Abstract
Background
The heart progressively remodels over the life course, yet longitudinal data characterizing such remodeling in the community are limited.
Methods and Results
Using multilevel modeling, we analyzed up to 4 serial echocardiographic observations obtained over a 16-year period in 4,062 Framingham Study participants (mean age 45 years, 54% women; 11,485 person-observations). We related LV wall thickness (LVWT), LV systolic (LVDS) and diastolic (LVDD) dimensions and fractional shortening (FS) to age, sex, body mass index (BMI), blood pressure (BP, including antihypertensive medication use), smoking, and diabetes (separate analyses for each echocardiographic measure). With advancing age, LV dimensions decreased, whereas FS and LVWT increased concomitantly. Male sex, BMI, and BP indices/hypertension treatment were significantly related to both greater LV dimensions and LVWT. The effect of age on cardiac remodeling was influenced by key covariates (P>0.05 for all interactions): women and individuals with diabetes experienced greater age-associated increases in LVWT; presence of diabetes or a higher BP was associated with a lesser decrease in LV diastolic dimensions with increasing age; antihypertensive medication use was a marker of an attenuated increase in FS with aging.
Conclusions
Cardiac remodeling over the adult life course is characterized by a distinct pattern of increasing LVWT, decreasing LV dimensions and increasing FS with advancing age. Overall, female sex, greater BP load, and presence of diabetes serve to attenuate this remodeling pattern. These observations suggest a mechanism for the preponderance of women with hypertension and individuals with diabetes among patients with diastolic heart failure.
Keywords: aging, cardiac remodeling, heart failure
BACKGROUND
The human heart undergoes dynamic, incremental alterations in structure and function over the life course, a phenomenon referred to as cardiac remodeling. These alterations are associated with changes in the cellular and extracellular composition of myocardial tissue – processes that can involve cardiomyocyte hypertrophy, apoptosis, and regeneration.1-3 The morphology of this progressive cardiac remodeling may be characterized by changes in multiple measurable echocardiographic dimensions. Accordingly, several cross-sectional studies suggest that the cardiac remodeling that occurs with advancing age involves increasing left ventricular (LV) wall thickness and may also involve decreasing LV cavity diameter in the setting of globally preserved or even increased systolic function.4-7 Although common patterns of cardiac remodeling might be attributed to aging-related processes, they are associated with adverse clinical consequences.8 In fact, such remodeling may contribute to the higher incidence of heart failure in older adults and, particularly, heart failure that presents with a normal ejection fraction.6,9 Therefore, prospectively investigating LV remodeling over the life course could elucidate the evolution of alterations in cardiac structure and function that antedate symptomatic myocardial dysfunction by years to decades.
We have previously described the tracking of LV mass over the adult life course, highlighted the age-associated increase in cardiac mass and underscored the adverse impact of cardiovascular risk factors (notably obesity, hypertension and diabetes) on such increases in LV mass with aging.10 However, LV mass is a composite variable that only indirectly reflects variation in the multiple components of LV morphology, including LV internal dimensions as well as wall thickness. As such, tracking of LV mass may not adequately capture distinctive patterns of LV remodeling that may be the consequence of disparate trajectories of changes in LV wall thickness versus LV dimensions. In the present investigation, we evaluated the longitudinal tracking of LV wall thickness (LVWT), diastolic and systolic dimensions (LVDD and LVDS, respectively) and endocardial fractional shortening (FS), a measure of pump function, over the adult life course in a large community-based sample. Specifically, we used multilevel modeling to estimate growth curves tracking change in several indices of LV morphology, as captured by serial and routine echocardiograms performed over an extended follow-up period of up to 16 years. We also analyzed the degree to which these morphologic alterations were associated with traditional risk factors, many of which have been reported as possible determinants of LV remodeling in cross-sectional studies.5,11
METHODS
Study Sample
In 1971, the Framingham Offspring Study began with the enrollment of 5124 individuals, who were the children of the Original cohort of the Framingham Heart Study, and the spouses of the children.11 Routine examinations of the Offspring cohort are performed approximately every 4 years, and include a medical history focusing on the incidence of new-onset cardiovascular events since the previous examination, a targeted physical examination that includes anthropometry and blood pressure measurements using a standardized protocol, and phlebotomy for obtaining specimens used for the laboratory assessment of cardiovascular risk factors. Attendees at Offspring cohort examination cycles 2 (1979-1982), 4 (1987-1990), 5 (1991-1995), and 6 (1996-1998) underwent routine transthoracic echocardiography. Of the 4,556 unique attendees (15,216 observations) at these examinations, we excluded individuals who were >25 or ≥75 years old at the time of their examination (N=12; 344 observations), and those with prevalent or incident myocardial infarction or heart failure (N=269; 763 observations), which are conditions that can affect LV measurements based on M-mode echocardiography. We also excluded observations if participants had atrial fibrillation or valvular disease (N=22; 312 observations), were missing clinical covariates for analyses (N=7; 58 observations), were missing echocardiographic measurements (N=183; 2,251 observations, or had extreme outlier values for these measurements (N=1; 3 observations). Thus, 4,062 unique individuals providing 11,485 observations were included in the analyses involving longitudinal tracking of LV indices.
