dogs (7,8,11–16).
In the present case, the patient was diagnosed with feline erythropoietic porphyria based on pathognomonic postmortem findings (4). Although an acquired form of porphyria had been considered, involvement of teeth and diffuse involvement of bones was consistent with a congential or genetic form, as porphyrin deposition occurs only at sites of active mineralization (17).
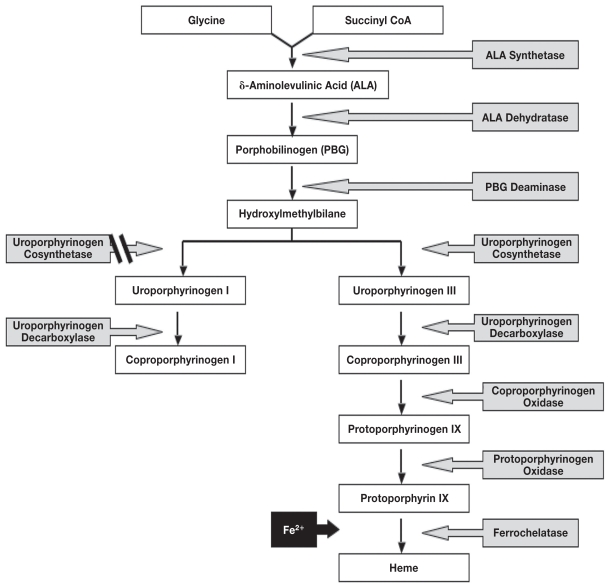
Porphyrins are the major precursor to heme, a constituent of hemoglobin, myoglobin, catalase, peroxidase, and P450 cytochromes (3,5). Heme synthesis occurs within erythropoietic cells and within hepatocytes. The primary site of the defect occurs within the bone marrow or liver (1–3). Clinical signs are typically a result of tissue accumulation of heme in high concentrations. Eight enzymes are involved in the heme pathway (Figure 3). The enzyme deficiency noted in association with porphyria generally allows for sufficient heme production as there is adequate residual enzyme capacity (1–3).
Figure 3.
Heme biosynthetic pathway showing component enzymes and porphyrins. Note that non-enzymatic conversion of hydroxylmethylbilane results in accumulation of Type I porphyrin isomers.
In humans, there are at least 7 types of porphyria that are classified depending on the specific enzyme affected. A general classification of hepatic or erythropoietic porphyria is assigned based on the primary site of the defect (1–3). Human porphyria is generally considered to be genetically determined. Clinical signs are dependent on the location at which the heme precursors accumulate and the chemical properties of the intermediates (1,2).
Three forms of human porphyria are of interest in the context of this case; congenital erythropoietic porphyria (CEP); porphyria cutanea tarda (PCT); and, erythropoietic protoporphyria (EPP). Congenital erythropoietic porphyria involves an autosomal recessive mode of inheritance; is rarely reported (18). Deficient activity of uroporhyrinogen III cosynthase (UCoS) results in accumulation of non-physiological porphyrin isomers, uroporphyrin I, and coproporphyrin I. Clinical signs associated with human CEP include cutaneous photosensitivity, anemia (secondary to hemolysis), hypertrichosis of the face and extremities, and erythrodontia. Phenotypic variability is reported and is dependent on the level of residual UCoS activity, the degree of hemolysis, the extent of reactive erythropoiesis, and exposure to UV light (1,2,18). Deficiency of UCoS activity results in accumulation of the enzyme’s substrate, hydroxylmethylbilane. Hydroxylmethylbilane is converted non-enzymatically to uroporphyrinogen I, which can then undergo decarboxylation by uroporphyrinogen decarboxylase to form coproporphyrinogen I. However, the next reaction in the heme biosynthetic pathway cannot proceed, as the enzyme is stereospecific for type III isomers (Figure 3). The type I porphyrin isomers accumulate and are auto-oxidized to their corresponding porphyrins which catalyze phototoxic reactions (1).
Feline CEP is the only true form of porphyria reported in cats; however, a feline precursor porphyria with neurological signs and persistent delta aminolevulinic aciduria has been documented (19). Feline CEP is rare and few cases have been documented. Feline porphyria is thought to be similar to CEP in humans and cattle as it is associated with deficient UCoS activity and accumulation of type I isomers of uroporphyrin and coproporphyrin (4,5,20–23).
