Abstract
Methylphenidate (MPH) is an amphetamine derivative widely prescribed for the treatment of attention deficit–hyperactivity disorder. Recent concern over its genotoxic potential in children [11] spurred a study on the effects of chronic MPH treatment in a nonhuman primate population and the studies reported here were conducted in conjunction with that study in the same animals. Here, the focus was on the ability of juvenile rhesus monkeys to learn how to perform a battery of operant behavioral tasks while being treated chronically with MPH. Performance of the National Center for Toxicological Research (NCTR) Operant Test Battery (OTB) was used to quantify the learning of tasks thought to model specific aspects of cognitive function including learning, motivation, color and position discrimination, and short-term memory. The OTB tasks designed to assess these specific behaviors included Incremental Repeated Acquisition (IRA), Progressive Ratio (PR), Conditioned Position Responding (CPR), and Delayed Matching-to-Sample (DMTS), respectively. Juvenile males (n = 10/group) pressed levers and press-plates for banana-flavored food pellets. Subjects were treated orally, twice a day, five days per week (M–F) for 66 weeks with escalating doses (0.15 mg/kg initially, increased to 2.5 mg/kg for the low dose group and to 12.5 mg/kg for the high dose group) and tested in OTB tasks 30 to 60 min after the morning dose. The findings indicate that MPH at doses up to 2.5 mg/kg twice per day were well tolerated (performance was no different than controls) but at doses of 12.5 mg/kg twice per day there was a significant decrement in OTB performance, characterized by decreases in both percent task completed and response rates for all tasks. These effects of MPH seem primarily due to decreases in motivation to perform for food, which is not surprising given the well known appetite suppressing effects of amphetamine-like stimulants. Thus, the current data do not strongly suggest cognitive impairments following chronic MPH administration.
Keywords: Methylphenidate, Juvenile, Operant, Learning, Memory, Attention
1. Introduction
The chronic administration of methylphenidate (MPH) in the pediatric population for attention deficit-hyperactivity disorder (ADHD) is prevalent in today's society. MPH acts mainly by blocking the dopamine transporter [40] to facilitate an increase in extracellular dopamine [40]. This neurochemical effect is believed to compensate for a deficiency in extracellular dopamine concentration [41] which acts on either pre- [13] or post-synaptic dopamine receptors [5]. However, the long-term effects of this psychostimulant remain unknown [38]. Behavioral outcomes for subjects receiving MPH over extended periods of time are equivocal [14] and its long-term administration may have effects on systems other than the CNS including the cardiovascular system [42] or may exacerbate undiagnosed conditions such as Tourette's syndrome [37]. High doses produce effects opposite to those intended and include agitation, hallucinations, psychosis, lethargy, seizures, tachycardia, dysrhythmias, hypertension, and hyperthermia [16]. Bedford et al. [4] have shown that high doses of MPH produce stereotypic behaviors in monkeys, such as increased vocalization and self-grooming and intense idiosyncratic behavior, while others have reported adverse side effects in children [3,12] but positive behavioral and cognitive effects at lower doses (for review see [1,39]).
In addition to its noted behavioral effects, MPH was recently reported to increase metrics of mutagenesis in a population of pediatric patients with ADHD treated with MPH over a period of 3 months [11]. Therefore, studies were initiated to rigorously explore the genotoxic effects of chronic MPH treatment in a primate model (findings to be reported elsewhere [22]) and, in conjunction we sought to determine the concomitant behavioral effects. For this effort the National Center for Toxicological Research's (NCTR) Operant Test Battery (OTB) was employed to assess cognitive function in adolescent monkeys. The doses of MPH administered were adjusted to attain clinically relevant plasma levels (low dose group) and plasma levels well beyond (high dose group).
The OTB has been used extensively to assess aspects of cognitive function in both primates and children [8,30,31] and to monitor both acute and chronic drug effects in monkeys [19,25,26]. The tasks and the particular brain functions they are thought to model include the: Incremental Repeated Acquisition task (IRA), learning; Progressive Ratio task (PR), motivation; Conditioned Position Responding task (CPR), color and position discrimination; Delayed Matching-To-Sample task (DMTS), short-term memory. Children and well-trained rhesus monkeys perform similarly on OTB tasks [27,25,26]. This is particularly important when extrapolating the neurobehavioral effects of drugs and toxicants from monkeys to humans. Additionally, the demonstration that several measures of OTB performance correlate significantly with measures of intelligence in children serves to highlight the human relevance of such measures in animals [29,7]. Here, OTB training required that subjects perform to a certain criteria at each level of training before moving on to the next training level (Table 1). The age of the animals used in the present study was chosen to model those comparable to pediatric patients.
Table 1.
Operant test battery training schedule.
| Task | Session time (min) | Number of reinforcements | Points earned | Cumulative points |
|---|---|---|---|---|
| TIRA level 1 | 50 | 40 | 1 | 1 |
| TIRA level 2 | 50 | 40 | 1 | 2 |
| TIRA level 3 | 50 | 40 | 1 | 3 |
| TIRA level 4 | 50 | 40 | 1 | 4 |
| IRA | 50 | 120 | 1 | 5 |
| TCP level 1 | 60 | 100 | 1 | 6 |
| TCP level 2 | 60 | 100 | 1 | 7 |
| TCP level 3 | 60 | 100 | 1 | 8 |
| TCP level 4 | 60 | 100 | 1 | 9 |
| TCP level 5 | 60 | 1000 | 5(1 pt. per 200 reinforcements) | 10–14 |
| CPR | 10 | 60 | 1 | 15 |
| TMTS level 1 | 50 | 100 | 1 | 16 |
| TMTS level 2 | 50 | 100 | 1 | 17 |
| TMTS level 3 | 50 | 100 | 1 | 18 |
| TMTS level 4 | 50 | 100 | 1 | 19 |
| TMTS level 5 | 50 | 1000 | 5(1 pt. per 200 reinforcements) | 20–24 |
| DMTS 1st delay set | 50 | 120 | 1 | 25 |
| TPR | 10 | 100 | 1 | 26 |
| PR | 10 | 100 | 1 | 27 |
| DMTS delay sets 2–10 | 50 | 120 | 1 per delay set | 28–36 |
This table shows the OTB training paradigm including task and training level, session length, number of possible reinforcers available per training level, training score points earned per level and cumulative training score (cumulative points). The day following completion of TIRA level 4, the full IRA task commenced. Following three IRA sessions, the TCP task was added to the training regimen on the next test day. Once subjects had completed the TCP task at level 5, the full CPR task began and tasks began alternating each test day such that on one test day the IRA task was presented and the next test day the CPR task was presented. After 1000 CPR reinforcers had been earned, the CPR task session length was reduced from 60 to 10 min and it was paired with TMTS task (50 min) which it preceded. At that point the schedule was IRA on one test day and CPR/ TMTS on the next test day. Following completion of TMTS at level 5, the DMTS task began. At this point the schedule was IRA on one test day and CPR/DMTS on the next test day. The day following the pairing of DMTS with CPR, the TPR task was introduced with and preceding the IRA task. Final OTB task administration was PR/IRA tasks on one test day and CPR/DMTS on the following test day.
2. Methods
2.1. Subjects
The experimental subjects were 30 male rhesus monkeys, approximately two years old at the beginning of the experiment, an age approximately equivalent to six-to-eight-year old children. The animals were randomly assigned (10/group) to either the vehicle control, low dose or high dose groups and housed individually. Subjects were removed from their home cages and placed into restraint/transport chairs (Primate Products, Miami, FL) for subsequent daily weighing, dosing, behavioral testing and blood sampling as needed. Standard nonhuman primate chow (Purina Mills, Richmond, IN) was given once a day following behavioral sessions (M–F) and on weekends at approximately the same time of day (3–4 p.m.) and adjusted weekly to insure that animals gained approximately 0.1 kg per month. Fresh fruit and multivitamins (USA Drug, Little Rock, AR) were provided 5 days a week and water was available ad libitum in the home cages.