The study protocols for Offspring examinations were approved by the Institutional Review Board at the Boston University Medical Center, and all attendees at the examinations provided written informed consent.
Echocardiographic Measures
Study participants underwent standardized routine transthoracic echocardiography, as detailed elsewhere.10 The equipment used to obtain echocardiographic measures differed across examination cycles: Hoffrel 201 ultrasound receiver (with Aerotech transducer) at cycle 2; Hewlett-Packard (Model 77020AC) ultrasound machine at cycles 4 and 5; and, Hewlett-Packard (Sonos 1000) machine at cycle 6. All echocardiograms were evaluated by an experienced technician or cardiologist using a standardized reading protocol. End-diastolic LV septal (SWT), posterior wall (PWT) thickness, and LV diameter at the end of diastole (LVDD) and systole (LVDS) were obtained by using a leading-edge technique and averaging M-mode measurements from atleast 3 cardiac cycles.12 Total LV wall thickness (LVWT) was calculated as the sum of SWT and PWT. Fractional shortening (FS) was calculated according to the following formula: FS = [(LVDD–LVDS) / LVDD ] × 100. Relative wall thickness (RWT), a commonly used measure of LV hypertrophy, was calculated as LVWT divided by LVDD. For secondary analyses, LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were derived from M-mode measures using the Teichholz method;12 LV ejection fraction (LVEF) was defined as ([LVEDV LVESV]/LVEDV) ×100.
Statistical Analyses
Estimating individual growth curves for LV indices
Multi-level statistical modeling allows the analysis of data that vary at multiple levels. It is applicable to longitudinal data where repeated measurements are obtained on different occasions (level 1) within the same individual (level 2). The models allow for estimation of the overall pattern of change over time and also for the impact of risk factors on the temporal pattern. Compared to traditional regression models, this analytical approach has the advantage of accommodating participants with missing data at some of the serial examinations and facilitates analyses using the maximum number of observations in a longitudinal investigation.13 Results of formal testing indicated that the relationship between the echocardiographic variables and covariates were similar for participants with data collected at all examination cycles compared to participants who may have had missing values at later examination cycles (data not shown).
We estimated growth curves for each morphologic index of LV remodeling (LVWT, LVDD, LVDS, and FS) using multi-level statistical modeling (SAS PROC MIXED; using an unstructured correlation matrix). Growth curves were also estimated for RWT, a variable derived from measures of both LVWT and LVDD. We used forward direct entry to analyze the associations of each LV index with common clinical covariates that have been related to measures of LV remodeling in cross sectional studies: age, sex, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), antihypertensive treatment, smoking status, and diabetes mellitus. In analyses where the relation of the LV measure with SBP and DBP was discordant, we additionally examined the association of the same LV measure with the difference between SBP and DBP, which is also known as the pulse pressure. Examination cycle was included as a covariate in all analyses to account for variation in LV indices across examinations due to variation in the echocardiographic instrumentation used. Random intercepts and random effects of age were fitted for all models to reflect different starting values and different relationships with age for each LV index measured in each participant.
We also examined the quadratic effect of age, which was not found to reach statistical significance. We fit a series of clinically pre-specified models, with direct entry of candidate variables. Biologically plausible statistical interactions between age, sex, and other clinical risk factors were also investigated. Separate growth curves were graphically displayed separately for men and women to demonstrate the tracking of LV indices over time.
Association of Clinical Risk Status on Longitudinal Tracking of LV Structure and Function
To clarify the association of the overall burden of clinical risk factors on patterns of longitudinal tracking, we additionally estimated sex-specific associations stratified by ‘high’ versus ‘low’ risk factor status: high risk factor status was defined as having hypertension (SBP ≥140 mm Hg or DBP ≥90 mm Hg or taking antihypertensive treatment), obesity (BMI ≥30 kg/m2, or diabetes (fasting glucose ≥126 mg/dL or taking medication for diabetes); low risk factor status was defined as not having hypertension, not being obese, and not having diabetes. The covariates used to define ‘high’ versus ‘low’ risk factor status (i.e., hypertension, obesity, and diabetes) were selected based on their significant associations with LV measures in the final regression models. To graphically illustrate the effect of risk factor burden on longitudinal tracking of LV measures, we plotted the mean value of each LV measure versus age for men and women in the high and low risk factor groups separately (where values were adjusted for specific combinations of risk factors according to their statistical significance in the final regression model produced for each LV measure). Median values of SBP and DBP among groups with and without hypertension were used in the regression equation to determine the graphically depicted mean outcome values for individuals with and without hypertension, respectively.