Feline CEP appears to be of autosomal dominant inheritance (20,21). Clinical features include anemia and pink-brown discoloration of the dentition, bones, and other tissues. The abnormal porphyrin accumulation in erythrocytes is thought to result in red cell destruction and phagocytosis within the spleen. Macrocytic and hypochromic anemia is noted in association with decreased hemoglobin production. Anemia can vary from mild to severe and life-threatening (20–22). Anisocytosis, poikilocytosis, target cells, normoblasts, and Howell-Jolly bodies may be identified (20). In bovine studies, a negative correlation between erythrocyte porphyrin concentrations and erythrocyte survival times has been reported (24).
Splenomegaly, as noted in the present case, may result from erythrophagocytosis and extramedullary hematopoiesis. Brown discoloration of tissue is noted secondary to porphyrin deposition. The pigment fluoresces bright pink or red in UV light (20,21). Renal disease has been reported in 2 related cases of feline CEP characterized by changes consistent with glomerulonephritis (20). The color of the urine may vary from light brown to red. Postmortem findings in feline CEP may reveal brown discoloration of bones, marrow, and other major organs. Upon histological section, the pigment appears as aggregates of golden brown material and does not stain with Prussian blue. Marked hemosiderosis may be present (20,21). Feline porphyria was last reported in 1975 as a study of CEP in a family of Siamese cats (20). The principal elevated porphyrins detected were Type I isomers of uroporphyrin and coproporphyrin. Elevated protoporphyrin and porphyrin precursor (porphobilinogen and δ-aminolevulinic acid) concentrations were detected. Porphyrins were demonstrated within the blood, viscera, teeth, bones, and excreta. The cats demonstrated severe macrocytic hypochromic anemia and hepatosplenomegaly. Renal disease with uremia and proteinuria was also noted. Episodes of nasal and ocular discharge occurred during the summer months. These signs were suspected to have been associated with photosensitivity (20).
Porphyrin concentrations were significantly higher in these cats compared to earlier reports. No evidence of severe anemia, photosensitivity, renal disease, or deposition of porphyrins within the viscera was detected previously (4,20,21). Accumulation of porphyrins in soft tissues may have occurred secondary to phagocytosis of porphyrin containing erythrocytes throughout the reticuloendothelial cells within the affected organs. The renal changes may have occurred through a similar mechanism. However, uroporphyrin or other hemoglobin related compounds may have resulted in damage to the tubular and glomerular epithelial cells and mesangium (20). Signs of anemia and hemolysis noted in these cats were thought to be associated with extremely high erythrocyte porphyrin concentrations (20,24).
The case of feline porphyria described in the present report appears to be most similar to the cases described previously in 1975. The patient had demonstrated signs characteristic of feline CEP including brown discoloration of the teeth and bones (Figure 1 C, D), hemolytic anemia, hematopoietic hyperplasia, extramedullary hematopoeisis, and fluorescence of the teeth, bones, and urine. Additional signs noted were considered as being atypical; specifically, severe hepatic disease with intracytoplasmic pigment and the unusual “charcoal-like” renal calculi (Figure 2). No signs of photosensitivity were noted; however, the cat had been kept indoors. It was speculated that the dark brown-orange cytoplasmic pigment in the patient’s hepatocytes represented an accumulation of porphyrins and that the severe vacuolar hepatopathy with marked nodular hyperplasia and hepatocellular loss could have occurred secondary to the pigment accumulation. Hepatic accumulation of porphyrins may have been associated with a primary hepatic porphyria similar to the human forms of porphyria, such as PCT; or, as speculated in an earlier study, the pigment may have been transported into the hepatocytes by phagocytosis of erythrocytes (20). Significant hepatic disease in association with accumulation of porphyrins within the liver has not been reported in cats. The erythrocyte morphological changes (Heinz bodies, acanthocytes, schistozytes) noted in the present case may have been related to hemolysis associated with porphyria; however, these changes may have occurred in association with hepatic disease (25).
A recent human report assessed unusual black charcoal-like renal calculi for porphyrin content. The calculi exhibited infrared spectra (IRS) similar to characteristic IRS for hemoglobin. Two types of porphyrins were detected, coproporphyrin and hepatacarboxyl-porphyrin. The human patients with these porphyrin-containing calculi had been affected with either porphyria or chronic renal failure (26). A similar process was suspected to have occurred in the present case; however, confirmatory testing was not performed. High concentrations of porphyrins in the urine may have resulted in the formation of porphyrin-containing calculi.