2.2. Treatment and testing
All mg/kg doses were administered twice a day, i.e. 2.5 mg/kg× 2 per day for the low dose group. Subjects were dosed orally twice daily (separated by 4 h) in four groups (1st group at 8 a.m. and 12 p.m.; 2nd group at 9 a.m. and 1 p.m.; 3rd group at 10 a.m. and 2 p.m.; 4th group at 11 a.m. and 3 p.m.) five days per week (M–F) for 66 weeks. OTB sessions commenced on the first day of MPH dosing, 30 to 60 min following the morning treatment. Exposure assessments (plasma level determinations) were carried out for MPH and its major metabolite, ritalinic acid (RA) (Table 2), according to the method of Doerge et al. [10]. Monkeys were administered MPH orally at the indicated doses and after a week at a given dose blood samples were drawn 45 min later to obtain an approximation of maximal plasma levels (Cmax). The 45 min collection time was based on a previous dose range-finding study. At week 23 dose escalation (Table 2) was necessary because MPH plasma levels were not at the expected clinical range for the low dose group or between 5 and 10 times higher for the high dose group. Dose escalation occurred as follows: 0.15 mg/kg was increased to 2.5 mg/kg from weeks 23 to 31 for the low dose group and 1.5 mg/kg was increased to 12.5 mg/kg from weeks 23 to 27 for the high dose group. Thus, dose escalation occurred concurrent with OTB training/testing before a stable target dose was established. However, only those subjects that had reached a particular level of OTB performance prior to the week 23 dose escalation were included in the statistical analysis for given tasks.
Table 2.
Timetable of dose escalation and plasma levels of MPH and RA in ng/ml.
| Weeks | Low MPH dose (mg/kg) | High MPH dose (mg/kg) | Low MPH dose plasma concentration (ng/ml) | High MPH dose plasma concentration (ng/ml) | Low MPH dose ritalinic acid plasma concentration (ng/ml) | High MPH dose ritalinic acid plasma concentration (ng/ml) |
|---|---|---|---|---|---|---|
| 0 | 0.00 | 0.00 | 0.02±0.0 | 0.0±0 | 0.0±0 | 0.02±0.02 |
| 3 | 0.15 | 0.30 | 0.31±0.06 | 0.56±0.05 | 15.88±2.73 | 44.620±9.91 |
| 5 | 0.15 | 0.30 | 0.31±0.07 | 0.92±0.21 | 20.99±3.45 | 48.46±10.23 |
| 7 | 0.15 | 0.75 | 0.32±0.02 | 1.46±0.19 | 26.72±3.69 | 112.37±19.06 |
| 8 | 0.15 | 0.75 | 0.19±0.02 | 2.03±0.39 | 23.48±4.26 | 222.14±52.43 |
| 9 | 0.15 | 1.50 | 0.23±0.05 | 2.94±0.44 | 21.77±4.20 | 254.98±72.12 |
| 12 | 0.15 | 1.50 | 0.26±0.04 | 3.13±0.47 | 16.29±1.85 | 152.52±35.20 |
| 16 | 0.15 | 1.50 | 0.19±0.02 | 2.07±0.38 | 17.50±3.38 | 127.27±29.57 |
| 20 | 0.15 | 1.50 | 0.49±0.05 | 7.23±1.92 | 37.36±5.79 | 236.31±44.37 |
| 23 | 0.30 | 3.00 | 1.27±0.51 | 11.02±3.19 | 73.10±13.51 | 482.96±67.82 |
| 24 | 0.30 | 3.00 | Ø | 68.41±37.22 | Ø | 575.89±56.14 |
| 26 | 0.60 | 6.00 | 1.72±0.16 | 49.84±16.63 | 155.21±35.99 | 725.98±76.98 |
| 28 | 1.25 | 12.50 | 3.74±0.44 | 51.93±12.19 | 337.98±50.51 | 716.32±74.97 |
| 32 | 2.50 | 12.50 | 13.61±6.56 | 90.86±33.39 | 219.95±59.54 | 891.06±133.31 |
| 36 | 2.50 | 12.50 | 4.24±0.49 | 40.84±14.84 | 249.28±42.80 | 633.83±89.43 |
| 40 | 2.50 | 12.50 | 6.70±1.09 | 97.76±38.60 | 250.54±43.97 | 813.32±114.74 |
| 44 | 2.50 | 12.50 | 6.59±1.24 | 102.16±47.21 | 127.75±26.56 | 648.29±105.60 |
| 56 | 2.50 | 12.50 | 3.46±0.25 | 134.55±68.29 | 159.08±37.02 | 787.17±120.53 |
All mg/kg doses were administered twice daily. Group averages and standard errors of the means are shown normalized to vehicle group measurements. Exposure assessment was performed during the week indicated by analyzing plasma collected 45 min after administration of the first dose of the day one week after a dose change. The limits of quantification (LOQ) were approximately 0.1 ng/ml for MPH and 0.03 ng/ml for RA and the limits of detection (LOD) were approximately 0.03 and 0.009 ng/ml, respectively. The Ø symbol denotes that levels were not determined.
Dextro-levo-Methylphenidate hydrochloride (Mallinckrodt Chemicals, St. Louis, MO, USA) was suspended in an orange flavored drink (Prang, Bio-Serv, Frenchtown, NJ) which also served as the vehicle for the control group. Prang solution was composed of 75 g Prang powder dissolved in 1500 mL of Millipore filtered water. Low dose MPH (2.5 mg/kg) was concentrated in a 5 mg/ml Prang solution and the dose volume was 0.5 ml/kg. High dose MPH (12.5 mg/kg) was concentrated in a 25 mg/ml Prang solution and the dose volume was 0.5 ml/kg.
All behavioral tasks were conducted in primate operant test chambers using behavior panels that included press-plates and response levers (described in [35,36]). Banana-flavored food pellets (190 mg, Bio-Serv, Frenchtown, NJ) served as positive reinforcers. Subjects were rotated through eight identical behavior chambers such that no animal was placed in the same chamber for two consecutive test days in order to prevent chamber preference and for all subjects to equally experience any possible confound associated with a particular chamber. Each operant task was preceded by its own training component i.e. Training Incremental Repeated Acquisition (TIRA) for the IRA task and Training Conditioned Position Responding (TCPR) for the CPR task (see Table 1 for OTB training procedure). Following the successful training of one task, training for a new task was added to the testing schedule. Initially, subjects were trained to press levers in a Training Incremental Repeated Acquisition (TIRA) task. Once subjects had completed 3 sessions performing the IRA task, Training Conditioned Position Responding (TCPR) was added to the testing schedule on alternate test days and continued until criteria were met for the CPR task. At this point in training the Training Delayed Matching-To-Sample (TMTS) was added to the testing schedule and continued until criteria were met for the DMTS task, at which time the Training Progressive Ratio (TPR) task was added and continued until criteria were met for the PR task (see Table 1). Behavioral schedules alternated daily such that the final test schedule was: PR (motivation) task (10 min) preceded the IRA (learning) task (50 min) on one test day and the CPR (color and position discrimination) task (10 min) preceded the DMTS (short-term memory) task (50 min) on the next test day.
2.2.1. Training IRA
The goal of the IRA task was to test the subjects' ability to learn a particular lever sequence which changed each test session. During TIRA animals progressed through four training levels using the method of successive approximation. Beginning with level 1, a single press on any one of four extended response levers resulted in reinforcement delivery into a pellet trough. One response lever was randomly deactivated at each training level until only one lever remained active at level 4. Each training level required the attainment of 40 reinforcers. (Presses on deactivated levers had no programmed consequences). This training was generally completed in a few sessions. If subjects did not complete TIRA in one session, they began the next training session at the level at which they stopped on the previous day.
