Abstract
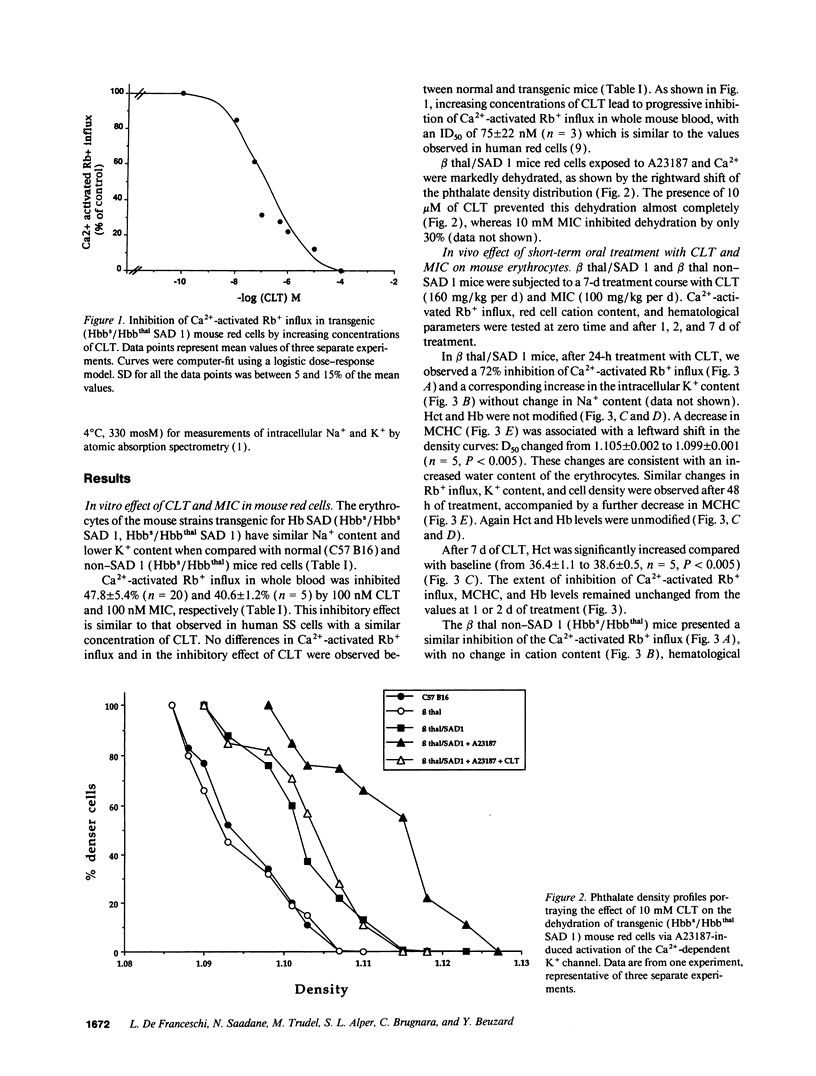
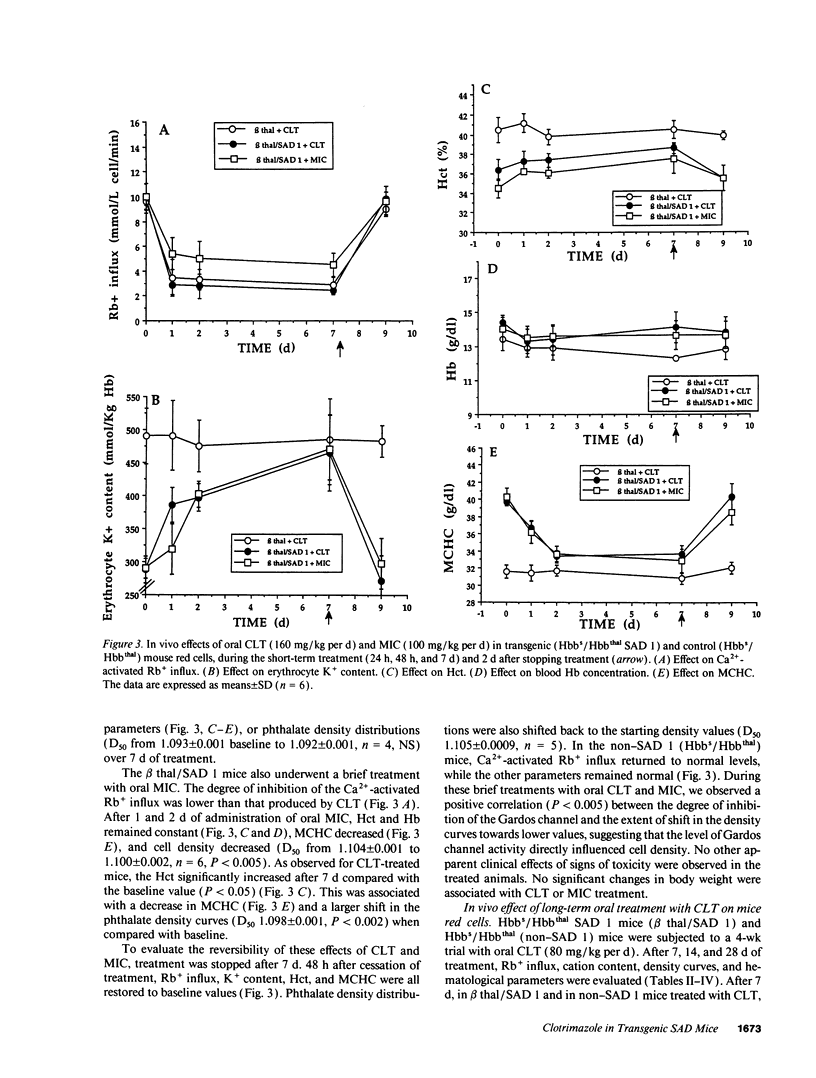
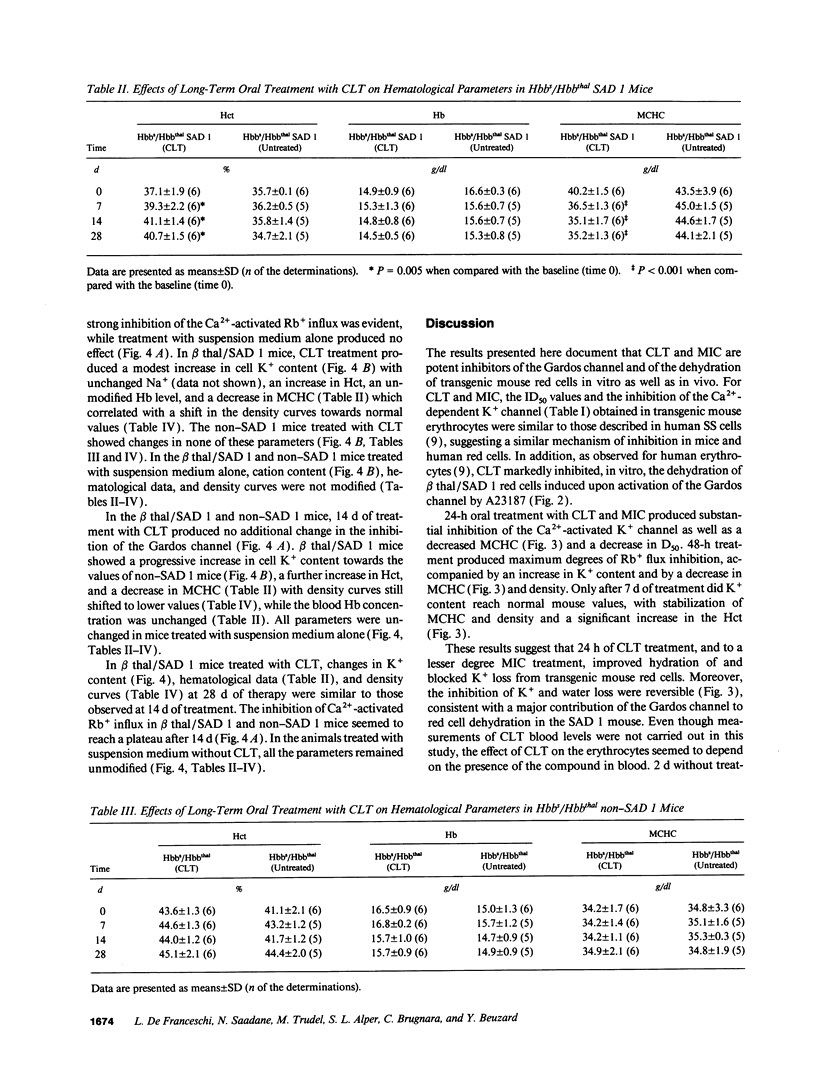
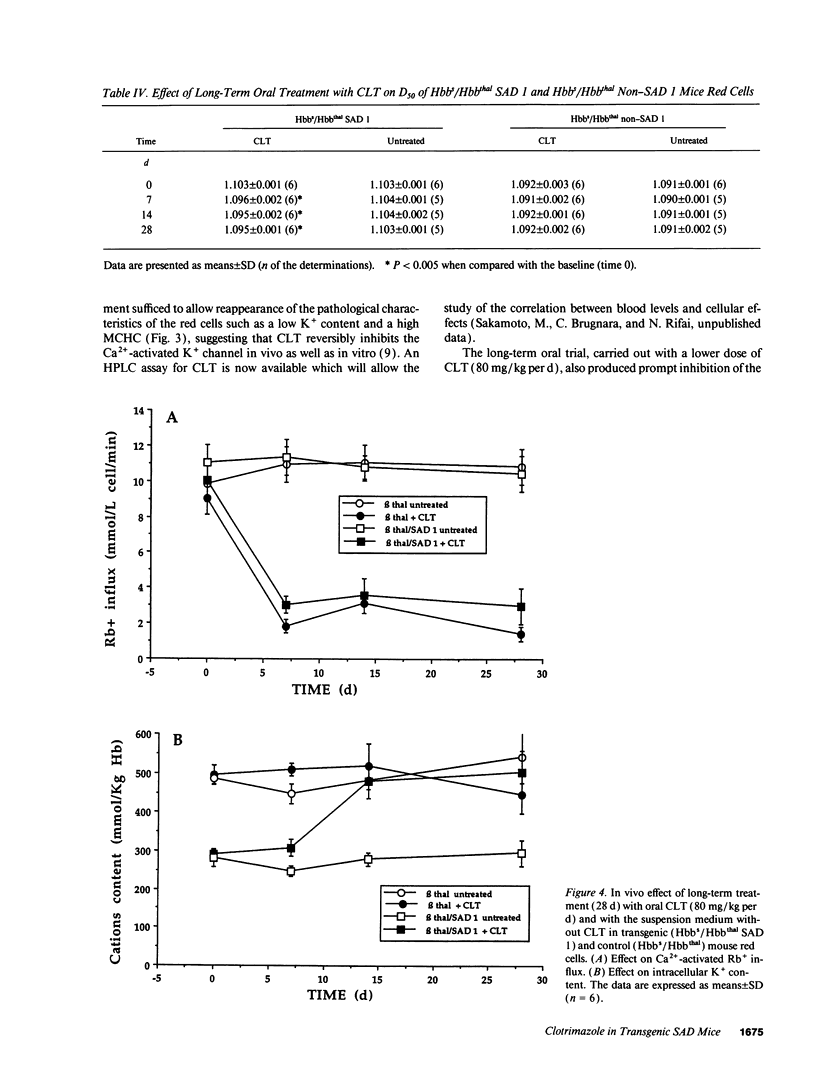
Prevention of red cell K+ and water loss is a therapeutic strategy for sickle cell disease. We have investigated in vitro and in vivo the effects of clotrimazole (CLT) and miconazole (MIC) on transgenic mice red cells expressing hemoglobin SAD. CLT blocked the Gardos channel (ID50 75 +/- 22 nM; n = 3) and the A23187-induced dehydration of Hbbs/Hbbthal SAD 1 mouse erythrocytes in vitro. Oral treatment with CLT (160 mg/kg per d) and MIC (100 mg/kg per d) inhibited the Gardos channel in both SAD 1 and control (Hbbs/Hbbthal) mice. In the SAD 1 mice only, cell K+ content increased, and mean corpuscular hemoglobin concentration and cell density decreased. After 7 d of treatment, the hematocrit of SAD 1, CLT-treated animals also increased. All changes were fully reversible. Long-term treatments of SAD 1 mice with oral CLT (80 mg/kg per d for 28 d) lead to sustained increases in cell K+ content and hematocrit and sustained decreases in mean corpuscular hemoglobin concentration and cell density, with no changes in animals treated with vehicle alone. Thus, CLT and MIC