Secondary Analyses
In secondary analyses, we repeated all multivariable analyses after excluding individuals with Type 1 diabetes, given its predisposition for premature LV remodelling and dysfunction.14 In order to clarify the independent effects of obesity and diabetes, which often coexist, we compared the relation of BMI to LV indices in multivariable models with and without adjustment for diabetes. Since parameters of LV geometry, particularly LVWT and LVDD, are influenced by body size, we also repeated multivariable analyses of LVWT and LVDD using models adjusting for height and weight (instead of BMI). Multi-level regression analyses were additionally performed using volumetric assessments of cavity size, derived from direct M-mode measurements using the Teichholz method,12 including: LVEDV, LVESV, and LVEF.
All analyses were performed using SAS version 9.2, and a two-tailed P value of >0.05 was considered significant. S-PLUS and Excel were used to create the graphical displays.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
The baseline characteristics of our study sample are shown in Table 1.
Table 1.
Clinical and echocardiographic characteristics of the study samples used to characterize clinical correlates of longitudinal tracking of LV remodeling indices.*
| Men (N=1851) |
Women (N=2211) |
|
|---|---|---|
| Clinical Characteristics | ||
| Age, years | 45±10 | 45±10 |
| Body mass index, kg/m2 | 26.9±3.7 | 25.0±5.0 |
| Systolic blood pressure, mmHg | 126±16 | 119±17 |
| Diastolic blood pressure, mmHg | 81±9 | 75±9 |
| Pulse pressure, mmHg | 45±11 | 44±12 |
| Antihypertensive treatment, % | 10.0 | 8.9 |
| Hypertension, % | 27.8 | 18.5 |
| Smoking, % | 40.1 | 34.9 |
| Diabetes, % | 5.8 | 3.3 |
| Echocardiographic Characteristics | ||
| LV wall thickness, mm | 19.4±2.5 | 16.8±2.3 |
| LVDD, mm | 51.1±3.8 | 46.2±3.5 |
| LVDS, mm | 33.0±3.6 | 28.9±3.3 |
| Fractional shortening, % | 35.9±3.6 | 37.8±3.8 |
LV, left ventricular; LVDD, left ventricular diameter in diastole; LVDS, left ventricular diameter in systole.
Samples characteristics were obtained at the first attended examination cycle.
Patterns of Longitudinal Change in LV Structure and Function: Unadjusted
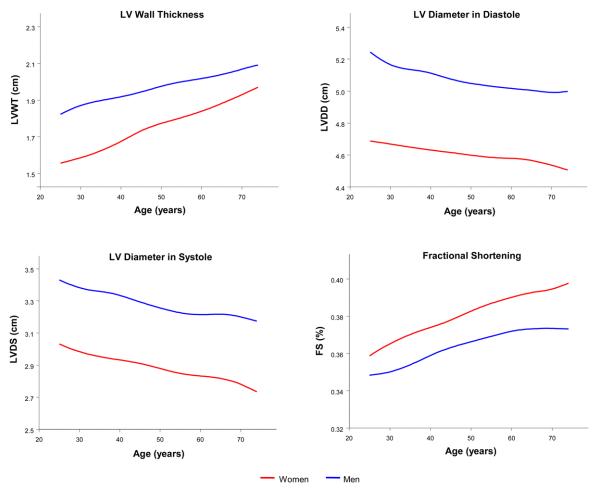
In both sexes, LV wall thickness progressively increased, both LVDD and LVDS decreased, whereas FS increased with advancing age (Figure 1).
Figure 1.
Unadjusted LV wall thickness, diameter in diastole, diameter in systole, and fractional shortening with increasing age for men and women.
Multivariable Clinical Correlates of Longitudinal Tracking of LV Structure and Function
LV wall thickness
Over the 16-year observation period, several clinical factors were positively related to progressively increasing LVWT: age, sex, BMI, SBP, DBP, antihypertensive treatment, and diabetes (Table 2). We observed statistically significant interactions of age with sex (P>0.0001), BMI (P=0.021), and diabetes (P=0.018). Women had a greater increase in LVWT with advancing age relative to men (Figure 1), as did participants with diabetes compared to those without diabetes (Table 2). Notably, LVWT increased more among individuals with higher compared to lower BMI. Similar to findings for LVWT, progressively increasing RWT was associated with age, BMI, DBP, antihypertensive treatment, diabetes, and also smoking (Supplement Table 1). As with LVWT, women had a greater increase in RWT with age relative to men (Supplement Figure 1).
Table 2.