2.2.1.1. The IRA task
Following the completion of TIRA, the `full' IRA task (described in detail in [35,36]) was conducted as follows: each test session began at IRA component 1 (IRA1) during which only one (randomly chosen) of the four responses levers was correct. Responses to this lever resulted in the delivery of a reinforcer and illumination of a `correct response' indicator light after which the subject could continue the task; incorrect responses (pressing on any of one the three incorrect levers) resulted in illumination of an `incorrect response' indicator light and presentation of the same lever-pressing `problem.' After criterion performance at IRA1 (20 reinforcers), a 30 second time-out followed after which the task difficulty was `incremented' to a 2-lever sequence (IRA2). Here, a press to a lever that was different from the correct lever at IRA1 was required, followed by responding to the lever correct during IRA1; thus, subjects had to add a response element (lever press) to the previous lever `sequence'. Errors did not `reset' the response requirement: error correction was permitted. After criterion performance at IRA2 (completion of 20 errorless sequences — not necessarily consecutive — the criteria to advance for all subsequent IRA levels), a 30 second time-out followed after which the task was increment to a three lever sequence (IRA3) and so on up to a six lever sequence (IRA6) or the task timed out. Thus within each IRA session task difficulty increased from a 1 lever sequence (IRA1) to a 6 lever sequence (IRA6) depending on the subjects' mastery of the task. After three consecutive IRA sessions (50 min/session maximum) during which subjects completed IRA1, subjects began performing a Training Conditioned Position Responding (TCPR) task on alternate test days. The dependent measures assessed for the IRA task were percent task completed (PTC), response rate (RR), and accuracy (ACC).
2.2.2. Training Conditioned Position Responding (TCPR) and CPR task
The CPR task assessed color and position discrimination. At TCPR level 1 all three press-plates were illuminated with one of four colors (red, yellow, green or blue) and a press to any of them resulted in reinforcer delivery. At level 2 only two of the three press-plates were illuminated (randomly, but with the same color) and a press to either of them resulted in reinforcer delivery. Responses to darkened press-plates had no programmed consequences. At level 3, the center press-plate was initially illuminated with one of the four colors, selected randomly and presses to it resulted in its immediate extinction and the simultaneous illumination of a specific side plate with the same color (left = red or yellow; right = blue or green). A response to the illuminated side key resulted in reinforcer delivery. At level 4, the center press-plate was initially illuminated with one of the four colors, presented randomly and a single press to it resulted in its immediate extinction and the simultaneous illumination of a single side-press plate. If the center press-plate color had been red or yellow, the left press-plate was illuminated white. If the center press-plate had been blue or green, then the right press-plate was illuminated white. A single response to the correct (illuminated) press-plate resulted in reinforcer delivery. At level 5, the center press-plate was randomly illuminated with one of the four colors and, in contrast to level 4, a press to the center press-plate resulted in its immediate extinction and the simultaneous illumination of both side keys, white. A response to the left press-plate was reinforced only if the center color had been either red or yellow and a response to the right press-plate was reinforced only if the center color had been either blue or green. Responding to the incorrect side press-plate resulted in a 10-second time out (all press-plates dark) and re-presentation of the same problem (color) on the center press-plate. The same `problem' was presented to the subject until it was solved correctly, after which the center press-plate was randomly illuminated (new problem) with one of the four colors. If subjects did not complete a level in a given session, they began the next TCPR session at the level at which they stopped on the previous TCPR session, and proceeded until 100 reinforcement pellets were delivered. (Note that TCPR sessions alternated with the IRA task between days and thus the TCPR task was not presented on consecutive days.) After subjects earned 1000 reinforcers at TCPR level 5, the `full' CPR task was presented in which the problems (initial colors) were presented randomly each trial, regardless of whether the previous trial had been completed correctly or not and the maximum number of reinforcers obtainable was capped at 100 per session. The final CPR task required subjects to make observing responses to the initial red, yellow, blue or green cue color and then chose the left press-plate after the presentation of either a red or yellow cue or the right press-plate after the presentation of either a blue or green cue. After the subjects had earned 1000 reinforcers under the full CPR schedule, the CPR session length was reduced from 60 to 10 min and the maximum number of reinforcers obtainable was decreased to 60. The Training Matching-to-Sample (TMTS, 50 min) task was added at this time, 1 min following the CPR task. The dependent measures assessed for the CPR task were percent task completed (PTC), response rate (RR) and accuracy (ACC).
2.2.3. Training Matching-to-Sample (TMTS)
TMTS (50 min sessions) also consisted of five levels. Subjects had to earn 100 reinforcers for each of the first four levels and 1000 at fifth level. If subjects did not complete TMTS in a given session, they began the next TMTS training session at the level at which they stopped during the previous TMTS training session. At TMTS level 1 all three press-plates were illuminated with an identical white-on-black geometric symbol chosen randomly from seven possibilities: square, circle, triangle, plus sign, minus sign, vertical line, or an `X'. A press to any of the illuminated press-plates resulted in reinforcer delivery. At level 2, the center press-plate and one of the side press-plates (chosen randomly) were illuminated with the same symbol (selected randomly). A press to either of the illuminated press-plates resulted in reinforcer delivery. Responses to darkened press-plates had no programmed consequences. At level 3 only the center press-plate was illuminated at the beginning of each trial and presses to it resulted in its immediate extinction and the simultaneous illumination of one of the three press-plates (location was random) with the same symbol. A response to this illuminated press-plate resulted in reinforcer delivery. At level 4 only the center press-plate was illuminated at the beginning of each trial and a single response to it resulted in its immediate extinction and the simultaneous illumination of two press-plates, one with a matching symbol and the other with a non-matching symbol. A single response to the press-plate illuminated with the `matching' symbol resulted in reinforcer delivery and presentation of a new matching problem whereas a response to the non-matching symbol resulted in a 10-sec time out followed by re-presentation of the same matching problem. At level 5 the center press-plate was illuminated as in level 4 and responses to it resulted in its immediate extinction and simultaneous illumination of all three press-plates, one with the `matching' symbol, the others with non-matching symbols. Responses to the matching symbol resulted in reinforcer delivery and presentation of the next trial whereas responses to incorrect symbols were followed by a 10 second time-out during which all press-plates were dark and after which another trial using the same symbol was presented (i.e., the same problem was presented). After 1000 reinforcers had been obtained at TMTS level 5, the `full' Delayed Matching-to-Sample (DMTS) task (50 min) was presented in which the maximum number of reinforcers obtainable per session was 120. The difference between TMTS level 5 and the DMTS task is that the problems following choice responses were all presented randomly, regardless of whether the choices had been correct or incorrect and the addition of the recall delay intervals following the sample stimulus presentation.
2.2.4. Delayed Matching-to-Sample (DMTS)
The DMTS task assessed short-term memory by testing the subjects' ability to match a choice stimulus with the preceding sample stimulus. Thus, if the sample stimulus for a given trial was a triangle, the subject had to choose the triangle from the three presented choice stimuli, only one of which would be the triangle. At `full' DMTS, six different recall delays (chosen from specific delay sets) were utilized. Delay values were chosen from these sets pseudo randomly and interposed after initial (observing) responses were made to the sample stimuli: no more than 20 reinforcers were allowed for each of the six delay values. Initially, the six delay values were all set to 0 s (delay set 1). When overall choice accuracy (i.e., collapsed across all delay values) at this delay configuration was 60% or greater for three consecutive DMTS sessions during which at least 50 trials were completed, the 2nd delay set was used: (0, 0, 0, 1, 1, and 1=three values were set to 0 s and three to 1 s). In this manner, the following time delay sets (ten sets total) were incorporated (in the following order) into the DMTS task as subjects demonstrated criterion performance: 0, 0, 1, 1, 2, and 2 s; 0, 1, 1, 2, 2, and 4 s; 0, 1, 1, 2, 4, and 8 s; 0, 1, 2, 4, 8, and 16 s; 0, 2, 4, 8, 16, and 32 s; 0, 4, 8, 16, 32, and 48 s; 0, 8, 16, 32, 48, and 64 s; 0, 16, 32, 48, 64, and 80 s. The dependent measures assessed for the DMTS task were percent task completed (PTC), response rate (RR), and accuracy (ACC). The DMTS task was presented 1 min after the CPR task. Following the first DMTS session, Training Progressive Ratio (TPR, 10 min) was paired with and preceded the IRA task on the next test day.
