Abstract
We have generated transgenic mice expressing the human apolipoprotein CII (apoCII) gene under the transcriptional control of the human cytochrome P-450 IA1 (CYPIA1) promoter. Human apoCII transgenic (HuCIITg) mice exhibited significant basal expression of the transgene (plasma apoCII level = 26.1 +/- 4 mg/dl) and showed further induction of transgene expression after treatment with beta-naphthoflavone. Unexpectedly, HuCIITg mice were hypertriglyceridemic and human apoCII levels correlated strongly to triglyceride levels (R = 0.89, P < 0.0001). Triglyceride levels (mg/dl +/- SEM) were elevated compared to controls in both the fed (804 +/- 113 vs 146 +/- 18, P < 0.001) and fasted (273 +/- 39 vs 61 +/- 4, P < 0.001) states. HuCIITg mice accumulated triglyceride-rich very low density lipoproteins (VLDL) with an increased apoC/apoE ratio. Tracer kinetic studies indicated delayed clearance of VLDL-triglyceride, and studies using Triton inhibition of VLDL clearance showed no increase in VLDL production. Plasma from these mice activated mouse lipoprotein lipase normally and radiolabeled VLDL were normally hydrolyzed. However, HuCIITg VLDL showed markedly decreased binding to heparin-Sepharose, suggesting that apoCII-rich, apoE-poor lipoprotein may be less accessible to cell surface lipases or receptors within their glycosaminoglycan matrices. HuCIITg mice are a promising model of hypertriglyceridemia that suggests a more complex role for apoCII in the metabolism of plasma triglycerides.
Full text
PDF




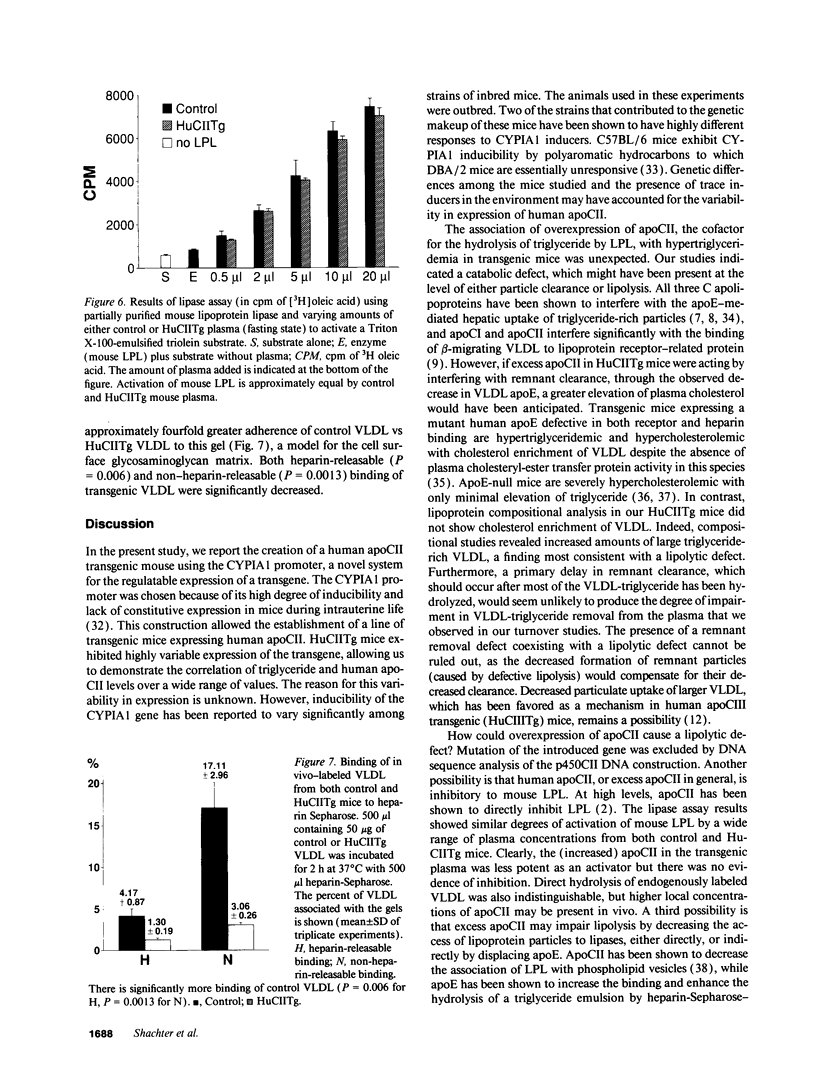


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalto-Setälä K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., Breslow J. L. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest. 1992 Nov;90(5):1889–1900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin J., Freeze H., Tejada P., Brown W. V. A comparison of molecular properties of hepatic triglyceride lipase and lipoprotein lipase from human post-heparin plasma. J Biol Chem. 1978 May 10;253(9):2912–2920. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Brinton E. A., Eisenberg S., Breslow J. L. Elevated high density lipoprotein cholesterol levels correlate with decreased apolipoprotein A-I and A-II fractional catabolic rate in women. J Clin Invest. 1989 Jul;84(1):262–269. doi: 10.1172/JCI114149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. V., Baginsky M. L. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972 Jan 31;46(2):375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- Clark A. B., Quarfordt S. H. Apolipoprotein effects on the lipolysis of perfused triglyceride by heparin-immobilized milk lipase. J Biol Chem. 1985 Apr 25;260(8):4778–4783. [PubMed] [Google Scholar]
- Cox D. W., Breckenridge W. C., Little J. A. Inheritance of apolipoprotein C-II deficiency with hypertriglyceridemia and pancreatitis. N Engl J Med. 1978 Dec 28;299(26):1421–1424. doi: 10.1056/NEJM197812282992601. [DOI] [PubMed] [Google Scholar]
- Dey A., Westphal H., Nebert D. W. Cell-specific induction of mouse Cyp1a1 mRNA during development. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7446–7450. doi: 10.1073/pnas.86.19.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. N., Le N. A., Gibson J. C. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects. Effect of weight loss. J Clin Invest. 1985 Feb;75(2):614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Fielding C. J., Olivecrona T., Shore V. G., Fielding P. E., Egelrud T. Cofactor activity of protein components of human very low density lipoproteins in the hydrolysis of triglycerides by lipoproteins lipase from different sources. Biochemistry. 1973 Apr 24;12(9):1828–1833. doi: 10.1021/bi00733a026. [DOI] [PubMed] [Google Scholar]