Clinical correlates of longitudinal tracking of left ventricular wall thickness.
| Covariates | Coefficient | 95% CI |
|---|---|---|
| Age (10 year increase) * | — | — |
| men, with diabetes | 0.48 | (0.28, 0.67) |
| men, without diabetes | 0.25 | (0.18, 0.31) |
| women, with diabetes | 0.75 | (0.55, 0.95) |
| women, without diabetes | 0.52 | (0.46, 0.58) |
| Male Sex † | 1.73 | (1.63, 1.82) |
| BMI (5 kg/m2 increase) † | 0.58 | (0.53, 0.63) |
| SBP (10 mm Hg increase) | 0.11 | (0.08, 0.15) |
| DBP (10 mm Hg increase) | 0.12 | (0.07, 0.18) |
| Antihypertensive treatment | 0.28 | (0.16, 0.40) |
| Diabetes † | 0.21 | (0.00, 0.42) |
CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure. The coefficients represent the increase in left ventricular wall thickness in mm per increase in the continuous covariates (or presence versus absence of categorical covariates). All models also adjusted for examination cycle and significant interaction terms; any non-significant covariates were retained in the model if they contributed to a significant interaction term.
Given presence of a significant BMI interaction, coefficients are for individuals with a BMI of 25 kg/m2.
Given presence of a significant age interaction, coefficients are for individuals at age 50 years.
LV internal dimensions
Correlates of long-term tracking were similar for LVDD and LVDS, with positive associations being observed with BMI and inverse relations with age. Blood pressure measures were associated with LV diastolic dimensions (Table 3). Of note, LVDD was associated positively with SBP but inversely with DBP, with the coefficients being opposite in direction and of a similar magnitude, suggesting a potential pulse pressure effect. Accordingly, longitudinal tracking of LVDD was positively related with pulse pressure (Table 3).
Table 3.
Clinical correlates of longitudinal tracking of left ventricular diameter in diastole.
| Covariates | Coefficient | 95% CI |
|---|---|---|
| Age (10 year increase) | — | — |
| with diabetes | −0.11 | (−0.45, 023) |
| without diabetes | −0.60 | (−0.70, −0.50) |
| Male Sex * | 4.38 | (4.17, 4.58) |
| BMI (5 kg/m2 increase) | — | — |
| men | 0.83 | (0.66, 1.00) |
| women | 1.05 | (0.94, 1.17) |
| SBP (10 mm Hg increase) | 0.24 | (0.17, 0.30) |
| DBP (10 mm Hg increase) | −0.27 | (−0.38, −0.17) |
| Diabetes † | −0.31 | (−0.72, 0.10) |
CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure. The coefficients represent the increase in left ventricular diameter in diastole in mm per increase in the continuous covariates (or presence versus absence of categorical covariates). All models also adjusted for examination cycle and significant interaction terms; any non-significant covariates were retained in the model if they contributed to a significant interaction term. In a model that included pulse pressure instead of SBP and DBP, the coefficient of mm change in LVDD per 10 mm Hg increase in pulse pressure was 0.31 (95% CI 0.21, 0.40) for men and 0.19 (95% CI 0.12, 0.27) for women.
Given presence of a significant BMI interaction term, coefficients are for individuals with a BMI of 25 kg/m2.
Given presence of a significant age interaction term, coefficients are for individuals at age 50 years.
The association of age with both LVDD and LVDS varied with diabetes status, such that LV diameter decreased more in people without diabetes compared to participants with diabetes (Tables 3 and 4). For LVDD, there was an interaction between sex and BMI (P=0.03), indicating that the association of BMI with LVDD was of larger magnitude in women compared to men (Table 3). For LVDS, the interaction between age and antihypertensive treatment was also statistically significant (P=0.02): the age-associated decrease in LVDS was attenuated in individuals on antihypertensive treatment.
Table 4.
Clinical correlates of longitudinal tracking of left ventricular diameter in systole.
| Covariates | Coefficient | 95% CI |
|---|---|---|
| Age (10 year increase) | — | — |
| diabetes, on antihypertensive treatment | 0.09 | (−0.27, 0.46) |
| no diabetes, on antihypertensive treatment | −0.39 | (−0.61, −0.18) |
| diabetes, not on antihypertensive treatment | −0.17 | (−0.51, 0.18) |
| no diabetes, not on antihypertensive treatment | −0.66 | (−0.74, −0.57) |
| Male Sex | 3.69 | (3.51, 3.88) |
| BMI (5 kg/m2 increase) | 0.67 | (0.57, 0.76) |
| Antihypertensive treatment * | −0.18 | (−0.45, 0.08) |
| Diabetes * | −0.02 | (−0.42, 0.39) |
CI, confidence interval; BMI, body mass index. The coefficients represent the increase in left ventricular diameter in systole in mm per increase in the continuous covariates (or presence versus absence of categorical covariates). All models also adjusted for examination cycle and significant interaction terms; any non-significant covariates were retained in the model if they contributed to a significant interaction term.
Given presence of a significant age interaction, coefficients are for individuals at age 50 years.
Fractional shortening
Longitudinal correlates of FS included age, sex, SBP, DBP, and antihypertensive treatment (Table 5). Notably FS increased progressively with advancing age and FS values were consistently higher in women compared to men (Figure 1, Table 5). As with LVDD, FS was associated positively with SBP and negatively with DBP. Models incorporating pulse pressure confirmed a positive relation with pulse pressure (Table 5). Although overall tracking of FS was positively related to antihypertensive treatment, there was a statistically significant interaction between age and antihypertensive treatment (P=0.008), suggesting that FS increased less in individuals who were being treated for hypertension compared to those not being treated (Table 5).
