Abstract
Background
Hematopoietic chimerism induces transplantation tolerance across allogeneic and xenogeneic barriers, but has been difficult to achieve in the pig-to-primate model. We have now utilized swine with knockout of the gene coding for α-1,3-galactosyltransferase (GalT-KO pigs) as bone marrow donors in an attempt to achieve chimerism and tolerance by avoiding the effects of natural antibodies to Gal determinants on pig hematopoietic cells.
Methods
Baboons (n = 4; Baboons 1 to 4 = B156, B158, B167, and B175, respectively) were splenectomized and conditioned with TBI (150 cGy), thymic irradiation (700 cGy), T cell depletion with rabbit anti-thymocyte globulin (rATG) and rat anti-primate CD2 (LoCD2b), and received FK506 and supportive therapy for 28 days. All animals received GalT-KO bone marrow (1 to 2 × 109 cells/kg) in two fractions on days 0 and 2, and were thereafter monitored for the presence of pig cells by flow cytometry, for porcine progenitor cells by PCR of BM colony-forming units, and for cellular reactivity to pig cells by mixed lymphocyte reaction (MLR). In vitro antibody formation to LoCD2b and rATG was tested by ELISA; antibody reactivity to GalT-KO pig cells was tested by flow cytometry and cytotoxicity assays. Additionally, Baboons 3 and 4 received orthotopic kidney transplants on days 17 and 2, respectively, to test the potential impact of the protocol on renal transplantation.
Results
None of the animals showed detectable pig cells by flow cytometry for more than 12 h post-BM infusion. However, porcine progenitor cell engraftment, as evidenced by pig-derived colony forming units in the BM, as well as peripheral microchimerism in the thymus, lymph node, and peripheral blood was detected by PCR in baboons 1 and 2 for at least 28 days post-transplant. ELISA results confirmed humoral immunocompetence at time of transplantation as antibody titers to rat (LoCD2b) and rabbit (ATG) increased within 2 weeks. However, no induced antibodies to GalT-KO pig cells or increased donor specific cytotoxicity was detectable by flow cytometry. In contrast, baboons 3 and 4 developed serum antibodies to pig cells as well as to rat and rabbit immunoglobulin by day 14. Retrospective analysis revealed that although all four baboons possessed low levels of antibody-mediated cytotoxicity to GalT-KO cells prior to transplantation, the two baboons (3 and 4) that became sensitized to pig cells (and rejected pig kidneys) had relatively high pre-transplantation titers of anti–non-Gal IgG detectable by flow cytometry, whereas baboons 1 and 2 had undetectable titers.
Conclusions
Engraftment and specific non-responsiveness to pig cells has been achieved in two of four baboons following GalT-KO pig-to-baboon BMT. Engraftment correlated with absence of preformed anti–non-Gal IgG serum antibodies. These results are encouraging with regard to the possibility of achieving transplantation tolerance across this xenogeneic barrier.
Keywords: bone marrow, chimerism, GalT-Ko, tolerance, xenotransplantation
Introduction
Organ transplantation remains the only definitive cure for end-stage organ failure, yet there currently exists a critical shortage of human organs. Xenotransplantation of organs from pigs could provide a potential solution to this shortage. Pig organs are attractive due to their unlimited availability, physiologic compatibility with humans, and relative lack of ethical issues surrounding their use, as compared to non-human primates. However, interspecies transplantation poses additional immune barriers to those seen with human-to-human allogeneic transplantation [1].
Reliance on high-dose, non-specific pharmacologic immunosuppression for pig xenograft acceptance in primates results in serious and often fatal side effects, including fulminate infection and malignancy [2]. Thus, overcoming the immune challenges posed by clinical xenografts may depend on inducing immunological tolerance [3]. We have previously reported success in the induction of allogeneic tolerance through administration of donor bone marrow to produce mixed lymphohe-matopoietic chimerism in rodent [4,5], pig [6], and non-human primate models [7], as well as in recent clinical trials [8,9]. Tolerance has also been achieved by this modality for concordant xenografts, including rat-to-mouse [10] and baboon-to-cynomolgus monkey [11]. However, successful induction of tolerance through mixed chimerism across the discordant pig-to-non-human primate barrier has not yet been achieved.
Previous attempts using bone marrow (BM) [12,13] or mobilized peripheral blood progenitor cells (PBPC) [14–16] following ex vivo immunoadsorption of natural anti-Gal antibodies have demonstrated only transient chimerism. In these studies, the rapid reappearance of anti-Gal antibodies was thought to preclude long-term hematopoietic engraftment [3,16]. The recent production of αl-3-Gal transferase gene knock-out (GalT-KO) pigs through nuclear transfer has circumvented the problems posed by anti-Gal antibodies [17,18]. Using these animals as donors in a previous study, we have demonstrated successful multilineage peripheral blood microchimerism for 9 to 16 days in three animals. However, one recipient died on post-transplant day 6 and the remaining two recipients never recovered baseline immune function [19]. In the current study, we have employed an attenuated non-myeloablative conditioning regimen to improve survival and immunocompetence in order to determine the effect of the Gal knock-out on engraftment.
Materials and methods
Animals
Recipients were Papio hamadryas baboons (n = 4 = B156, B158, B167, B175) of known ABO blood type and body weight 8 to 15 kg (Manheimer Foundation, Homestead, FL, USA) (Table 1). Bone marrow cell donors were Massachusetts General Hospital (MGH) inbred GalT-KO miniature swine (n = 4), produced as previously described [19,20]. All swine were of SLADD (hereafter DD) swine leukocyte antigen haplotype, between 13 and 31 months old, and weighing between 78 and 91 kg. All animal care was performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1996). The protocol was approved by the MGH subcommittee of research animal care.
Table 1.
Summary of characteristics and courses of recipient baboons in this study
| Baboon 1 (B156) | Baboon 2 (B158) | Baboon 3 (B167) | Baboon 4 (B175) | |
|---|---|---|---|---|
| Recipient age (yr) | 4 | 5 | 5 | 2.5 |
| Recipient weight (kg) | 10.8 | 15.3 | 15.0 | 8.0 |
| Recipient sex | F | F | F | F |
| Donor age (months) | 13 | 12 | 27 | 31 |
| Donor weight (kg) | 78 | 82 | 73 | 91 |
| BM Tx (cells/kg) | D0: 5 ×108 | D0: 3.75 × 108 | D0: 5 × 108 | D0: 5 × 108 |
| D2: 5 × 108 | D2: 3.75 × 108 | D2: 5 × 108 | D3: 5 ×108 | |
| Kidney Tx | No | No | D17 | D2 |
| Native ureter untied | N/A | N/A | D25/8 | D13/11 |
| Increase in anti–non-Gal IgG | No | No | Yes | Yes |
| Increase in cytotoxicity | No | No | Yes | Yes |
| CVF | No | No | No | Yes |
| Porcine-derived CFUs in BM | Yes | Yes | No | No |
| Day of death | D28. Anaphylactic reaction to LoCD2b | D187. Elective sacrifice | D153. Elective sacrifice | D26. |
Surgical procedures
All surgical procedures, including renal transplantation, splenectomy, intravenous or intra-arterial line insertions and bone marrow biopsies were performed under general anesthesia, as described previously [20,21]. In brief, GalT-KO donor bone marrow was harvested by curetting long bones and vertebral bodies from the donor pig, mincing and agitating fragments at room temperature using a rotatory shaker at high speed. Marrow cells were filtered and washed by centrifugation. Red cells in the pellet were lysed with ammonium chloride potassium (ACK) lysing buffer. The cells were resuspended in Hanks medium, washed again by centrifugation, counted, adjusted to the required concentration and stored at 4° until infusion, at which time they were washed and filtered once more [22].