2.2.5. Training Progressive Ratio (TPR) and PR
The PR task was used to assess aspects of appetitive motivation. During performance of TPR (designated PR 1+0) each lever press (right lever) resulted in reinforcer delivery (continuous reinforcement or CRF). After 100 reinforcers were obtained at TPR, subjects were presented the `full' PR schedule (designated PR 1+1) in which the first reinforcer of each session was obtained after a single lever press and the response requirement for each subsequent reinforcer was increased by one. Thus, the second reinforcer required 2 lever presses, the third reinforcer required three lever presses, and so on until the maximum number of reinforcers (100) had been earned or the session timed out (10 min). TPR/PR sessions were combined with IRA sessions such that TPR/PR was presented first, followed 1 min later by IRA (50 min). The dependent measures for the PR task were PTC and RR.
2.2.6. OTB dependent variables
The final test schedule was PR/IRA on one test day followed by CPR/DMTS the next test day. PTC was determined by the formula: (# reinforcers earned/# reinforcers possible)×100. RR was determined by: total number of responses made/total running time (i.e., minus timeouts) in seconds. ACC was determined by: (# of correct choices/# total choices)×100.
2.2.6.1. Cumulative training scores
Training levels were given scores as follows (Table 1). TIRA training level 1=score of 1, level 2=score of 2, level 3=3, and level 4=4. When animals met criteria for the full IRA task, the cumulative training score was 5. TCP training scores were similar: level 1=a score of 1, level 2=a score of 2, level 3=3, and level 4=4. Combining these with the TIRA/IRA training scores thus yields cumulative training scores of 6, 7, 8, and 9, respectively. TCP level 5 was assigned an additional 5 scores, 1 for each 200 reinforcers earned. Thus, subjects were given a score of 1 while performing for the first 200 reinforcers (out of 1000), 2 for performing for the second 200, 3 for the third 200, 4 for the fourth 200 and 5 for the last 200. Thus, when subjects were performing at TCP level 5, their cumulative training scores were 10, 11, 12, 13 and 14 and when they began performing full CPR, their cumulative training score was 15. TMTS training scores were also similar: level 1=score of 1, level 2=score of 2, level 3=3, and level 4=4. Thus, the cumulative score for TMTS up through level 4 is 19. At TMTS training level 5, there were an additional 5 scores assigned as for TCP level 5. A score of one was given after the first 200 reinforcers, a score of 2 was given for the second 200, 3 for the third 200, 4 for the fourth 200 and 5 for the last 200. Thus, when subjects were performing at TMTS level 5, their cumulative training score was 24 and when they began performing full DMTS (all 6 recall delays set at 0 s), their cumulative training score was 25. For the TPR/PR tasks, a score of 1 was given for the first 100 reinforcers earned and at full PR the score given was 2, making the cumulative training score 27. At the second DMTS delay set (0, 0, 0, 1, 1, and 1 s), the cumulative score was=28 and the score was incremented by one for the addition of each of the eight subsequent delay sets (see Section 2.2.4) to a maximum cumulative training score of 36.
2.3. Statistical analysis
Statistical analysis of overall performance in the OTB was assessed using a mixed procedure, type 3 fixed effect tests for Overall Score and DMTS recall delay sets (SAS software). Analysis of task endpoints (PTC, ACC, RR,) were assessed using a repeated measures model fit by use of a mixed procedure in the SAS system. The model included terms for Dose and Week and their interaction. The covariance structure on the repeated observations within each animal that was fit was first-order autoregressive.
3. Results
3.1. Methylphenidate and ritalinic acid plasma levels
As indicated in Table 2, MPH and RA plasma levels increased with administered dose and were relatively stable at a given dose, although there is considerable variability as would be expected with oral drug administration. A marked increase in MPH plasma levels occurred in both groups at week 23 when MPH doses increased from 0.15 to 0.3 mg/kg for the low dose group and 1.5 to 3.0 mg/kg for the high dose group. At 3.0 mg/kg (high dose group), MPH plasma levels were equivalent to or higher than those typically seen in human adolescents being treated for ADHD (~10 ng/ml following 0.3 mg/kg in humans, see [23]). At week 25 doses were again increased from 0.3 to 0.6 mg/kg for the low dose group and from 3.0 to 6.0 mg/kg for the high dose group, resulting in MPH plasma levels of 1.72 and 49.84 ng/ml, respectively, during week 26. At this time operant performance began to decrease in the high dose group. At week 27 the low dose was increased again to 1.25 mg/kg and at week 31 it was increased to 2.5 mg/kg where it remained for the remainder of the study (low dose plasma MPH levels ranged from 13.61 to 3.46 ng/ml during the last 6 months of the study). At week 27 the high dose was increased to 12.5 mg/kg where it remained (MPH plasma levels varied from 51.93 ng/ml, in week 28, to 134.55 ng/ml, in week 56). Thus, for the latter part of the study, MPH plasma levels in the low dose group were approximately equivalent to those seen clinically in children with ADHD and those in the high dose group were approximately 10 times higher. The plasma concentrations of RA mirrored those of MPH, albeit significantly higher, and are included as accompaniment of MPH metabolism.
3.2. Overall training scores
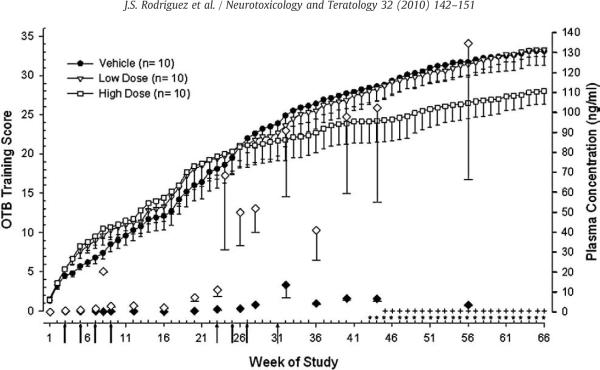
Fig. 1 shows the overall OTB training scores for all three treatment groups. A cumulative score was calculated (Table 1) for each subject and reflects which OTB training stage a subject had reached. Analyses revealed a difference in the number of weeks required to progress through OTB training. The vehicle and low dose MPH groups attained higher training scores as compared to the high dose group beginning at week 43 (for vehicle) and week 45 (for low dose) and this difference was maintained through week 66, F(66,1977)=25.18, p<0.05. By week 43 the low (2.5 mg/kg b.i.d.) and the high dose groups (12.5 mg/kg b.i.d.) had been receiving their maximal doses for 12 and 16 weeks, respectively (Table 2).
Fig. 1.
MPH effects on overall performance in the OTB as measured by cumulative training score for weeks 1–66. Analysis of group means (±sem) revealed that the vehicle group attained a significantly higher overall score in the OTB as compared to the high dose group for weeks 43–66 (*, p<0.05). Similarly, the low dose group attained a higher overall score than the high dose group for weeks 45–66 (+, p<0.05). Closed and open diamonds represent MPH plasma concentrations (right y-axis) for the low and high dose MPH groups, respectively. Number of subjects is given in parentheses. Arrows indicate changes in MPH doses (all doses given twice daily) as follows: weeks 1–2: 0.15 mg/kg for low and high dosed groups; 1st arrow, weeks 3–4: low=0.15 mg/kg, high=0.30 mg/kg; 2nd arrow, weeks 5–6: low=0.15 mg/kg, high=0.45 mg/kg; 3rd arrow, weeks 7–8: low=0.15 mg/kg, high=0.75 mg/kg; 4th arrow, weeks 9–22: low=0.15 mg/kg, high=1.50 mg/kg; 5th arrow, weeks 23–24: low=0.30 mg/kg, high=3.0 mg/kg; 6th arrow, weeks 25–26: low=0.60 mg/kg, high=6.0 mg/kg; 7th arrow, week 27–30: low=1.25 mg/kg, high=12.5 mg/kg; 8th arrow, week 31 and beyond: low=2.5 mg/kg, high=12.5 mg/kg.