Table 5.
Clinical correlates of longitudinal tracking of fractional shortening.
| Covariates | Coefficient | 95% CI |
|---|---|---|
| Age (10 year increase) | — | — |
| on antihypertensive treatment | 0.17 | (−0.12, 0.46) |
| not on antihypertensive treatment | 0.58 | (0.47, 0.69) |
| Male Sex | −1.71 | (−1.92, −1.50) |
| SBP (10 mm Hg increase) | 0.23 | (0.14, 0.31) |
| DBP (10 mm Hg increase) | −0.21 | (−0.35, −0.07) |
| Antihypertensive treatment * | 0.41 | (0.07, 0.76) |
CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure. The coefficients represent the increase in the fractional shortening in % per increase in the continuous covariates (or presence versus absence of categorical covariates). All models also adjusted for examination cycle and significant interaction terms; any non-significant covariates were retained in the model if they contributed to a significant interaction term. In a model that included pulse pressure instead of SBP and DBP, the coefficient of % change in fractional shortening per 10 mm Hg increase in pulse pressure was 0.23 (P<0.0001).
Given presence of a significant age interaction, coefficients are for individuals at age 50 years.
Association of Clinical Risk Status on Longitudinal Tracking of LV Structure and Function
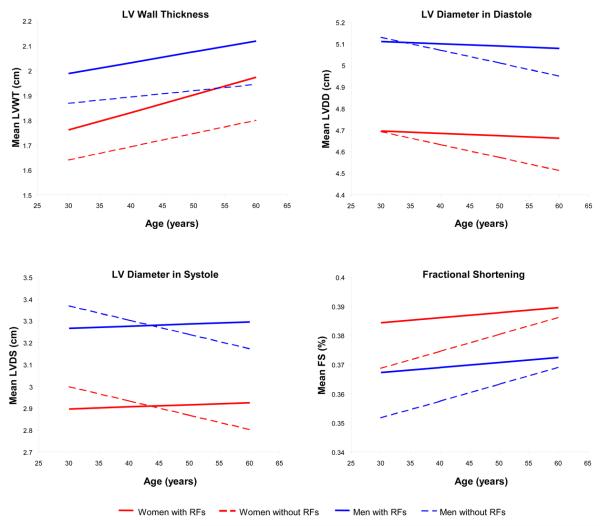
Consistent with the age interactions described above, longitudinal tracking of LV measures was not the same in individuals with hypertension, obesity, or diabetes compared to individuals without these risk factors (Figure 2, Supplement Figure 2). Both women and men in the higher clinical risk group demonstrated greater increases in LVWT and RWT with advancing age than their lower risk counterparts. Whereas LV dimensions decreased over time in the low-risk group, LVDD decreased to a much lesser extent and LVDS even slightly increased in the high-risk group. Accordingly, FS did not increase as much in high- compared to low-risk individuals with aging.
Figure 2.
Adjusted mean values of LV wall thickness, diameter in diastole, diameter in systole, and fractional shortening with increasing age are shown for men and women, with and without risk factor burden (i.e., hypertension, obesity, and diabetes).
Secondary Analyses
In analyses excluding individuals with Type 1 diabetes (N=3; 8 observations), results remained unchanged (data not shown). The association of BMI with indices of LV remodeling was similar in multivariable models with and without adjustment for diabetes (data not shown), and as reflected by results in the total sample. In multivariable analyses of LVWT and LVDD that included adjustment for height and weight as clinical covariates (instead of BMI), the direction and magnitude of associations between clinical covariates and LV structural indices were similar to those in analyses adjusting for BMI (Supplement Tables 2 and 3). In analyses of derived volumetric measures, the clinical covariates associated with longitudinal tracking of LVEDV were similar to those associated with LVDD; similarly, the clinical correlates of LVESV were similar to those of LVDS, as were those of LVEF to FS (Supplement Tables 4-6).
DISCUSSION
Although data from numerous cross-sectional studies have suggested that the human heart remodels over the life course, and likely in association with certain cardiovascular risk factors, a comprehensive longitudinal assessment of cardiac remodeling in the community has been limited. Lieb and colleagues recently reported on the correlates of longitudinal changes in LV mass in the Framingham cohort.10 Alterations in LV mass, a composite measure of LV morphology, represent the summation of multiple discrete aspects of LV remodeling – which can demonstrate either concordant or discordant trajectories of change over time. Therefore, in the present investigation, we extend prior work by using serial echocardiographic observations to evaluate longitudinal tracking of the components of LV mass (cavity dimensions and wall thickness) in addition to LV systolic function.