Renal transplants
Baboons 3 and 4 received orthotopic kidney transplant to test whether, as in allogeneic renal transplantation [7], early renal transplant might promote tolerance induction. These animals received transplants as previously described [21], on days 17 and 2, respectively.
Conditioning regimen
All animals were subjected to a modification of a non-myeloablative conditioning regimen that has been previously reported [19]. In brief, the baboons were splenectomized and exposed to 150 cGy of total body irradiation (TBI) on day −7, T cell depleted with LoCD2b (rat anti-primate CD2 IgG2b; Immerge BioTherapeutics) at 4 mg/kg on days −5 and −4, and rabbit anti-thymocyte globulin (rATG; Genzyme) at 20 mg/kg on day −3, and exposed to 700 cGy of thymic irradiation (TI) on day −2. Heparin (10 U/kg/h IV) was given prior to the start of transplantation to achieve a target activated clotting time (ACT) of 130 to 150s, as a prophylactic measure against thrombotic microangiopathy (TM) [23–25]. Prostacylin (PGI2; 20 ng/kg/h IV) was administered for 7 days, starting on day -2, also to prevent TM. All recipient animals received an infusion of 3 to 5 × 108 nucleated bone marrow cells/kg over 2.5 h on day 0 and again on day 2, except for Baboon 4, which received the second infusion on day 3. The animals were immunosuppressed in the peritransplant period (day -6 to 28) with Tacrolimus (FK506, Fujisawa) at 0.15 to 0.30 mg/kg/day IV with subsequent dose titrations to achieve a target FK506 blood level of 30 to 40 ng/ml. A bolus dose of recombinant porcine cytokines p-SCF (30 μg/kg) and p-IL-3 (30 μg/kg) was injected directly into the bag of porcine BM cells given on day 0 in order to promote pig cell proliferation and engraftment in the recipient BM stromal environment [12,26]. Both pig cytokines were then administered from day 0 to 28, each at 30 μg/kg IV, per day. One of the four experimental animals, Baboon 4, received Cobra Venom Factor (CVF) (Advanced Research Technologies, San Diego, CA, USA) on day -1 through 7 for complement inhibition (Table 1).
Clinical laboratory studies
Blood cell counts, chemistries, coagulation assays and immunosuppressive drug blood levels were carried out at regular intervals. Animals were given electrolyte supplementation to maintain normal blood electrolyte levels. Packed red blood cells (RBC) were given if the hematocrit was < 20%, and platelets were given if the platelet count fell below 20 000 cells/μl and the animal manifested symptoms of thrombocytopenia. Animals with indwelling IV catheters were given cefazolin (25 mg/kg BID IV), ganciclovir (5 mg/kg/day IV), and levofloxacin (10 mg/kg qd IV). Oral Cimetidine (5 mg/kg/day) was administered for gastrointestinal prophylaxis.
Colony formation assays
In vitro progenitor cell assays were performed according to methods described previously [26]. Briefly, mononuclear cells from bone marrow aspirate were plated in duplicate at concentrations of 25 000 cells in 35-mm petri dishes (Nunc, Naperville, IL) in a total volume of 1.5 ml of methylcellulose-based medium (Methocult H4230; Stem Cell Technologies, Vancouver, British Columbia, Canada). To select for porcine cell specific proliferation, the methylcellulose-based media was enriched with 11 ng/ml recombinant porcine stem cell factor (pSCF, BioTransplant, Inc., Charlestown, MA, USA), 0.85 ng/ml recombinant porcine interleukin-3 (pIL3, BioTransplant, Inc.), and 2 ng/ml recombinant porcine granulocyte macrophage colony stimulating factor (GM-CSF, Amgen, Inc., Thousand Oaks, CA, USA) and 0.85 U/ml recombinant human erythropoietin (Amgen Inc.). Following 10 to 14 days of incubation in 5% CO2 at 37 °C, each culture dish was visually scored through an inverted microscope and evaluated for the presence and frequency of granulocyte-macrophage colony-forming units (CFU-GM), granulocyte, erythroid, macrophage, megakaryocyte-colony forming units (CFU-GEMM), and burst-forming units-erythroid (BFU-E). Distinct colony types were manually picked for PCR analysis to detect the presence of porcine cytochrome b porcine DNA (see below).
DNA assays
A polymerase chain reaction (PCR) assay that amplifies the porcine cytochrome b gene was used to detect porcine DNA from bulk tissue samples and from CFUs grown from bone marrow of baboon recipients [19]. The cytochrome b primer sequence (Cyt-F1: 5′ GCC TAT TCA TCC ACG TAG GC 3′, Cyt-R1: 5′ CAT TCT ACG AGG TCT GTT CCG 3′) amplifies ubiquitous cytochrome b DNA found in porcine mitochondria. The microchimerism assay for detection of pig DNA was performed on peripheral blood, thymic, and BM tissues. Peripheral blood and BM cells were processed for PBMC, frozen at −80 °C, and thawed at time of genomic DNA extraction. We isolated genomic DNA with the DNeasy Tissue Kit (Qiagen, Valencia, CA, USA), measured the quantity of template using the Hoeffer TKO-100 Fluorometer (Hoeffer Scientific Instruments, San Francisco, CA, USA), and denatured the sample by incubating at 100 °C for 10 min. To validate template quality and quantity, PCR reactions using baboon GAPDH primers were performed as well. The final reaction mixture (100 μl) consisted of 1 × GeneAmp PCR Buffer II (Applied Biosystems, Carlsbad, CA, USA), 20 mM MgCl2, 400 μM dNTP mix, 200 nm of both forward and reverse primers, 2.5 U AmpliTaq Gold 5 U/ul, and 250 ng of sample DNA. Porcine DD positive control DNA was obtained from porcine CFU cells. The PCR reaction tubes were placed in a PerkinElmer Gene-Amp 9600 Thermal Cycler and run at 95 °C for 9 min. Forty cycles of replication, with the following thermal profile, were carried out: 96 °C for 10 s, 59 °C for 30 s, and 72 °C for 30 s. The reaction concluded with a 72 °C incubation for 5 min. We detected the amplification product by gel electrophoresis. Expected product size was 290 bp for baboon GAPDH and 210 bp for porcine cytochrome b. Samples were considered negative for porcine DNA in the presence of a strong signal for baboon GAPDH, indicating sufficient quantity and quality of DNA template. We used porcine BM aspirate-derived CFUs as the positive control, and naïve baboon BM aspirate-derived CFUs as the negative control.