The hepatic changes in the present case bear similarities to PCT and EPP; PCT being the most common form of human porphyria (1–3,27). Porphyria cutanea tarda is characterized by hepatic deficiency of uroporphyrinogen decarboxylase (UROD). It is usually an acquired condition and may occur in association with liver disease. Oxidation of the enzyme’s substrate to corresponding porphyrins contributes to the clinical signs (1,3,18,27). Liver damage may result secondary to hepatic accumulation of porphyrins. Histopathologic examination of liver sections often demonstrates siderosis, lipidosis, necrosis, and chronic inflammatory changes (2,3,27,28). It is possible that a primary hepatic disease or another systemic condition contributed to decreased enzyme function and porphyrin accumulation in the cat discussed in this report.
In human EPP, a genetic defect results in a partial deficiency of ferrochelatase (FeC) activity. The bone marrow is the primary affected site. Liver involvement is common as the concentration of porphyrins may exceed the hepatic excretory capacity with resultant hepatic accumulation and toxicity (1,2,18).
FeC is the enzyme involved in a rate dependent step and utilizes protoporphyrin IX as a substrate (Figure 3) (1,29). Protoporphyrin IX accumulates within various organs, including the liver, where it can result in cholestatic disease and cirrhosis (1). Protoporphyrin-IX is highly hydrophobic and porphyrinuria does not occur with EPP (30,31).
Acquired forms of EPP have recently been reported in dogs (7,8); however, EPP has not been documented in cats. It is reasonable to speculate that the liver changes described in the present case had occurred as a result of a hepatic accumulation of porphyrins. Although high tissue concentrations of protoporphyrins have been noted previously in cases of feline CEP, a transient or inherited form of EPP was unlikely in the present case, as there was evidence of porphynuria (4,20). However, a similar process may have contributed to hepatic porphyrin accumulation and hepatotoxicity.
Footnotes
Reprints will not be available from the authors.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Sassa S, Kappas A. Molecular aspects of the inherited porphyrias. J Intern Med. 2000;247:169–178. doi: 10.1046/j.1365-2796.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 2.Kauppinen R. Porphyrias. Lancet. 2005;365:241–252. doi: 10.1016/S0140-6736(05)17744-7. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann Y, Puy H. Human hereditary hepatic porphyrias. Clinica Chimica Acta. 2002;325:17–37. doi: 10.1016/s0009-8981(02)00276-0. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko JJ. The porhyrias and the porphyrinurias. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm’s Veterinary Hematology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 1002–1007. [Google Scholar]
- 5.Basrur PK. Genetics in Veterinary Medicine. Vaspar Press; 2003. pp. 101–103. [Google Scholar]
- 6.Schalm OW, Jain NC, editors. Schalm’s Veterinary Hematology. 4th ed. Philadelphia: Lea & Febiger; 1986. pp. 646–647. [Google Scholar]
- 7.Greijdanus-van der Putten SW, van Esch E, Kamerman J, Ballering LA, van den Dobbelsteen DJ, T de Rijk EP. Drug-induced protoporphyria in beagle dogs. Toxicol Pathol. 2005;33:720–725. doi: 10.1080/01926230500351392. [DOI] [PubMed] [Google Scholar]
- 8.Kroeze EJ, Zentek J, Edixhoven-Bosdijk A, Rothuizen J, van den Ingh TS. Transient erythropoietic protoporphyria associated with chronic hepatitis and cirrhosis in a cohort of German shepherd dogs. Vet Rec. 2006;158:120–124. doi: 10.1136/vr.158.4.120. [DOI] [PubMed] [Google Scholar]
- 9.Nezamzadeh R, Seubert A, Pohlenz J, Brenig B. Identification of a mutation in the ovine uroporphyrinogen decarboxylase (UROD) gene associated with a type of porphyria. Anim Genet. 2005;36:297–302. doi: 10.1111/j.1365-2052.2005.01301.x. [DOI] [PubMed] [Google Scholar]
- 10.Pawliuk R, Tighe R, Wise RJ, Mathews-Roth MM, Leboulch P. Prevention of murine erythropoietic protoporphyria-associated skin photosensitivity and liver disease by dermal and hepatic ferrochelatase. J Invest Dermatol. 2005;124:256–262. doi: 10.1111/j.0022-202X.2004.23529.x. [DOI] [PubMed] [Google Scholar]