Features of Progressive LV Remodeling: Overview
We observed a distinct pattern of longitudinal tracking of LV structure and function over the adult life course in both sexes. First, LV wall thickness steadily increased with age, which is consistent with the higher frequency of LV hypertrophy seen among older adults in cross-sectional studies.4 Such an increase in LVWT may result from cardiomyocyte hypertrophy or apoptosis with replacement fibrosis or both.1,2 Second, we observed progressive, steady declines in both LVDS and LVDD with increasing age, suggesting an overall decrease in LV cavity size concurrent with the increase in LV wall thickness. These findings provide confirmatory longitudinal evidence of previously reported cross-sectional trends5 and provide key information on the longitudinal time course of such change. Smaller LV volumes in older individuals could contribute to the lower hemodynamic tolerance for a preload challenge seen in older compared to younger adults.15 Finally, we observed a progressive increase in FS with advancing age, which may be attributable to a greater decline in LVDS than LVDD. This finding is similar to the results of cross-sectional studies7,16 and may reflect a compensatory increase in myocardial contractility to maintain cardiac output in the face of decreasing LV volumes.
Interactions of Risk Factors with Age and the Impact on LV Remodeling
The association of age with measures of LV remodeling varied by sex and the presence of major clinical risk factors including hypertension, obesity, and diabetes. Although cross-sectional and autopsy studies have previously suggested that age-related LV remodeling differs between men and women,2,16,17 prior data regarding the impact of clinical risk factors have been scant.
Sex Differences in Cardiac Remodeling
Overall, men had greater LVWT than women at baseline and over the life course, but women experienced a greater age-associated increase in LVWT as noted above. The latter finding is consistent with the sexual dimorphism seen in a longitudinal analysis of LV mass, as recently reported by Lieb and colleagues.10 Accumulating evidence suggests that estrogens play an important role in LV remodeling, particularly since functional estrogen receptors have been shown to reside in the myocardium of both men and women;18 estrogen binding to these receptors can result in a variety of genomic and non-genomic effects that influence metabolic and vascular as well as intracellular pathways.19 Accordingly, the sex-related findings in this study may be related to the post-menopausal withdrawal of estrogen exerting either direct or indirect effects on myocardial remodeling.19,20 Additionally, an augmented pulsatile load in older women may induce a more pronounced cardiac remodeling response.21 Furthermore, LV hypertrophic remodeling has been noted to be more pronounced in women than men in pressure overload states,22 potentially due to differential gene expression of extracellular matrix components;23 sex-biased gene expression may similarly influence age-associated changes in LVWT.19
Impact of Body Mass Index
As with male sex, higher BMI was also directly associated with greater LVWT and larger LV dimensions over the long term. These findings are consistent with cross-sectional and autopsy studies indicating that obesity is associated with concentric as well as eccentric hypertrophy.24-26 Although obesity is typically associated with eccentric LV remodeling, concurrent LV wall thickness may be an earlier manifestation of remodeling, possibly related to hemodynamic, inflammatory, and neurohormonal effects of adiposity that are additive to the effects of coexistent hypertension.24-26 The association of BMI with LVDD was of greater magnitude in women compared to men. Prior cross-sectional studies have observed similar relations of BMI to LV cavity size in both men and women.26,27 With respect to the longitudinal effects of BMI on alterations in cardiac structure, it is possible that hormonal or other sex-based differences contribute to absolute increases in BMI having a relatively greater impact on LV remodeling in women versus men. Overall, the association of BMI with measures of cardiac structure is consistent with cross-sectional reports and may be related to mechanical, paracrine, and/or systemic effects of adiposity on LV remodeling.28 In addition, higher BMI may be associated with larger cardiac chamber dimensions due to the direct correlation of body size with total body volume.29,30 Notably, there was no significant association of BMI with systolic function, as reflected by FS.
Relations of Blood Pressure
Of note, the associations of blood pressure measures to LV measures were not completely concordant with the effects of aging on these measures. Higher blood pressure indices and the use of antihypertensive treatment (a marker of greater severity and chronicity of BP elevation) were associated with greater LVWT, which is similar to the aging effects on LVWT and not unlike the longitudinal effects of blood pressure on LV mass.10 Higher pulse pressure and use of antihypertensive treatment correlated with greater LVDD and LVDS, respectively. Also, individuals on antihypertensive treatment experienced a lesser decrease in LVDS with age and a lesser increase in FS, which is consistent with the hypothesis that LV mid-wall contractility progressively declines – prior to a detectable reduction in endocardial contractility – in the setting of chronic hypertension.31 Thus, although arterial stiffening is often considered a hallmark of cardiovascular aging,32 our data suggest that elevated pulsatile load is associated with longitudinal effects on LV dimensions that are opposite in direction to the independent effects of age per se. Although hypertension is recognized as primarily associated with concentric remodeling, prior studies have also reported eccentric hypertrophy coexisting with concentric remodeling in a large proportion of individuals with hypertension.33,34 The extent to which concentric versus eccentric hypertrophy develops in the setting of hypertension may depend on factors specific to the type as well as chronicity of hemodynamic load on the LV.33
Impact of Diabetes
The impact of diabetes on longitudinal LV remodeling was similarly discordant in terms of directionality of relations when compared to that of aging. Consistent with cross-sectional reports,35,36 the presence of diabetes was associated with greater LVWT but also a greater age-associated increase. The presence of diabetes, however, was associated with lesser decrements in both LVDD and LVDS with age. Nevertheless, there was no statistical significant relation of diabetes with longitudinal tracking of LV systolic function, as reflected by FS.