Detection of porcine DNA in CFUs was performed in a similar fashion, save for the DNA extraction step. In brief, we picked all CFUs that grew on pig enriched cytokine media within 14 days of plating. Colony growth on the human cytokine media enriched media plates served as a positive control for assay integrity. Colonies were picked individually, or in pools of 10 provided that all colonies were of the same type (i.e. CFU-GM, CFU-GEMM, BFU-E), and suspended in 90 μl of sterile PBS in a microcentrifuge tube. After spinning and pelleting the colonies, we aspirated the supernatant but left approximately 10 μl of PBS with the cell pellet prior to proceeding to DNA isolation. Genomic DNA was extracted from the colonies by resuspending the cells in 40 μl of lysis buffer (1 × HotStart Taq Buffer (Qiagen), 0.45% NP40 (Sigma), 0.45% Tween 20 (Sigma), 100 μg/ul proteinase K (Qiagen)), incubating at 55 °C for 1 h, and 100 °C for 10 min. 10 μl of the lysate was used for each PCR reaction. The cytochrome b PCR reaction was carried out identically to the method described for bulk tissues and blood (see above), except that the reaction volume was reduced from 100 to 50 μl.
All DNA isolation and PCR setups were performed under a laminar flow hood in a room separate from the site of DNA amplification and post-PCR analyses. Appropriate precautions were used to prevent specimen contamination.
Mixed lymphocyte reaction
The mixed lymphocyte reaction (MLR) in vitro assay has been previously described [27]. Briefly, heparinized whole blood from the baboon BM recipient was processed for isolation of peripheral blood mononuclear cells (PBMC) and diluted to a concentration of 4 × 106 cells/ml. Stimulator PBMCs were isolated in a similar fashion from GalT-KO DD miniature swine, as well as from third party baboon and swine (SLAA or SLACC) as controls. All stimulator cells were then irradiated at 25 Gy. To test the proliferative response of cells from the baboon BM recipient against GalT-KO and third party PBMCs, we plated 100 μl of responder PBMCs in triplicate on a 96-well flat-bottom plate. We then stimulated with an equal number of irradiated stimulator PBMCs. The plates were incubated for 5 days at 37 °C, pulsed with 3H-thymidine (25 μCi/well), and then harvested onto filter mats using a Cell Harvester (Harvester 96® Mach II). We assessed the incorporation of 3H-thymidine into proliferating cells by adding scintillation fluid and reading the radioactive emissions, per well, with a Microbeta counter. The number of counts per minute (CPM) could be normalized into the stimulation index (SI), by dividing the CPM in all wells by the CPM within the self-stimulator wells. Post-transplantation MLRs were performed after day 28, at which point the animals were no longer treated with FK506.
Flow cytometry for detection of chimerism and quantification of T cell depletion
Monoclonal antibodies to specific cell subsets were used to determine the phenotype of pig or baboon cells during immunologic recovery or during subsequent stages of engraftment. To characterize baboon cell populations, we used antibodies against CD3 (Mouse Anti-Human CD3, BD Pharmingen), CD4/CD8 for T cell subsets (BD Simultest); CD20 for B cells (BD Pharmingen); and Class I (FITC Mouse Anti-Human MLA ABC, Abd Serotec). For detection of pig cells among baboon cells, we used 1030H1-19-BIO (mouse anti-pig leukocyte, IgM, Arn et al., unpublished), 2.27.3A-BIO (mouse anti-pig class I IgG [28]), and 2.12.3-BIO (allele-specific, anti-pig class Id IgM [28]). In addition, we utilized a series of mAbs previously produced and characterized in this laboratory [29–32], to detect defined subsets of pig mononuclear cells, including the porcine equivalents of Class I, CD1, CD5, CD9, CD16, and SWC172. Briefly, 100 μl of whole blood was washed and stained by incubating for 30 min at room temperature with the appropriate fluorescein isothiocyanate (FITC) conjugated anti-baboon mAb or biotin conjugated anti-pig mAb. Cells were washed twice and then incubated with phycoerythrin conjugated strepavidin (PESA) for 15 min at room temperature, lysed with FACS lysing solution (BD Biosciences, San Jose, CA) and washed twice more. Cells were read on FACScan (Becton Dickinson, Mountain View, CA, USA) for detection of cell-bound antibody and data was analyzed using WinList mode analysis software (Verity Software House, Topsham, ME, USA). The percentage of cells staining with each mAb was determined by comparison to staining of PBMC from the naïve pig “positive control”, naïve baboon “negative control”, and staining of porcine PBMC with irrelevant isotype controls (2.27.3A and 2.12.3).
Assays of humoral immunity
BM recipient baboons were tested for (1) antibody production and binding to BM donor cells, and (2) antibody-mediated cytotoxicity. Serum titers of induced anti–non-Gal baboon IgG and IgM antibodies were measured by flow cytometry. Briefly, 1 × 106 PBMCs from a GalT-KO pig were incubated with decomplemented baboon sera for 30 min at 4 °C in the dark. Cells were then washed twice with FACS media and incubated with rabbit anti-human IgG or goat anti-human IgM conjugated to FITC for 30 min at 4 °C in the dark. Cells were washed another two times, read on FACScan, and analyzed using WinList mode analysis software for detection of cell-bound antibody.
To assess humoral immunocompetence at the time of transplantation, we measured induced anti-LoCD2b and anti-rATG IgG and IgM by ELISA. Either LoCD2b or ATG was diluted in carbonate-bicarbonate buffer (Sigma-Aldrich, St Louis, MO, USA) to a final concentration of 5 μg/ml. ELISA Maxisorp plates (Nunc, Rochester, NY, USA) were coated with 100 μL/well of the LoCD2b or ATG dilutions and incubated overnight at 4 °C. Plates were then washed thrice with Dulbecco's Phosphate-Buffered Saline/polyoxyethylenesorbitan monolaurate (D-PBS, Cellgro, Manassas, VA / Tween, Sigma- Aldrich, St. Louis, MO, USA) and blocked with 1% Bovine serum albumin (BSA, Fisher, Pittsburgh, PA, USA), 125 μl/well, for 1 h at room temperature. After blocking, plates were washed again, loaded with 100 μl/well of serial dilutions of baboon serum and incubated at 37 °C for 1 h. After washing, 100 μl/well of horseradish peroxidase (HRP)-conjugated anti-human IgG (Southern Biotech, AL) was added and incubated for 1 h at 37 °C. Plates were then washed, loaded with 100 μl/well of ABTS developing solution (Southern Biotech, Birmingham, Al), and incubated for 10 to 15 mins at room temperature. Plate development was arrested by adding 50 μl/well of 1% sodium dodecyl sulfate (SDS, Ambion, Foster City, CA, USA). The plates were read for anti-LoCD2b and ATG antibody at 405 nm.