- 11.With TK. Porphyrias in animals. Clin Haematol. 1980;9:345–370. [PubMed] [Google Scholar]
- 12.Matilla A, Molland EA. A light and electron microscopic study of the liver in case of erythrohepatic protoporphyria and in griseofulvin induced porphyria in mice. J Clin Pathol. 1974;27:698–709. doi: 10.1136/jcp.27.9.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gralla EJ, Fleischman RW, Luthra YK, et al. Toxic effects of hexachoro-benzene after daily administration to beagle dogs for one year. Toxicol Appl Pharmacol. 1977;40:227–239. doi: 10.1016/0041-008x(77)90093-x. [DOI] [PubMed] [Google Scholar]
- 14.Cantoni L, Di Padova C, Rovagnati P, Ruggieri R, Dal Fiume D, Tritapepe R. Bile secretion and liver microsomal mixed function oxidase system in mice with griseofulvin-induced hepatic protoporphyria. Toxicology. 1983;27:27–39. doi: 10.1016/0300-483x(83)90073-2. [DOI] [PubMed] [Google Scholar]
- 15.Frater Y, Brady A, Lock EA, De Matteis F. Formation of N-methyl protoporphyrin in chemically-induced protoporhyria. Studies with a novel porphyrogenic agent. Arch Toxicol. 1993;67:179–185. doi: 10.1007/BF01973305. [DOI] [PubMed] [Google Scholar]
- 16.Smith AG, Clothier B, Francis JE, Gibbs AH, De Matteis F, Hider RC. Protoporphyria induced by the orally active iron chelator 1,2-diethyl-3-hydroxipyridin-4-one in C57BL/10ScSn mice. Blood. 1997;89:1045–1051. [PubMed] [Google Scholar]
- 17.Thompson K. Bones and joints. In: Maxie MG, editor. Pathology of Domestic Animals. 5th ed. New York: Elsevier Saunders; 2007. p. 48. [Google Scholar]
- 18.Gross U, Hoffmann GF, Doss MO. Erythropoietic and hepatic porphyrias. J Inherit Metab Dis. 2000;23:641–661. doi: 10.1023/a:1005645624262. [DOI] [PubMed] [Google Scholar]
- 19.Watson AD. Feline precursor porphyria, characterized by persistent delta aminolevulinic aciduria. J Small Anim Pract. 1990;31:393–397. [Google Scholar]
- 20.Giddens WE, Jr, Labbe RF, Swango LJ, Padgett GA. Feline congenital erythropoietic porphyria associated with severe anemia and renal disease. Clinical, morphologic, and biochemical studies. Am J Pathol. 1975;80:367–386. [PMC free article] [PubMed] [Google Scholar]
- 21.Glenn BL, Glenn HG, Omtvedt IT. Congenital porphyria in the domestic cat (Felis catus): Preliminary investigations on inheritance pattern. Am J Vet Res. 1968;29:1653–1657. [PubMed] [Google Scholar]
- 22.Mackey L. Haematology of the cat. In: Archer RK, Jeffcott LB, editors. Comparative Clinical Haematology. Oxford: Blackwell Scientific Publications; 1977. pp. 462–463. [Google Scholar]
- 23.Nicholas FW. Veterinary Genetics. Oxford: Clarendon Pr; 1987. pp. 86–87. [Google Scholar]
- 24.Kaneko JJ, Zinkl JG, Keeton KS. Erythrocyte porphyrin and erythrocyte survival in bovine erythropoietic porphyria. Am J Vet Res. 1971;32:1981–1985. [PubMed] [Google Scholar]
- 25.Christopher MM. Disorders of feline red blood cells. In: Bonagura JD, editor. Kirk’s Current Veterinary Therapy XIII Small Animal Practice. Philadelphia: WB Saunders; 2000. pp. 421–424. [Google Scholar]
- 26.Traba Villameytide ML. Porphyrins in renal calculi (review) Actas Urol Esp. 2005;29:163–169. doi: 10.1016/s0210-4806(05)73218-6. [DOI] [PubMed] [Google Scholar]
- 27.Bleasel NR, Varigos GA. Porphyria cutanea tarda. Australas J Dermatol. 2000;41:197–206. doi: 10.1046/j.1440-0960.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- 28.Palmieri C, Vigushin DM, Peters TJ. Managing malignant disease in patients with porphyria. QJM. 2004;97:115–126. doi: 10.1093/qjmed/hch027. [DOI] [PubMed] [Google Scholar]
- 29.Anderson KE, Sassa S, Bishop DF, Desnick RJ. The porphyrias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 8th ed. New York: McGraw-Hill; 2001. pp. 2991–3062. [Google Scholar]
- 30.Hindmarsh JT, Oliveras L, Greenway DC. Biochemical differentiation of the porphyrias. Clin Biochem. 1999;32:609–619. doi: 10.1016/s0009-9120(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 31.Meerman L. Erythropoietic protoporphyria. An overview with emphasis on the liver. Scand J Gastroenterol Suppl. 2000;232:79–85. [PubMed] [Google Scholar]