3.3. Incremental Repeated Acquisition task endpoints
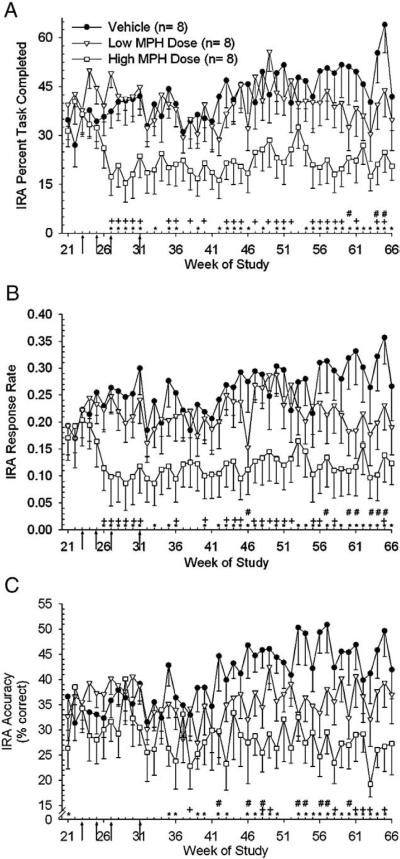
Statistical analysis for the IRA task was performed for weeks 21–66. Week 21 was selected for the beginning of the IRA analysis to maximize the number of subjects (n=8/group) which had progressed furthest through OTB training prior to dose escalation at week 23. Analysis of IRA percent task completed (PTC) revealed an effect of treatment [F(2,106)=30.90, p<0.0001] and week [F(45,1040)=2.06, p<0.0001] between groups and these differences were apparent at doses of 1.25 mg/kg (2×day for low dose group) and 12.5 mg/kg (2×day for high dose group). Post-hoc analysis showed the vehicle group performed better than the low dose and high dose groups for 3 and 30 weeks, respectively (Fig. 2A). (Note that the differences observed between the low dose group and vehicle group were at 3 of the latter weeks analyzed, which implies possible sensitization.) Similar differences were also found between the low and high dose groups for this endpoint with the low dose group performing better than the high dose group for 25 weeks (Fig. 2A). Analysis of IRA response rate (RR) data revealed an effect of treatment [F(2,104)=24.64, p<0.0001] and week [F(45,1264)=2.08, p<0.0001] between groups and these differences were apparent at doses of 0.60 mg/kg (2×day for low dose group) and 6.0 mg/kg (2×day for high dose group). Post-hoc analysis showed that the vehicle group responded faster than the low dose group for 7 weeks and the high dose group for 33 weeks, during weeks 21–66 (Fig. 2B). Similar differences were also found between the low and high dose groups for the IRA RR endpoint with the low dose group responding faster than the high dose group for 21 weeks (Fig. 2B). Analysis of IRA accuracy (ACC) revealed an effect of treatment [F(2,208)=45.56, p<0.0001] and week [F(45,1010)=1.44, p<0.05) between groups with differences occurring at weeks following final dose attainment. Post-hoc analysis showed the vehicle group performed better than the low dose and high dose groups for 8 and 25 weeks, respectively (Fig. 2C). Differences were also found between the low and high dose groups for the IRA ACC endpoint with the low dose group performing better than the high dose group for 8 weeks during the analysis period (Fig. 2C).
Fig. 2.
Arrows indicate the dose changes denoted as arrows 5–8 in Fig. 1 legend. A) Analysis of the means (±sem) of IRA Percent Task Completed (PTC) for weeks 21 through 66 revealed that the vehicle group performed better than both the low dose group (#, p<0.05) and the high dose group (*, p<0.05) when indicated by symbols. The low dose group performed better than the high dose group when indicated (+, p<0.05). Note: For the IRA task the number of animals per group is 8 and not 10 due to the fact that data for only those subjects that had completed the subsequent CPR training prior to dose escalation at week 23 were analyzed. B) Analysis of the means (±sem) of response rate (RR) revealed the vehicle group responded faster than both the low dose group (#, p<0.05) and high dose group (*, p<0.05) when indicated by symbols. The low dose group responded faster than the high dose group when indicated (+, p<0.05). C) Analysis of the means (±sem) of IRA Accuracy (ACC) revealed the vehicle group performed better than both the low dose group (#, p<0.05) and high dose group (*, p<0.05) when indicated by symbols. The low dose group performed better than the high dose group when indicated (+, p<0.05).
3.4. Conditioned Position Responding task endpoints
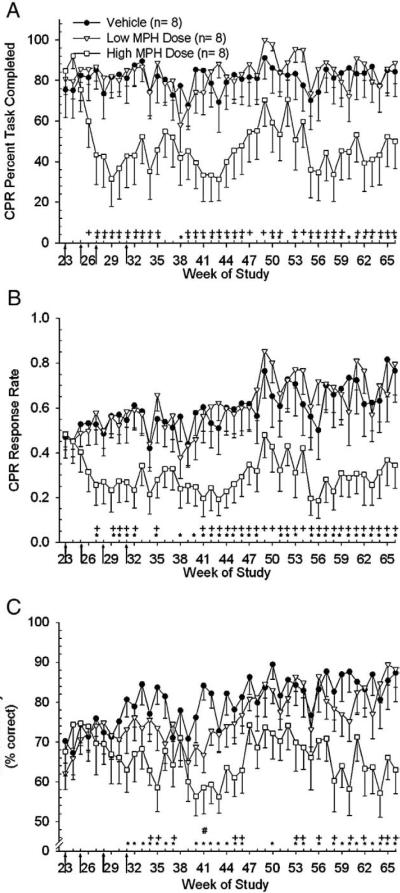
Statistical analysis for the CPR task was performed for weeks 23–66. Analysis of endpoints for the CPR task began at week 23, instead of 21, because performance of the CPR task began on average 2 weeks after subjects began performance of the IRA task. Data for the same subjects utilized in the IRA analysis (above) were included in the CPR analysis (n=8/group). Analysis of CPR PTC data revealed an effect of treatment [F(2,88.5)=27.93, p<0.0001] and week [F(43,995)=2.12, p<0.0001] between groups with differences apparent at doses of 1.25 mg/kg (2×day for low dose group) and 12.5 mg/kg (2×day for high dose group). Post-hoc analysis showed the vehicle group performed better than the high dose group for 33 weeks during weeks 23–66 (Fig. 3A) but not different than the low dose group. Similar differences were also found between the low and high dose groups for this endpoint with the low dose group performing better than the high dose group for 35 weeks during weeks 23–66 (Fig. 3A). Analysis of CPR RR data revealed an effect of treatment [F(2,82.4)=26.29, p<0.0001] and week [F(43,995)=2.78, p<0.0001] between groups with differences apparent at doses of 0.60 mg/kg (2×day for low dose group) and 6.0 mg/kg (2×day for high dose group). Post-hoc analysis showed the vehicle group performed no differently than the low dose group but responded faster than the high dose group for 31 weeks during weeks 23–66 (Fig. 3B). Similar differences were also found between the low and high dose groups for the CPR RR endpoint with the low dose group responding faster than the high dose group for 32 weeks (Fig. 3B). Analysis of CPR ACC data revealed an effect of treatment [F(2,121)=24.0, p<0.0001] and week [F(43,951)=2.16, p<0.0001] between groups. Post-hoc analysis showed the vehicle group performed more accurately than the low and high dose groups for 1 and 27 weeks, respectively, during weeks 23–66 (Fig. 3C). The low dose group was more accurate than the high dose group for 14 weeks during the same period.
Fig. 3.