Cytotoxity mediated by anti-donor or anti-third party antibody was detected by a two-stage complement dependent lymphocytotoxicity assay described previously [33]. In brief, decomplemented sera samples taken prior to conditioning and in the weeks following transplantation were serially diluted from 1 : 2 to 1 : 512 in cytotoxicity media (Medium 199 (Cellgrow, Herndon, VA, USA) supplemented with decomplemented FBS). Dilutions of each sera sample were plated in 25 μl volumes on a 96-well round bottom plate. Additional wells were plated with 25 μl of cytotoxicity media only, to serve as controls for complement and media. All wells were then plated with 25 μl of GalT-KO cells (5 × 106cells/ml) and the plate was incubated in a CO2 incubator for 15 min at 37 °C. Plates were washed with 125 μl of cytotoxicity media and incubated again for 30 mins after the addition, and thorough mixing, of 25 μl of rabbit complement (diluted 1 : 8 in media). 200 μl of FACS cold media and 10 μl of 7-AAD (10 μg/ml, Sigma) were added to each well prior to transferring the contents to FACS tubes for acquisition on the FACScan. Cytotoxicity was measured by determining the percent killing within each tube. FPS sera, media and cell only wells, and media, cell and complement only wells served as negative controls to determine background cytotoxicity. We used sera from SLAAC swine that had sensitized to DD as the positive control. FACS analysis was carried out using Winlist. We observed good reproducibility of the relative cytotoxicity between samples in each assay and moderate variability in background killing, ranging from 5 to 20%.
Results
T cell depletion
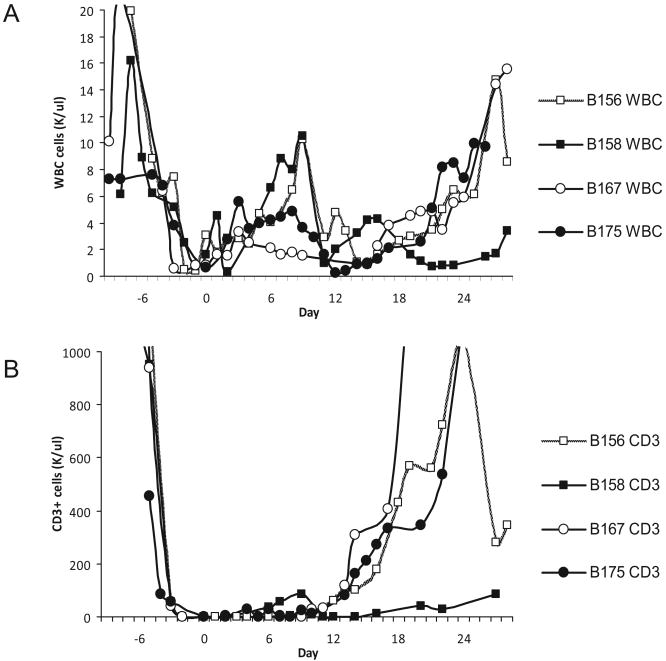
The T cell depleting regimen of radiation, LoCD2b, and ATG successfully depressed the WBC count to an average value of 1.5K cells/μl and CD3 + T cells to 0.25 cells/μl by day 0 (Fig. 1). T cells began to re-emerge within 10 days following transplant, except for Baboon 2, where T cell recovery began as early as day 6. We gave Baboon 2 an additional dose of ATG on day 10 when T cells had climbed to 83 cells/μl, to maintain CD3 + counts below 100 cells/μl (Fig. 1). On day 27, we administered a third dose of LoCD2b to Baboon 1 to condition the animal for a second BM transplant. The animal expired on day 28, prior to transplantation (see below).
Fig. 1.
Cellular recovery: (A) WBC showed recovery within a week of transplant for all animals. B158 (Baboon 1) had a suspected low grade infection following transplant, with a relative leukocytosis, that resolved with empiric antibiotic coverage; (B) T cells were depleted by irradiation, ATG and LoCD2b, which began 1 week before BM transplant, and reached maximal depletion by day -1. All animals maintained CD3 + T cell counts < 100/μl for 10 days post-transplant, except for B158 (Baboon 2), which manifested an accelerated recovery and subsequently received a second dose of ATG on day 10. Baboon 1 received a third dose of LoCD2b on D27 in preparation for an additional infusion of GalT-KO bone marrow (presumably the cause of its anaphylactic death).
Clinical course of Baboons receiving GalT-KO bone marrow transplants
Baboons 1 and 2, which received bone marrow transplants only, survived 28 days and 194 days, respectively. Baboon 1 tolerated the BM infusions well but developed mild hypotension (100 to 120/80 to 90) following the institution of prostacyclin therapy. We discontinued the prostacyclin beginning on day 6 and observed a return to baseline blood pressure. LDH was followed closely, as were coagulation assays, to ensure that removing prostacyclin did not result in TM.
Neither PB nor BM macrochimerism could be detected on flow cytometry on day 14, which raised concerns as to whether a sufficient dose of BM cells had been transplanted. We planned on giving an additional third dose of bone marrow cells on day 29, with T cell depletion prior to transplantation. However, in the process of infusing the third dose of LoCD2b 2 days prior to the second transplant, the animal became acutely hypotensive. Despite resuscitative efforts, the animal developed neurogenic shock (i.e. bradycardia, hypotension (40/20), loss of brain stem reflexes) within 24 h of the insult and was sacrificed. Necropsy revealed grossly normal heart, lung, brain, kidney and GI tract, without evidence of hemorrhage or embolic sequeli. Pathology of tissues showed only a lymphohistiocytic infiltration in the lungs, suggestive of nonspecific interstitial pneumonitis. Given these findings, and the acute decompensation that was observed, we suspected that animal might have been sensitized to LoCD2b following its administration on days −5 and −4, and that it had developed a severe anaphylactic reaction following the third infusion of LoCD2b on day 27 (see “Discussion”, below).
Baboon 2 had a considerably longer clinical course, despite developing a suspected low grade infection shortly following BM transplant, which was treated empirically with metronidazole (500 mg/day) and fluconazole (180 mg/day) from days 8 to 15. While cultures obtained prior to administering antibiotics, and in the subsequent weeks, were all negative, the animal appeared clinically improved after broadening antibiotic coverage. Due to persistent anorexia and suspected weight loss, Baboon 2 was started on TPN on day 9. The animal was weaned from TPN on day 19 when it demonstrated improved PO intake.