Taken together, several specific risk factors were associated with longitudinal changes in LV structure and function that were different in directionality compared to the effects of aging itself. This trend was most evident with respect to LV cavity dimensions, which progressively decreased with aging but were actually increased with higher BP and less likely to decrease in the setting of diabetes or hypertension treatment. Interestingly, these same risk factors have been reported in association with the presence of heart failure with preserved ejection fraction (HFPEF), a type of heart failure that occurs predominantly in later life.37-39 Furthermore, individuals with HFPEF are more likely to have greater LV end-diastolic volume compared to individuals of similar age range but without heart failure.37,38 Therefore, aging alone may be associated with a specific pattern of progressive LV remodeling that is altered by the early and chronic exposure to certain risk factors; such alterations in the typical course of LV remodeling may, in turn, contribute to the risk for overt heart failure in older age.
Limitations and Strengths
Several limitations of our investigation merit consideration. The change in echocardiographic instrumentation across examinations raises issues regarding comparability of serial measurements. For this reason, we adjusted for the ‘examination cycle’ as a covariate in all our analyses. In addition, the Framingham laboratory maintained a limited number of readers over serial echocardiographic examinations, and studies were subject to rigorous quality control with respect to image acquisition and adherence to the measurements protocol. As part of the quality control process at the sixth examination cycle, intra- and inter-reader correlations were assessed for LVDD (r>0.97 and r>0.96) and FS (r>0.90 and r>0.72) but not for LVWT or LVDS. Any differences in LV remodeling indices across examination cycles are likely to have resulted in random misclassification and therefore likely to bias our findings toward the null (i.e. result in a lack of associations). Doppler-based echocardiographic measures can provide important information about alterations in diastolic function, as well as volume and pressure load, that likely occur in the setting of cardiac remodeling. However, Doppler-derived measures were unavailable at the Framingham examination cycles that were included in the present study. Lastly, our study sample included predominantly middle-aged to elderly individuals of European ancestry. Thus, the generalizability of our findings to other age and racial/ethnic groups is unknown.
Notwithstanding these limitations, our study had several strengths. The present investigation included a large sample derived from a community-based cohort followed closely over two decades. This longitudinal design allowed the use of a multilevel modeling analysis, which facilitates the evaluation of serial echocardiographic measures and the analysis of progressive long-term alterations in LV remodeling indices.
Conclusions
Cardiac remodeling over the life course may be characterized by a distinct pattern of progressive changes in the structure and function of the LV. These changes include LV wall thickening, shrinking cavity dimensions, and increased fractional shortening. The presence of certain risk factors in midlife, including hypertension and diabetes, can serve to alter this typical pattern of LV remodeling. Further research is needed to investigate how such alterations in the course of LV remodeling may contribute to the risk for common cardiovascular diseases, particularly heart failure, in later life.
Clinical Summary.
The human heart undergoes dynamic, incremental alterations in structure and function over the lifespan, a phenomenon referred to as cardiac remodeling. Examining course and correlates of cardiac remodeling in aging adults is critical for elucidating the pathways by which older age predisposes to cardiac dysfunction and, particularly, heart failure in the setting of a preserved ejection fraction. Using longitudinal data collected from participants in the Framingham Offspring Study (up to 4 serial echocardiographic observations per individual, totaling 11,485 observations) and multilevel statistical modeling, we observed that left ventricular (LV) cavity dimensions (end-systolic more than end-diastolic) decreased with advancing age, whereas LV wall thickness and fractional shortening increased. Women and individuals with diabetes experienced greater age-associated increases in LV wall thickness. However, the presence of diabetes or a higher blood pressure level was associated with a lesser decrease in LV dimensions with older age. Similarly, treatment with antihypertensive medication was a marker of an attenuated increase in fractional shortening with aging. Together, these findings indicate that cardiac remodeling over the adult life course is characterized by a distinct pattern of increasing LV wall thickness, decreasing LV dimensions, and increasing fractional shortening. Notably, female sex, greater blood pressure load, and presence of diabetes serve to attenuate this remodeling pattern. Overall, these observations suggest a mechanism by which women with hypertension and individuals with diabetes may be particularly predisposed to heart failure with a preserved ejection fraction in later life.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the Ellison Medical Foundation (SC) and the National Heart, Lung and Blood Institute's Framingham Heart Study (Contract No. N01-HC-25195) and the following grants: 6R01-NS 17950, R01HL080124 (RSV).