Arrows indicate the dose changes denoted as arrows 5–8 in Fig. 1 legend. A) Analysis of the means (±sem) of CPR PTC for weeks 23–66 revealed that the vehicle group completed more of the task than the high dose group on weeks indicated (*, p<0.05). There no differences between the vehicle and low dose groups. The low dose group completed more of the task than the high dose group when indicated (+, p<0.05). Note: For the CPR task the number of animals per group is 8 and not 10 due to the fact that data for only those subjects that had completed CPR training prior to dose escalation at week 23 were analyzed. B) Analysis of the means (±sem) of CPR RR for week's 23–66 revealed that the vehicle group responded faster than the high dose group during weeks indicated (*, p<0.05). There no differences between the vehicle and low dose groups. The low dose group responded faster than the high dose group when indicated (+, p<0.05). C) Analysis of the means (±sem) of CPR ACC for week's 23–66 revealed that the vehicle group performed better than the high dose group during weeks indicated (*, p<0.05). There was a significant difference between the vehicle and low dose group ACC during week 41 only (#, p<0.05). The low dose group performed better than the high dose group on weeks indicated (+, p<0.05).
3.5. Progressive Ratio task endpoints
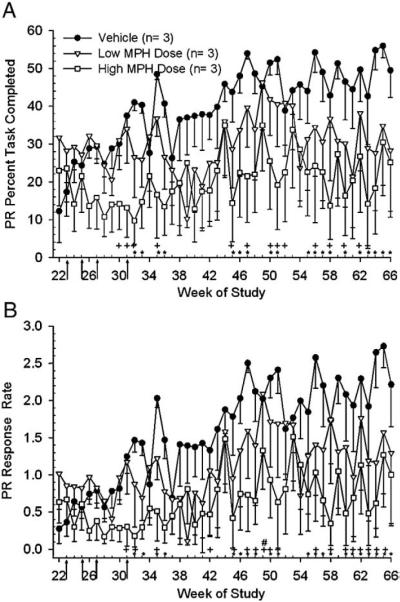
For the PR task only 3 subjects/group, from the 8/group noted above, had reached this level of OTB testing prior to dose escalation at week 23 and hence were the only subjects included in this statistical analysis, which was performed for weeks 22–66. Analysis of PR PTC revealed an effect of treatment [F(2,23.3)=13.97, p<0.0001] and week [F(44,281)=2.71, p<0.0001] with differences apparent at doses of 1.25 mg/kg (2×day for low dose group) and 12.5 mg/kg (2×day for high dose group). Post-hoc analysis showed the vehicle group completed more of the PR task for 19 weeks during weeks 22–66 (Fig. 4A) as compared to the high dose group. The low dose group also outperformed the high dose group in PR PTC for 14 weeks during the same period. There were no differences between the vehicle and low dose group for PR PTC. Analysis of PR RR endpoint revealed an effect of treatment [F(2,30.2)=19.93, p<0.0001] and week [F(44,280)=3.26, p<0.0001] between groups with differences occurring during weeks following final dose attainment. Post-hoc analysis revealed that the vehicle group responded faster (Fig. 4B) than the high dose group for 21 weeks during weeks 22–66. Only one week differed between the vehicle and low dose group for PR RR, week 41. For the RR endpoint, the low dose group responded faster than the high dose group for 18 weeks of the analysis period.
Fig. 4.
Arrows indicate the dose changes denoted as arrows 5–8 in Fig. 1 legend. A) Analysis of the means (±sem) of Progressive Ratio PTC for weeks 22–66 revealed that the vehicle group completed more of the task than the high dose group on weeks indicated (*, p<0.05). There were no significant differences between the vehicle and low dose groups. The low dose group completed more of the task than the high dose group on weeks indicated (+, p<0.05). Note: The number of animals per group is only 3 due to the fact that data for only those subjects that had completed PR training prior to dose escalation at week 23 were analyzed. B) Analysis of the means (±sem) of Progressive Ratio RR for week's 22–66. Analysis revealed that the vehicle group responded faster than the high dose group on weeks indicated (*, p<0.05). There was a significant difference between the vehicle and low dose group during week 49 only (#, p<0.05). The low dose group responded faster than the high dose group on weeks indicated (+, p<0.05).
3.6. Delayed-Matching-To-Sample task endpoints
Analysis of the attainment of the 10 recall delay sets (Fig. 5) for the DMTS task revealed better performance in the vehicle group than in either the low or high dose groups. Note that only those subjects that began the full DMTS task prior to dose escalation at week 23 were included in the statistical analysis. Statistical analysis of the 24 weeks of DMTS performance, which corresponds to the last 24 weeks of the 66 week period, revealed differences in the progression through the DMTS recall delay sets between the vehicle and low and high dose groups [F(23,526)=9.15, p<0.0001]. Post-hoc analysis revealed that the vehicle group attained a higher DMTS score versus the high dose group by week 13 and this difference continued throughout testing (Fig. 5). Differences between the vehicle and low dose group emerged at week 17 of DMTS testing and lasted for several weeks after which the performance of the low dose group improved and became no different from that of the vehicle group (Fig. 5).
Fig. 5.
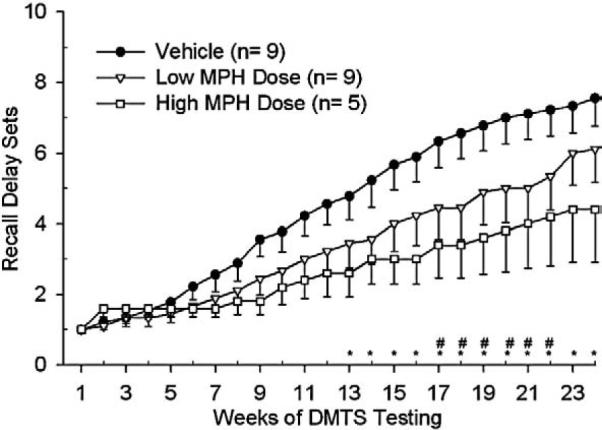
These results are of the 24 weeks of DMTS training which represent weeks 43–66 of OTB testing. DMTS recall delay set acquisition analysis revealed differences between the vehicle versus the low dose group on weeks 17–22 (#, p<0.05) and versus the high dose group on weeks 13–24 (*, p<0.05). The number of animals per group (in parentheses) reflects only those subjects that had reached the full DMTS task prior to dose escalation at week 23.
4. Discussion
The findings presented here indicate that in young rhesus monkeys substantial doses of MPH are necessary to attain clinically relevant plasma levels when given orally. Also, the concomitantly high RA plasma concentrations speak of the difference in MPH dose, when comparing between monkeys and children, required to reach the clinical range. Doses of 2.5 mg/kg (low dose group) were required to attain plasma levels in the human therapeutic range of 4–12 ng/ml [33]. On a mg/kg basis, this dose is approximately 10-fold higher than typical human oral doses [39] suggesting differences in gut absorption or first-pass metabolism between monkeys and humans although this is purely speculative. These plasma levels are similar to those reported in previous studies in adult rhesus monkeys where plasma levels were approximately 12 and 19 ng/ml after either acute or chronic oral treatment, respectively, with 3.0 mg/kg [10]. Interestingly, overt behavioral effects in the adults in the earlier study, such as lack of responding for food reinforcers and going-off feed for several days, were evident at much lower doses (less than 2 mg/kg given twice per day) than in the present study in pre-adolescents (personal observations). In the present study, this effect was not observed until doses reached 6.0 mg/kg/treatment and higher at around weeks 25–26 (high dose group). This effect was evident for the remainder of the study as indicated by the low response rates in all OTB tasks. Also of interest is the apparent increase in sensitivity to the effects of MPH with continued treatment at the low dose of 2.5 mg/kg. This is evidenced by significant decreases in IRA RR beginning at week 60 (when animals were a bit over 3 years of age) which was accompanied by a similar decrease in PTC and ACC. Similar phenomena seem also to have occurred in the PR task although the variability of those data precludes any clear determination of effect. Similar effects were not, however, noted for the CPR task, suggesting a specificity of effect on learning behavior and perhaps, appetitive motivation. These observations suggest that young animals are less sensitive to the behaviorally disruptive effects of MPH than are older animals: sensitivity increases as animals get older. Similar observations also have been noted in monkeys during studies on the acute effects of amphetamine [24] and cocaine [28] which like MPH are thought to exert their primary effects via enhancement of dopaminergic systems. Thus, the age-related reactivity of subjects to MPH may be linked to the developmental stage of the dopamine system at the time of exposure. However, chronic administration of MPH has also been shown to produce neurophysiological [43] and behavioral sensitization [9] and this may have also contributed to the findings of the present study.