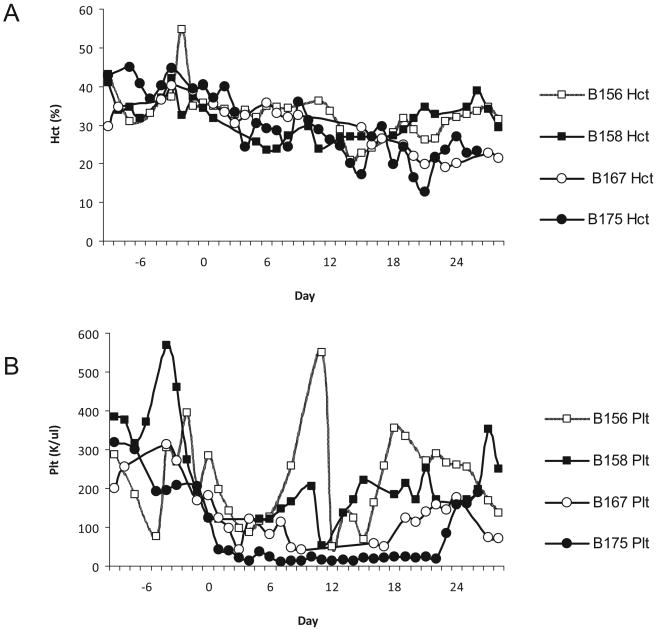
Neither Baboon 1 nor 2 required red blood cell or platelet transfusions in the post-transplant period (Fig. 2). Both animals developed moderate thrombocytopenia but never manifested any clinical signs of bleeding.
Fig. 2.
(A) Blood and platelet recovery: Despite frequent blood draws for in vitro assays, CBC, chemistries and coagulation panels, both animals that received only a BM transplant maintained a stable HCT above 20 without pRBC transfusions; (B) Platelet levels decreased following TBI, and reached the lowest counts 1 week following irradiation. Baboon 4 (B175) required extensive blood product support due to persistent anemia and thrombocytopenia. B156 experienced a sudden decrease in platelets on day 10, which was attributed to menses.
Clinical course of baboons receiving both GalT-KO bone marrow and kidney transplants
In the recent literature, it has been shown that allograft tolerance is strongly associated with the presence of multilineage peripheral blood chimerism at time of transplant [1] and that the presence of donor-derived CFUs in BM correlates with allograft tolerance in pigs [28]. We therefore attempted to introduce a GalT-KO kidney within the anticipated window of presence of porcine-derived CFUs in the baboon BM. Baboon 3 received an orthotopic GalT-KO kidney transplant on day 17 and began making urine immediately following the procedure. The native ureter was untied 25 days post-BM transplantation and 8 days post-kidney transplantation (hereafter day 25/8), due to renal failure of the transplanted kidney, as evidenced by a creatinine of 4.8.
Baboon 4 received a kidney transplant on day 2 and the second bone marrow infusion on day 3. The animal experienced a drop in platelets from 123 000 on day 0 to 13 000 on day 4 and a drop in hematocrit between days 2 and 4 to a nadir of 24.2% (Fig. 2). A platelet transfusion, packed RBC transfusion, and vitamin K injection was administered on day 4, leading to transient stabilization of platelet counts. The animal required multiple platelet and blood transfusion in the following 2 weeks. Evidence of kidney rejection necessitated that the native ureter be untied on day 13. At no point did creatinine levels increase to more than 2.3 mg/dl. LDH reached levels of 1620 and 2800 U/l on days 3 and 26, respectively, while remaining at a chronically elevated level of approximately 300 to 600 U/l during the other time points in the study. The animal expired on day 26. Necropsy revealed pulmonary edema, a gross enlargement of the transplanted kidney to three times its original size, with associated hemorrhagic changes, and thrombi in both the pleural and pericardial spaces.
Pig cell chimerism and engraftment in baboon peripheral blood, bone marrow and thymus
Multilineage peripheral blood macrochimerism (CD9 +, CD16 +, CD172 +) was observed in Baboon 4 in the 2 h immediately following BM transplant. While the initial multilineage chimerism was > 1%, the pig cell population quickly dwindled so that only CD9 + pig cells were present at 12 h. No chimerism was observed by day 1 and at no point did PB macrochimerism return. Previous studies, which employed a more rigorous conditioning regimen, including higher radiation exposure and increased immunosuppression, have documented returning peripheral macrochimerism between the first and third weeks following transplant [19]. Aside from Baboon 4, which was treated with CVF for 8 days in the peritransplantation period, no other animal developed chimerism detectable by flow cytometry at any point in the study.
Baboon 1 underwent a protocol bone marrow biopsy 2 weeks following transplantation (D16) (Table 2). Eight BFU-E colonies were observed in the pig cytokine enriched methocellulose media plates though none demonstrated porcine DNA by cytochrome b PCR. Baboon 1 underwent another biopsy on the day of death (D28). A total of 20 GM, 8 GEMM, and 300 BFU-E colonies grew from this aspirate on pig cytokine enriched media. The colonies were pooled in groups of 4 to 10; all samples were assayed by PCR and all were positive for porcine DNA. Of note, six out of six of the pooled GEMM colonies that grew from human cytokine enriched media were also positive for porcine DNA by PCR. None of the other colony types that grew on human cytokine media were positive. The naïve baboon CFU control and water control wells were likewise negative, for porcine DNA, suggesting that there was no porcine DNA contamination in the samples tested. PCR performed on tissues obtained on the day of death (day 28) of Baboon 1 showed porcine DNA in bone marrow, thymus and peripheral blood (Table 2).
Table 2.
Colony forming units containing porcine-derived DNA. B156 (Baboon 1) aspirate from day 28 was plated on both pig and human cytokine enriched media. Of the plates that were enriched with pig cytokines, 20 GM, 8 GEMM, and 300 BFUE colonies grew. Colonies were pooled in groups of 4 to 10 and assayed for porcine cytochrome B. All pooled colonies that were derived from pig cytokine media plates were positive for cytochrome. Six GEMM colonies were seen growing on human cytokine enriched media due to the cross reactivity between porcine and human cytokines, of which all were porcine derived. Plates containing B158 (Baboon 2) aspirate and pig cytokines had three pooled colonies growing by day 28, of which two (GM) were derived from porcine progenitors. B167 (Baboon 3) and B175 (Baboon 4) had no growth on any of the plates enriched with pig cytokines. B156 (Baboon 1), B158 (Baboon 2), and B167 (Baboon 3) had detectable porcine DNA in LN, thymus, PB (peripheral blood), and BM samples taken on various days, as determined by PCR
| Pig Cytokine Enriched Media | Human Cytokine Enriched Media | ||||||
|---|---|---|---|---|---|---|---|
| GM | GEMM | BFU | GM | GEMM | BFU | ||
| B156 | D16 | 0/0 | 0/0 | 0/8 | 0/1 | 0/1 | 0/1 |
| D28 | 6/6 | 6/6 | 1/1 | – | 6/6 | – | |
| B158 | D28 | 2/2 | 0/1 | 0/1 | 0/3 | – | 1/2 |
| D68 | 0/1 | 0/0 | 0/0 | 0/2 | – | – | |
| D104 | 0/3 | 0/0 | 0/0 | 0/1 | 0/1 | 0/2 | |
| B167 | D15 | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 | – |
| D58/40 | 0/0 | 0/0 | 0/0 | – | 0/5 | 0/6 | |
| D132/114 | 0/0 | 0/0 | 0/0 | 0/8 | 0/10 | 0/26 | |
| B175 | D26/24 | 0/0 | 0/0 | 0/0 | 0/35 | 0/70 | 0/53 |
| LN | Thymus | PB | PBMC | BM | CFU | ||
| B156 | D28 | Neg | + (DNA) | + (DNA) | – | + (DNA) | + (DNA) |
| B158 | D104 | + (cDNA) | – | – | – | + (cDNA) | Neg |
| D195 | + (cDNA) | + (cDNA) | – | – | – | Neg | |
| B167 | D42/24 | – | – | – | – | + (DNA) | – |
| D51/33 | – | – | + (DNA) | – | – | – | |
| D57/39 | – | – | Neg | – | – | – | |
| D58/40 | – | – | – | – | – | Neg | |
Baboon 2 BM aspirate obtained on D28 grew porcine-derived GM CFUs in porcine enriched cytokine media and BFU-E CFUs in human cytokine enriched media. Porcine CFU microchimerism was not detected beyond that point, despite repeated BM aspirates on days 68 and 104. RT-PCR on bulk tissues isolated from Baboon 2 demonstrated persistent microchimerism in lymph nodes, thymus, and bone marrow until D195 (Table 2).