Footnotes
From the Framingham Heart Study of the National Heart, Lung, and Blood Institute of the National Institutes of Health and Boston University School of Medicine.
Conflicts of Interest/Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–8. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 2.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995;26:1068–79. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy D. Left ventricular hypertrophy. Epidemiological insights from the Framingham Heart Study. Drugs. 1988;35(Suppl 5):1–5. doi: 10.2165/00003495-198800355-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes. Circulation: Cardiovascular Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 7.Nikitin NP, Loh PH, de Silva R, Witte KK, Lukaschuk EI, Parker A, Farnsworth TA, Alamgir FM, Clark AL, Cleland JG. Left ventricular morphology, global and longitudinal function in normal older individuals: a cardiac magnetic resonance study. Int J Cardiol. 2006;108:76–83. doi: 10.1016/j.ijcard.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–7. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 10.Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085–92. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–92. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 13.Leyland AH, Goldstein H. Multilevel Modeling of Health Statistics. John Wiley & Sons, Ltd; West Sussex: 2001. [Google Scholar]
- 14.Lo SS, Leslie RD, Sutton MS. Effects of type 1 diabetes mellitus on cardiac function: a study of monozygotic twins. Br Heart J. 1995;73:450–5. doi: 10.1136/hrt.73.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, Greenberg NL, Levine BD, Thomas JD. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol. 2006;290:H1454–9. doi: 10.1152/ajpheart.00902.2005. [DOI] [PubMed] [Google Scholar]
- 16.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–8. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–46. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R, Regitz-Zagrosek V. Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J. 2006;20:926–34. doi: 10.1096/fj.05-5148com. [DOI] [PubMed] [Google Scholar]
- 19.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex-Related Differences in Myocardial Remodeling. J Am Coll Cardiol. 2010;55:1057–1065. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res. 2003;57:388–94. doi: 10.1016/s0008-6363(02)00705-8. [DOI] [PubMed] [Google Scholar]
- 21.Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30:1863–71. doi: 10.1016/s0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 22.Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K, Devereux RB. Gender differences in left ventricular structure and function during antihypertensive treatment: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51:1109–14. doi: 10.1161/HYPERTENSIONAHA.107.107474. [DOI] [PubMed] [Google Scholar]
- 23.Villar AV, Llano M, Cobo M, Exposito V, Merino R, Martin-Duran R, Hurle MA, Nistal JF. Gender differences of echocardiographic and gene expression patterns in human pressure overload left ventricular hypertrophy. J Mol Cell Cardiol. 2009;46:526–35. doi: 10.1016/j.yjmcc.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–6. [PubMed] [Google Scholar]
- 25.Reisin E, Jack AV. Obesity and hypertension: mechanisms, cardio-renal consequences, and therapeutic approaches. Med Clin North Am. 2009;93:733–51. doi: 10.1016/j.mcna.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The Impact of Obesity on the Left Ventricle The Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorbala S, Crugnale S, Yang D, Di Carli MF. Effect of body mass index on left ventricular cavity size and ejection fraction. Am J Cardiol. 2006;97:725–9. doi: 10.1016/j.amjcard.2005.09.122. [DOI] [PubMed] [Google Scholar]
- 28.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, D'Agostino RB, Sr., O'Donnell CJ, Manning WJ. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–91. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation. 1981;64:477–82. doi: 10.1161/01.cir.64.3.477. [DOI] [PubMed] [Google Scholar]
- 30.Alexander JK, Dennis EW, Smith WG, Amad KH, Duncan WC, Austin RC. Blood volume, cardiac output, and distribution of systemic blood flow in extreme obesity. Cardiovasc Res Cent Bull. 1962;1:39–44. [PubMed] [Google Scholar]
- 31.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–8. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 33.Bella JN, Wachtell K, Palmieri V, Liebson PR, Gerdts E, Ylitalo A, Koren MJ, Pedersen OL, Rokkedal J, Dahlof B, Roman MJ, Devereux RB. Relation of left ventricular geometry and function to systemic hemodynamics in hypertension: the LIFE Study. Losartan Intervention For Endpoint Reduction in Hypertension Study. J Hypertens. 2001;19:127–34. doi: 10.1097/00004872-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Devereux RB, Bella J, Boman K, Gerdts E, Nieminen MS, Rokkedal J, Papademetriou V, Wachtell K, Wright J, Paranicas M, Okin PM, Roman MJ, Smith G, Dahlof B. Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE Study. Blood Press. 2001;10:74–82. doi: 10.1080/08037050152112050. [DOI] [PubMed] [Google Scholar]
- 35.Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 36.Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, Kitzman DW, Hopkins PN, Morgan D, Rao DC, Devereux RB. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102–7. doi: 10.1161/01.cir.103.1.102. [DOI] [PubMed] [Google Scholar]
- 37.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;49:972–81. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 38.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 39.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–7. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.