In earlier studies in young adult rhesus monkeys [20,21], chronic MPH administration at doses up to 1.6 mg/kg (orally, twice/day, M–F) was without effect on OTB performance over many months. In those studies, subjects had been performing the OTB tasks for many months prior to the initiation of treatment and thus, steady-state levels of performance prior to the onset of treatment served as each animals control performance. The absence of findings in that study is replicated somewhat in this study as evidenced by the fact that the low dose group showed minimal behavioral effects.
Additionally, investigations into the effects of MPH on the developing rodent nervous system and subsequent cognitive performance have recently appeared. Repeated oral doses (3 mg/kg) of MPH in pre- and periadolescent (postnatal day 22 to 40) rats has been reported to improve spatial learning and memory in the radial arm maze [44]. Similarly, in nonhuman primates, MPH reduced distractibility in young but not aged rhesus macaques in a Delayed-Matching-To-Sample task [32]. However, other studies of chronic oral MPH administration in periadolescent rats have reported prolonged impairments in novel recognition memory (3–5 mg/kg/b.i.d.) [17] and novel object exploration [15]. In addition to cognitive effects, early exposure to MPH can render adult rats less responsive to natural stimuli (sex and sucrose) and increase sensitivity to aversive stimuli [6]. Considering that almost 8% of youth aged 4 to 17 years have a diagnosis of ADHD and over half of those are taking stimulant (MPH or amphetamine) medication as treatment [18], it is imperative that the consequences of developmental exposure to MPH be thoroughly studied.
Here, we have shown that chronic exposure to doses of MPH that produced clinically relevant plasma levels were very well tolerated by pre-adolescent nonhuman primates with no evidence of behavioral disruption with the possible exception of a decrease in appetite noted during a year of treatment. This decrease in appetite manifested as a decrease in response rate in the IRA and PR tasks. Treatment with high doses, however, resulted in clear and continuous disruption of several operant behaviors. This effect manifested as a decrease in percent task completed which was associated with a decrease in both response rate and accuracy. Given that all of the tasks used in this study are food-reinforced, the noted decrease in response rate is likely a result of the well-known appetite suppressing effects of stimulants such as MPH. Animals not motivated by a given reinforcer are less likely to come under the stimulus control (rules) of tasks that employ that reinforcer and are, thus, not necessarily expected to perform those task well.
Therefore, it is possible that even the poor response accuracy noted in these animals is secondary to MPH's effect on task motivation. Alternatively, it is well known that amphetamine-like stimulants, including MPH, can induce stereotypical behaviors [34] which may interfere with task performance. While some stereotypes were observed in the current study (i.e. rotating in transport chairs and hyperlocomoting), they were not systematically assessed for these types of behaviors. The possibility that drug-induced stereotypic behavior contributed to the poor performance of our high dose animals remains a possibility. It is also important to acknowledge that the animals used in the present study were normal animals and not models of ADHD. Since a dominant theory about the etiology of ADHD suggests abnormalities of function in the dopaminergic system [2], it remains unknown how chronic exposure to therapeutic agents such as MPH might affect those systems in persons with ADHD. A perhaps reassuring observation of the present study is that, at least in normal (non-ADHD) nonhuman primates, prolonged treatment with MPH at doses likely to produce clinically relevant plasma MPH levels is not associated with findings of adverse effects as monitored in tasks designed to assess aspects of learning, visual discrimination and memory.
Acknowledgements
Funding: These studies were supported through an interagency agreement between the National Institute on Child Health and Human Development and the National Center for Toxicological Research, 224-05-0003 and a postdoctoral fellowship from the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the FDA (to J. Rodriguez).
Footnotes
Conflict of interest This declaration is for the purpose of disclosing any conflict of interest between authors and other parties. There are no conflict of interest for any authors involved.
References
- [1].Arnsten AF. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- [2].Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J. Clin. Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- [3].Barkley RA, McMurray MB, Edelbrock CS, Robbins K. Side effects of methylphenidate in children with attention deficit hyperactivity disorder: a systemic, placebo-controlled evaluation. Pediatrics. 1990;86:184–192. [PubMed] [Google Scholar]
- [4].Bedford JA, Marquis DK, Wilson MC. The effects of several anorexigenics on monkey social behavior. Pharmacol. Biochem. Behav. 1984;20(3):317–321. doi: 10.1016/0091-3057(84)90263-6. [DOI] [PubMed] [Google Scholar]
- [5].Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60(10):1111–1120. 15. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- [6].Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol. Psychiatry. 2003;54(12):1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- [7].Chelonis JJ, Daniels-Shaw JL, Blake DJ, Paule MG. Developmental aspects of delayed matching-to-sample task performance in children. Neurotoxicol. Teratol. 2000;22(5):683–694. doi: 10.1016/s0892-0362(00)00090-8. [DOI] [PubMed] [Google Scholar]
- [8].Chelonis JJ, Flake RA, Baldwin RL, Blake DJ, Paule MG. Developmental aspects of timing behavior in children. Neurotoxicol. Teratol. 2004;26:461–476. doi: 10.1016/j.ntt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [9].Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res. Bull. 2006;68(6):393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [10].Doerge DR, Fogle CM, Paule MG, McCullagh M, Bajic S. Analysis of methylphenidate and its metabolite ritalinic acid in monkey plasma by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2000;14(8):619–623. doi: 10.1002/(SICI)1097-0231(20000430)14:8<619::AID-RCM916>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [11].El-Zein RA, Abdel-Rahman SZ, Hay MJ, Lopez MS, Bondy ML, Morris DL, Legator MS. Cytogenetic effects in children treated with methylphenidate. Cancer Lett. 2005;230:284–291. doi: 10.1016/j.canlet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [12].Firestone P, Musten LM, Pisterman S, Mercer J, Bennett S. Short-term side effects of stimulant medication are increased in preschool children with attention-deficit/hyperactivity disorder: a double-blind placebo-controlled study. J. Child Adolesc. Psychopharmacol. 1998;8:13–25. doi: 10.1089/cap.1998.8.13. [DOI] [PubMed] [Google Scholar]
- [13].Fujita S, Okutsu H, Yamaguchi H, Nakamura S, Adachi K, Saigusa T, Koshikawa NJ. Altered pre- and postsynaptic dopamine receptor functions in spontaneously hypertensive rat: an animal model of attention-deficit hyperactivity disorder. Oral Sci. 2003;45(2):75–83. doi: 10.2334/josnusd.45.75. [DOI] [PubMed] [Google Scholar]
- [14].Goez H, Back-Bennet O, Zelnik N. Differential stimulant response on attention in children with comorbid anxiety and oppositional defiant disorder. J. Child Neurol. 2007;22(5):538–542. doi: 10.1177/0883073807303221. [DOI] [PubMed] [Google Scholar]