Baboons 3 and 4 had no detectable CFUs containing porcine DNA at any time point. Baboon 3 did however manifest peripheral microchimerism in peripheral blood and bone marrow samples until day 51, but not thereafter (Table 2).
Serum cytotoxicity assay and MLR
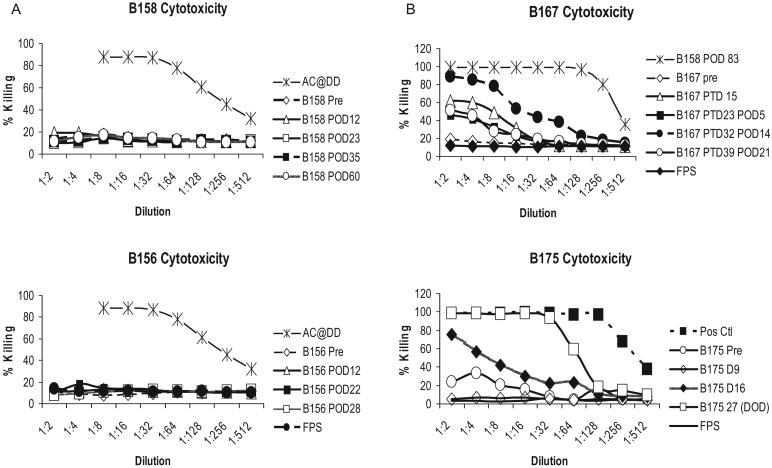
All four animals possessed low antibody mediated cytotoxicity directed against GalT-KO PBMCs prior to BM transplantation. Animals 1 and 2 maintained low cytotoxic titers for 28 days (day of death) and 2 months respectively (Fig. 3). In contrast, Baboons 3 and 4 developed an induced cytotoxic antibody response by day 15 (Fig. 3). While Baboon 4 received a kidney transplant on day 2, which may have sensitized the animal, Baboon 3 demonstrated increasing cytotoxic antibody titers on D15, prior to its kidney transplant on day 17.
Fig. 3.
Baboon anti-pig antibodies as measured by antibody-mediated cytotoxicity: All animals in this study had baseline low cytotoxicity that was indistinguishable from the negative control (fetal pig sera). (A) B156 (Baboon 1) and 158 (Baboon 2) maintained low levels of cytotoxicity during the study period with approximately 14% at a sera dilution of 1:2; (B) B167 (Baboon 3) and B175 (Baboon 4) in contrast, showed increasing cytotoxicity within 15 days following transplant. While B175 (Baboon 4) received a kidney transplant on day 2, which may have sensitized the animal, B165 (Baboon 3) demonstrated increasing cytotoxic antibody titers on D15, prior to its kidney transplant on day 17. Note: B158 was subsequently immunized with GalT-KO tissues to produce the positive control serum used in panel B.
Baboon 1 expired before a post-transplantation MLR could be performed. Baboon 2, however, demonstrated pig specific hyporesponsiveness following transplantation (Fig. 4). This animal manifested a robust response to third party allogeneic simulator cells as well as to DD pig cells prior to bone marrow transplantation. In the weeks following cessation of FK506, we repeated the MLR assay and observed a generalized loss of responsiveness to both allogeneic and xenogeneic cells. However, by D57, Baboon 2 regained significant reactivity to allogeneic stimulators, as demonstrated by a stimulation index approximately 15% that of pre-transplantation levels. In contrast, reactivity to porcine DD stimulators remained significantly suppressed until D57 (Fig. 4).
Fig. 4.
Mixed lymphocyte reaction (MLR): Pre-transplant MLR of B158 (Baboon 2) showed a robust proliferative response to both DD pig and allogeneic cell stimulators. By day 40, this animal showed hyporesponsiveness to allogeneic stimulators and a notable lack of reactivity to DD pig cells. By D57, the allogeneic response began to recover while stimulation by DD pig cells remained very low.
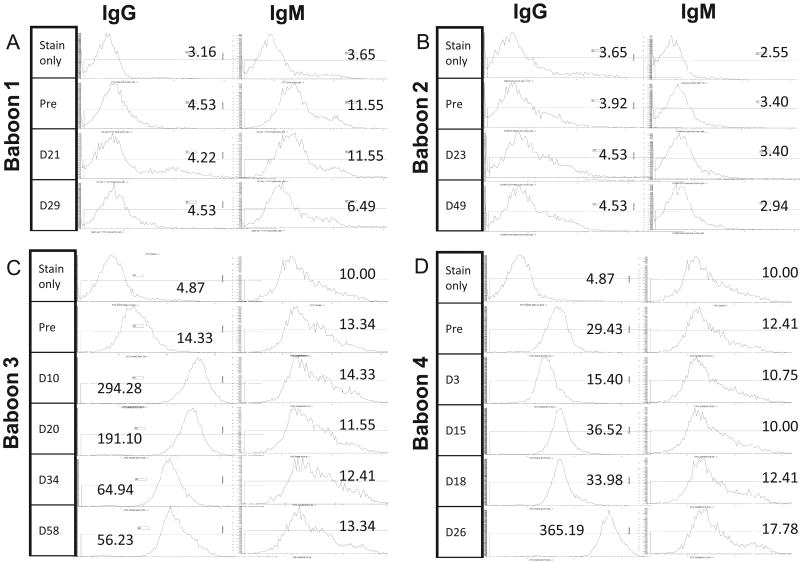
LoCD2b, ATG, and anti–non-Gal antibody responses
We examined pre-treatment and post-treatment antibody titers to LoCD2b by ELISA in all of the experimental animals since the development of acute shock in Baboon 1 following the third infusion of LoCD2b was suggestive of sensitization to the antibody. We also quantified serum titers of rATG and anti–non-Gal antibodies. Our assays showed induced IgG antibodies to LoCD2b and ATG in the sera of all animals within 2 weeks of BM transplant (Fig. 5), but hyporesponsiveness to pig antigens in both Baboon 1 and 2, as evidenced by consistently low levels of anti–non-Gal IgG (Fig. 6). In contrast, Baboons 3 and 4 were found to have pre-existing elevated titers of anti–non-Gal IgG before BM transplantation (Fig. 6). In the weeks following transplant, both of these baboons developed further increases in anti–non-Gal IgG titers and strong antibody-mediated cytotoxicity to GalT-KO cells, indicating sensitization to the transplanted BM cells (Figs 3 and 7). Animal 3 manifested a particularly robust increase in anti–non-Gal IgG by day 3 and slightly reduced titers after renal graft placement on day 17.