can reverse dehydration and K+ loss of SAD 1 mouse erythrocytes in vitro and in vivo, further supporting the potential utility of these drugs in the treatment of sickle cell anemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benos D. J. Intracellular analysis of sodium, potassium, and chloride in mouse erythrocytes. J Cell Physiol. 1980 Oct;105(1):185–187. doi: 10.1002/jcp.1041050120. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Ortiz O. E., Lew V. L. Evidence for a direct reticulocyte origin of dense red cells in sickle cell anemia. J Clin Invest. 1991 Jan;87(1):113–124. doi: 10.1172/JCI114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnara C., Bunn H. F., Tosteson D. C. Regulation of erythrocyte cation and water content in sickle cell anemia. Science. 1986 Apr 18;232(4748):388–390. doi: 10.1126/science.3961486. [DOI] [PubMed] [Google Scholar]
- Brugnara C., de Franceschi L., Alper S. L. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J Clin Invest. 1993 Jul;92(1):520–526. doi: 10.1172/JCI116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa M., Spalvins A., Nagel R. L. Volume-dependent and NEM-stimulated K+,Cl- transport is elevated in oxygenated SS, SC and CC human red cells. FEBS Lett. 1986 May 5;200(1):197–202. doi: 10.1016/0014-5793(86)80538-5. [DOI] [PubMed] [Google Scholar]