- [15].Heyser CJ, Pelletier M, Ferris JS. The effects of methylphenidate on novel object exploration in weanling and periadolescent rats. Ann. N. Y. Acad. Sci. 2004;1021:465–469. doi: 10.1196/annals.1308.066. [DOI] [PubMed] [Google Scholar]
- [16].Klein-Schwartz W. Pediatric methylphenidate exposures: 7-year experience of poison centers in the United States. Clin. Pediatr. (Phila) 2003;42(2):159–164. doi: 10.1177/000992280304200210. [DOI] [PubMed] [Google Scholar]
- [17].LeBlanc-Duchin D, Taukulis HK. Chronic oral methylphenidate administration to periadolescent rats yields prolonged impairment of memory for objects. Neurobiol. Learn. Mem. 2007;88(3):312–320. doi: 10.1016/j.nlm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- [18].Mayers R, Bagwell C, Erkulwater J. ADHD and the rise in stimulant use among children. Harv. Rev. Psychiatry. 2008;16(3):151–166. doi: 10.1080/10673220802167782. [DOI] [PubMed] [Google Scholar]
- [19].Morris P, Gillam MP, Allen RR, Paule MG. The effect of chronic cocaine exposure during pregnancy on the acquisition of operant behaviors by rhesus monkey offspring. Neurotoxicol. Teratol. 1996;18:155–166. doi: 10.1016/0892-0362(95)02031-4. [DOI] [PubMed] [Google Scholar]
- [20].Morris P, Gillam M, McCarty C, Paule MG. Effects of chronic methylphenidate on operant behavior in the rhesus monkey. Toxicologist. 1997;36(1):63. [Google Scholar]
- [21].Morris P, Gillam MP, Parker J, McCarty C, Paule MG. Effects of chronic methylphenidate on operant behavior in the rhesus monkey II. Toxicologist. 1998;42(1-S):304. [Google Scholar]
- [22].Morris SM, Dobrovolsky VN, Shaddock JG, Mittelstaedt RA, Bishop ME, Manjanatha MG, Shelton SD, Doerge DR, Twaddle NC, Chen JJ, Lin CJ, Paule MG, Slikker W, Jr., Hotchkiss CE, Petibone D, Tucker JD, Mattison DR. The genetic toxicology of methylphenidate hydrochloride in non-human primates. Mutat. Res. 2009;673(1):59–66. doi: 10.1016/j.mrgentox.2008.12.001. [DOI] [PubMed] [Google Scholar]
- [23].National Toxicology Program. U.S. Department of Health and Human Services NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Methylphenidate. 2005 NIH Publication No. 05-4473. [PubMed] [Google Scholar]
- [24].Paule MG. Age-related sensitivity to the acute behavioral effects of cocaine and amphetamine in monkeys. Neurotox. Teratol. 1997;19(3):241–242. [Google Scholar]
- [25].Paule MG. Validation of a behavioral test battery for monkeys. In: Buccafusco JJ, editor. Methods of Behavioral Analysis in Neuroscience. CRC Press LLC; Boca Raton, FL: 2001. pp. 281–294. [Google Scholar]
- [26].Paule MG. Taffe M, Weed MR, editors. Chronic drug exposures during development in nonhuman primates: models of brain dysfunction in humans. Frontiers in Bioscience Special Issue: Nonhuman Primate Models of Neuropsychopathology, Frontiers in Bioscience. 2005;10:2240–2249. doi: 10.2741/1693. [DOI] [PubMed] [Google Scholar]
- [27].Paule MG, Cranmer JM, Wilkins JD, Stern HP, Hoffman EL. Quantitation of complex brain function in children: preliminary evaluation using a nonhuman primate behavioral test battery. Neurotoxicology. 1988;9:367–378. [PubMed] [Google Scholar]
- [28].Paule MG, Gillam MP, Morris P. The effects of cocaine on nonhuman primate brain function are age-dependent. Ann. N.Y. Acad. Sci. 1998;844:178–182. [PubMed] [Google Scholar]
- [29].Paule MG, Chelonis JJ, Buffalo EA, Blake DJ, Casey PH. Operant test battery performance in children: correlation with IQ. Neurotoxicol. Teratol. 1999;21:223–230. doi: 10.1016/s0892-0362(98)00045-2. [DOI] [PubMed] [Google Scholar]
- [30].Popke EJ, Allen RR, Pearson EC, Hammond TG, Paule MG. Differential effects of two NMDA receptor antagonists on cognitive-behavioral development in nonhuman primates I. Neurotoxicol. Teratol. 2001;23:319–332. doi: 10.1016/s0892-0362(01)00156-8. [DOI] [PubMed] [Google Scholar]
- [31].Popke EJ, Allen RR, Pearson EC, Hammond TG, Paule MG. Differential effects of two NMDA receptor antagonists on cognitive–behavioral performance in young nonhuman primates II. Neurotoxicol. Teratol. 2001;23:333–347. doi: 10.1016/s0892-0362(01)00138-6. [DOI] [PubMed] [Google Scholar]
- [32].Prendergast MA, Jackson WJ, Terry AV, Jr., Kille NJ, Arneric SP, Decker MW, Buccafusco JJ. Age-related differences in distractibility and response to methylphenidate in monkeys. Cereb. Cortex. 1998;8(2):164–172. doi: 10.1093/cercor/8.2.164. [DOI] [PubMed] [Google Scholar]
- [33].Quinn D, Wigal S, Swanson J, Hirsch S, Ottolini Y, Dariani M, Roffman M, Zeldis J, Cooper T. Comparative pharmacodynamics and plasma concentrations of d-threo-methylphenidate hydrochloride after single doses of d-threo-methylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in a double-blind, placebo-controlled, crossover laboratory school study in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psych. 2004;43(11):1422–1429. doi: 10.1097/01.chi.0000140455.96946.2b. [DOI] [PubMed] [Google Scholar]
- [34].Roffman JL, Raskin LA. Stereotyped behavior: effects of d-amphetamine and methylphenidate in the young rat. Pharmacol. Biochem. Behav. 1997;58(4):1095–1102. doi: 10.1016/s0091-3057(97)00321-3. [DOI] [PubMed] [Google Scholar]
- [35].Schulze GE, McMillan DE, Bailey JR, Scallet AC, Ali SF, Slikker W, Jr., Paule MG. Acute effects of delta-9-tetrahydrocannabinol (THC) in rhesus monkeys as measured by performance in a battery of cognitive function tests. J. Pharmacol. Exp. Ther. 1988;245(1):178–186. [PubMed] [Google Scholar]
- [36].Schulze GE, McMillan DE, Bailey JR, Scallet AC, Ali SF, Slikker W, Jr., Paule MG. Acute effects of marijuana smoke on complex operant behavior in rhesus monkeys. Life Sci. 1989;45(6):465–475. doi: 10.1016/0024-3205(89)90096-9. [DOI] [PubMed] [Google Scholar]
- [37].Sleator EK. Deleterious effects of drugs used for hyperactivity on patients with Gilles de la Tourette syndrome. Clin. Pediatr. (Phila) 1980;19(7):453–454. doi: 10.1177/000992288001900704. [DOI] [PubMed] [Google Scholar]
- [38].Volkow ND, Insel TR. What are the long-term effects of methylphenidate treatment? Biol. Psychiatry. 2003;54:1307–1309. doi: 10.1016/j.biopsych.2003.10.019. [DOI] [PubMed] [Google Scholar]
- [39].Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am. J. Psychiatry 160. 2003:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- [40].Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. J. Atten. Disord. 2002;6(Suppl 1):S31–43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- [41].Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, Wong C, Swanson JM. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2007;64(8):932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- [42].Wilens TE, Hammerness PG, Biederman J, Kwon A, Spencer TJ, Clark S, Scott M, Podolski A, Ditterline JW, Morris MC, Moore H. Blood pressure changes associated with medication treatment of adults with attention-deficit/hyperactivity disorder. J. Clin. Psychiatry. 2005;66:253–259. doi: 10.4088/jcp.v66n0215. [DOI] [PubMed] [Google Scholar]
- [43].Yang PB, Swann AC, Dafny N. Chronic administration of methylphenidate produces neurophysiological and behavioral sensitization. Brain Res. 2007;1145:66–80. doi: 10.1016/j.brainres.2007.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu N, Weedon J, Dow-Edwards DL. Oral methylphenidate improves spatial learning and memory in pre- and periadolescent rats. Behav. Neurosci. 2007;121(6):1272–1279. doi: 10.1037/0735-7044.121.6.1272. [DOI] [PubMed] [Google Scholar]