Fig. 5.
LoCD2b antibody titers in B156 (Baboon 1). This animal had low levels of IgG and IgM anti-LoCD2b antibody prior to BM transplant, but developed elevated IgG titers by day 12 following transplant. High IgG titers persisted until day 28, when the animal expired following a third dose of LoCD2b. Suspicion of an anaphylactic reaction to LoCD2b prompted the retrospective analysis of anti-ATG and anti-LoCD2b serum antibody titers.
Fig. 6.
Pre-transplant anti-;non-Gal IgG antibody titers. (A, B) Both B56 (Baboon 1) and B158 (Baboon 2) had low levels of anti-non-Gal IgG prior to transplant, with a mode fluorescence intensity (MFI) comparable to the negative control. No significant change in MFI was noted in the weeks following transplantation. (C, D) In contrast, B167 (Baboon 3) and B175 (Baboon 4) had higher pre-existing levels of natural anti-non-Gal IgG, and demonstrated a clear increase in titers after BM transplantation.
Discussion
In previous studies from this laboratory, Sablinski et al. reported evidence for porcine bone marrow microchimerism for up to 300 days using Gal positive miniature swine bone marrow, but never saw evidence for peripheral blood chimerism and found no systemic effects of the microchimerism on immunologic parameters [12]. Since BM stem cells have been reported to lack the Gal antigen but give rise to Gal-positive progeny [34], we hypothesized that progeny of the engrafted stem cells may have been rapidly cleared by anti-Gal antibodies. Subsequently, a non-myeloablative conditioning regimen employing cyclosporine and/or anti-CD154, mycophenolate mofetil (MMF) and cobra venom factor (CVF) was found to be effective in inducing multilineage peripheral blood chimerism for up to 5 days following transfusion of high dose porcine PBMCs (2.7 to 4.6 × 1010 cells/kg) into baboons that had undergone extracorporeal immunoadsorption of anti-Gal antibodies [16]. In a subset of these animals, transient BM engraftment was seen on day 28 as shown by PCR and detectable pig DNA within BM-derived CFUs, but again without evidence of an effect on anti-donor immunity. Tseng et al. also reported early transient peripheral blood macrochimerism using a 30-fold lower dose of blood progenitor cells obtained from GalT-KO pig BM [19]. However, the only animal in the study that showed BM chimerism died on day 6 from accidental rupture of its arterial line, while neither of the two other animals showed evidence of chimerism by FACS, PCR, or CFU assays.
We report here that use of a modified T cell depleting regimen was successful in achieving BM chimerism at 28 days in two of four animals. We were able to achieve excellent T cell depletion with the pre-transplant regimen of 150 cGy TBI, splenectomy, 700 cGy TI, LoCD2b, and ATG. CD3 + T cells were undetectable on the day of transplant, and remained depressed without additional T cell depletion for at least 1 week following BM transplantation. The first clue that this regimen had also induced specific unresponsiveness to porcine cells was obtained from the observation of an apparent anaphylactic reaction to one of the anti-T cell antibodies that was administered a third time to one of these two animals. This reaction led us to examine the antibody responses of these animals to the T cell depleting reagents, and we were surprised to find that all of the animals had regained sufficient immunocompetence to produce high-titered antibody responses to both rat and rabbit IgG, despite the absence of responsiveness to pig cells, as assayed either by FACS or by complement-mediated cytotoxicity. These findings represent our first evidence that induction of specific xenogeneic unresponsiveness at the antibody level by the mixed chimerism approach, is possible in a pig-to-baboon model.
In contrasting the current protocol with previous conditioning regimens, the most significant differences lie in: (i) reduction of TBI from 300 cGy to 150 cGy; (ii) use of tacrolimus in lieu of cyclosporin as the calcineurin inhibitor; and (iii) elimination of anti-CD154, CVF, and MMF from the preparative and treatment regimens. These changes were made to model the conditioning regimen after a minimally myeoablative protocol which was successful in inducing bone marrow CFU chimerism in porcine BM hematopoietic cell allotransplantation at our center [35]. Furthermore, this modified conditioning regimen obviated the need for blood product support in both animals that became chimeric, and the putative infection in one of these animals (Baboon 2) was easily treated with broadened antibiotic coverage.
The transient BM engraftment observed in both Baboons 1 and 2 correlated with the absence of complement-mediated cytotoxicity to GalT-KO cells, hyporesponsiveness to pig cells by MLR (confirmed in Baboon 2 only), and persistently low anti–non-Gal IgG titers. In previous studies, there were concerns that the lack of an in vitro sensitization response may have been more reflective of immune incompetence than of a true marker for pig specific tolerance. Here, we have demonstrated that this BM transplant protocol is sufficient to induce sensitization to pig cells, as evidenced by increased anti–non-Gal IgG antibody titers and induced antibody-mediated cytotoxicity to GalT-KO cells by Baboons 3 and 4 (Figs 3 and 6). While the antibody response of Baboon 4 might potentially be explained by the additional kidney transplant performed on day 2, Baboon 3, which received its kidney transplant on day 17, had already developed a cytotoxic response and increasing antibody titers to pig by day 14 (Fig. 3). Given that the conditioning regimen for Baboon 3 was identical to that of Baboons 1 and 2, its early response serves as a control indicating that the response of this animal (and presumably of Baboon 4) was to the infused BM and not due to the transplanted kidney.
Our retrospective analysis on pre-treatment sera to determine the cause of death for Baboon 1 led to the realization that the two animals (Baboons 3 and 4) which failed to show evidence of BM chimerism or pig-specific hyporeactivity were also the two animals with pre-existing elevated anti–non-Gal IgG antibody titers. These findings suggest that either high levels of naturally occurring anti–non-Gal antibodies existed in these animals or that the animals were pre-sensitized to pig antigens prior to transplantation. The high levels of anti–non-Gal antibodies in Baboons 3 and 4 may have accelerated clearance of infused BM cells on transplantation, and thus precluded the transient BM engraftment that correlated with in vitro evidence of tolerance in Baboons 1 and 2. In future studies, it may be appropriate to incorporate antibody reducing agents in the conditioning regimen to test this hypothesis.
In conclusion, these studies provide encouraging data on transient engraftment using a minimally myeloablative conditioning protocol that decreases post-transplant morbidity. The ability to induce bone marrow chimerism appeared to correlate with absence of pre-transplant anti–non-Gal antibodies as detected by FACS analysis. Both animals in which such chimerism was detected also showed pig-specific hyporesponsiveness by complement-mediated cytotoxicity, MLR, and anti–non-Gal antibody FACS. We will now investigate the possibility that pre-screening recipients for low levels of anti–non-Gal IgG antibody and/or utilizing strategies to eliminate such antibodies, may increase the levels and frequency of porcine engraftment and, hopefully, lead to a reproducible means of inducing xenograft tolerance.