- Charache S., Walker W. G. Failure of desmopressin to lower serum sodium or prevent crisis in patients with sickle cell anemia. Blood. 1981 Nov;58(5):892–896. [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987 Nov;70(5):1245–1266. [PubMed] [Google Scholar]
- Fabry M. E., Costantini F., Pachnis A., Suzuka S. M., Bank N., Aynedjian H. S., Factor S. M., Nagel R. L. High expression of human beta S- and alpha-globins in transgenic mice: erythrocyte abnormalities, organ damage, and the effect of hypoxia. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12155–12159. doi: 10.1073/pnas.89.24.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Glader B. E., Nathan D. G. Cation permeability alterations during sickling: relationship to cation composition and cellular hydration of irreversibly sickled cells. Blood. 1978 May;51(5):983–989. [PubMed] [Google Scholar]
- Olivieri O., Vitoux D., Galacteros F., Bachir D., Blouquit Y., Beuzard Y., Brugnara C. Hemoglobin variants and activity of the (K+Cl-) cotransport system in human erythrocytes. Blood. 1992 Feb 1;79(3):793–797. [PubMed] [Google Scholar]
- Rosa R. M., Bierer B. E., Thomas R., Stoff J. S., Kruskall M., Robinson S., Bunn H. F., Epstein F. H. A study of induced hyponatremia in the prevention and treatment of sickle-cell crisis. N Engl J Med. 1980 Nov 13;303(20):1138–1143. doi: 10.1056/NEJM198011133032002. [DOI] [PubMed] [Google Scholar]
- Saitta M., Cavalier S., Garay R., Cragoe E., Jr, Hannaert P. Evidence for a DIOA-sensitive [K+,Cl-]-cotransport system in cultured vascular smooth muscle cells. Am J Hypertens. 1990 Dec;3(12 Pt 1):939–942. doi: 10.1093/ajh/3.12.939. [DOI] [PubMed] [Google Scholar]
- Schrier S. L. In sickle disease, unlike wine, dry is not good. J Clin Invest. 1993 Jul;92(1):1–1. doi: 10.1172/JCI116536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M., Saadane N., Garel M. C., Bardakdjian-Michau J., Blouquit Y., Guerquin-Kern J. L., Rouyer-Fessard P., Vidaud D., Pachnis A., Roméo P. H. Towards a transgenic mouse model of sickle cell disease: hemoglobin SAD. EMBO J. 1991 Nov;10(11):3157–3165. doi: 10.1002/j.1460-2075.1991.tb04877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waymouth C. Erythrocyte sodium and potassium levels in normal and anemic mice. Comp Biochem Physiol A Comp Physiol. 1973 Mar 1;44(3):751–766. doi: 10.1016/0300-9629(73)90139-4. [DOI] [PubMed] [Google Scholar]