Acknowledgments
We thank Ahmed Ghazi for significant contributions to the early phase of this work, including in vitro assays and animal care, Dr. John Hanekamp and Raimon Duran-Struuk D.V.M for critical reviews of the manuscript, and Rebecca Wark for expert assistance in preparation of the manuscript. Rabbit anti-thymocyte globulin was generously provided by Genzyme Corporation, Cambridge, MA.
References
- 1.Sachs DH, Sykes M, Robson M, Cooper DK. Xenotransplantation. Adv Immunol. 2001;79:129–223. doi: 10.1016/s0065-2776(01)79004-9. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Murase N, Tsakis A, et al. Clinical xenotransplantation. Xenotransplantation. 1994;1:3–7. doi: 10.1111/j.1399-3089.1994.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng YL, Sachs DH, Cooper DK. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation. 2005;79:1–9. doi: 10.1097/01.tp.0000146504.73727.13. [DOI] [PubMed] [Google Scholar]
- 4.Lee LA, Sergio JJ, Sykes M. Natural killer cells weakly resist engraftment of allogeneic, long-term, multilineage-repopulating hematopoietic stem cells. Transplantation. 1996;61:125–132. doi: 10.1097/00007890-199601150-00024. [DOI] [PubMed] [Google Scholar]
- 5.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CA, Fuchimoto Y, Scheier-Dolberg R, et al. Stable mixed chimerism and tolerance using a non-myeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 8.Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6:2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharabi Y, Aksentijevich I, Sundt TM, et al. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med. 1990;172:195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartholomew AM, Powelson J, Sachs DH, et al. Tolerance in a concordant nonhuman primate model. Transplantation. 1999;68:1708–1716. doi: 10.1097/00007890-199912150-00014. [DOI] [PubMed] [Google Scholar]
- 12.Sablinski T, Emery DW, Monroy R, et al. Long-term discordant xenogeneic (porcine-to-primate) bone marrow engraftment in a monkey treated with porcine-specific growth factors. Transplantation. 1999;67:972–977. doi: 10.1097/00007890-199904150-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kozlowski T, Monroy R, Xu Y, et al. Anti-Gal(alpha)1-3Gal antibody response to porcine bone marrow in unmodified baboons and baboons conditioned for tolerance induction. Transplantation. 1998;66:176–182. doi: 10.1097/00007890-199807270-00006. [DOI] [PubMed] [Google Scholar]
- 14.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 15.Buhler L, Alwayn IP, Basker M, et al. CD40-CD154 pathway blockade requires host macrophages to induce humoral unresponsiveness to pig hematopoietic cells in baboons. Transplantation. 2001;72:1759–1768. doi: 10.1097/00007890-200112150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Buhler L, Awwad M, Treter S, et al. Pig hematopoietic cell chimerism in baboons conditioned with a non-myeloablative regimen and CD154 blockade. Transplantation. 2002;73:12–22. doi: 10.1097/00007890-200201150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 18.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng YL, Dor FJ, Kuwaki K, et al. Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Xenotransplantation. 2004;11:361–370. doi: 10.1111/j.1399-3089.2004.00151.x. [DOI] [PubMed] [Google Scholar]
- 20.Barth RN, Yamamoto S, Lamattina JC, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation. 2003;75:1615–1624. doi: 10.1097/01.TP.0000064335.50622.20. [DOI] [PubMed] [Google Scholar]
- 21.Sablinski J, Latinne D, Gianello P. Xenotransplantation of pig kidneys to nonhuman primates. I: Development of the model. Xenotransplantation. 1995;2:264–270. [Google Scholar]
- 22.Pennington LR, Sakamoto K, Popitz-Bergez FA, et al. Bone marrow transplantation in miniature swine. I. Development of the model. Transplantation. 1988;45:21–26. doi: 10.1097/00007890-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Buhler L, Goepfert C, Kitamura H, et al. Porcine hematopoietic cell xenotransplantation in nonhuman primates is complicated by thrombotic microangiopathy. Bone Marrow Transplant. 2001;27:1227–1236. doi: 10.1038/sj.bmt.1703067. [DOI] [PubMed] [Google Scholar]
- 24.Pettitt AR, Clark RE. Thrombotic microangiopathy following bone marrow transplantation. Bone Marrow Transplant. 1994;14:495–504. [PubMed] [Google Scholar]
- 25.Alwayn IP, Buhler L, Appel JZ, et al. Mechanisms of thrombotic microangiopathy following xenogeneic hematopoietic progenitor cell transplantation. Transplantation. 2001;71:1601–1609. doi: 10.1097/00007890-200106150-00020. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski T, Monroy R, Giovino M, et al. Effect of pig-specific cytokines on mobilization of hematopoietic progenitor cells in pigs and on pig bone marrow engraftment in baboons. Xenotransplantation. 1999;6:17–27. doi: 10.1034/j.1399-3089.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 27.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186:497–506. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanoska D, Sun DC, Lunney JK. Production of monoclonal antibodies reactive with polymorphic and monomorphic determinants of SLA class I gene products. Immunogenetics. 1991;33:220–223. doi: 10.1007/BF01719247. [DOI] [PubMed] [Google Scholar]
- 29.Pescovitz MD, Lunney JK, Sachs DH. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985;134:37–44. [PubMed] [Google Scholar]
- 30.Pescovitz MD, Lunney JK, Sachs DH. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984;133:368–375. [PubMed] [Google Scholar]
- 31.Pescovitz MD, Hsu SM, Katz SI, et al. Characterization of a porcine CD1-specific mAb that distinguishes CD4/ CD8 double-positive thymic from peripheral T lymphocytes. Tissue Antigens. 1990;35:151–156. doi: 10.1111/j.1399-0039.1990.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan JA, Oettinger HF, Sachs DH, Edge AS. Analysis of polymorphism in porcine MHC class I genes: alterations in signals recognized by human cytotoxic lymphocytes. J Immunol. 1997;159:2318–2326. [PubMed] [Google Scholar]
- 33.Cosimi AB, Delmonico FL, Wright JK, et al. Prolonged survival of nonhuman primate renal allograft recipients treated only with anti-CD4 monoclonal antibody. Surgery. 1990;108:406–413. [PubMed] [Google Scholar]
- 34.Gojo S, Harper D, Down J, Awwad M, Cooper DK. Differential expression of Galalpha1,3Gal epitopes on fetal and adult porcine hematopoietic cells. Xenotransplantation. 2002;9:297–300. doi: 10.1034/j.1399-3089.2002.01048.x. [DOI] [PubMed] [Google Scholar]
- 35.Horner BM, Cina RA, Wikiel KJ, et al. Predictors of organ allograft tolerance following hematopoietic cell transplantation. Am J Transplant. 2006;6:2894–2902. doi: 10.1111/j.1600-6143.2006.01563.x. [DOI] [PubMed] [Google Scholar]