- Hegele R. A., Breckenridge W. C., Cox D. W., Maguire G. F., Little J. A., Connelly P. W. Interaction between variant apolipoproteins C-II and E that affects plasma lipoprotein concentrations. Arterioscler Thromb. 1991 Sep-Oct;11(5):1303–1309. doi: 10.1161/01.atv.11.5.1303. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Murase T., Takahashi K., Mori N., Kawakami M., Takaku F. Plasma apolipoprotein CII levels in hypertriglyceridemia. Metabolism. 1986 Aug;35(8):781–785. doi: 10.1016/0026-0495(86)90246-5. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y., Fielding C. J., Fielding P. E. A change in apolipoprotein B expression is required for the binding of apolipoprotein E to very low density lipoprotein. J Biol Chem. 1988 Feb 25;263(6):2744–2749. [PubMed] [Google Scholar]
- Ito Y., Azrolan N., O'Connell A., Walsh A., Breslow J. L. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990 Aug 17;249(4970):790–793. doi: 10.1126/science.2167514. [DOI] [PubMed] [Google Scholar]
- Jackson C. L., Bruns G. A., Breslow J. L. Isolation and sequence of a human apolipoprotein CII cDNA clone and its use to isolate and map to human chromosome 19 the gene for apolipoprotein CII. Proc Natl Acad Sci U S A. 1984 May;81(10):2945–2949. doi: 10.1073/pnas.81.10.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human P1-450 gene sequence and correlation of mRNA with genetic differences in benzo[a]pyrene metabolism. Nucleic Acids Res. 1985 Jun 25;13(12):4503–4520. doi: 10.1093/nar/13.12.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z. S., Brecht W. J., Miranda R. D., Hussain M. M., Innerarity T. L., Mahley R. W. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993 May 15;268(14):10160–10167. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaRosa J. C., Levy R. I., Herbert P., Lux S. E., Fredrickson D. S. A specific apoprotein activator for lipoprotein lipase. Biochem Biophys Res Commun. 1970 Oct 9;41(1):57–62. doi: 10.1016/0006-291x(70)90468-7. [DOI] [PubMed] [Google Scholar]
- Landis B. A., Rotolo F. S., Meyers W. C., Clark A. B., Quarfordt S. H. Influence of apolipoprotein E on soluble and heparin-immobilized hepatic lipase. Am J Physiol. 1987 Jun;252(6 Pt 1):G805–G810. doi: 10.1152/ajpgi.1987.252.6.G805. [DOI] [PubMed] [Google Scholar]
- Okey A. B. Enzyme induction in the cytochrome P-450 system. Pharmacol Ther. 1990;45(2):241–298. doi: 10.1016/0163-7258(90)90030-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg D. W., Leff T. Regulation of cytochrome P450 in cultured human colonic cells. Arch Biochem Biophys. 1993 Jan;300(1):186–192. doi: 10.1006/abbi.1993.1026. [DOI] [PubMed] [Google Scholar]
- Rosenberg D. W. Tissue-specific induction of the carcinogen inducible cytochrome P450 isoform, P450IAI, in colonic epithelium. Arch Biochem Biophys. 1991 Jan;284(1):223–226. doi: 10.1016/0003-9861(91)90288-t. [DOI] [PubMed] [Google Scholar]
- Shachter N. S., Zhu Y., Walsh A., Breslow J. L., Smith J. D. Localization of a liver-specific enhancer in the apolipoprotein E/C-I/C-II gene locus. J Lipid Res. 1993 Oct;34(10):1699–1707. [PubMed] [Google Scholar]
- Shelburne F., Hanks J., Meyers W., Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J Clin Invest. 1980 Mar;65(3):652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai K., Matsuoka N., Jackson R. L. Interaction of lipoprotein lipase with phospholipid vesicles. Role of apolipoprotein C-II and heparin. Biochim Biophys Acta. 1981 Sep 24;665(3):504–510. doi: 10.1016/0005-2760(81)90264-2. [DOI] [PubMed] [Google Scholar]
- Simonet W. S., Bucay N., Pitas R. E., Lauer S. J., Taylor J. M. Multiple tissue-specific elements control the apolipoprotein E/C-I gene locus in transgenic mice. J Biol Chem. 1991 May 15;266(14):8651–8654. [PubMed] [Google Scholar]
- Smit M., van der Kooij-Meijs E., Woudt L. P., Havekes L. M., Frants R. R. Exact localization of the familial dysbetalipoproteinemia associated HpaI restriction site in the promoter region of the APOC1 gene. Biochem Biophys Res Commun. 1988 May 16;152(3):1282–1288. doi: 10.1016/s0006-291x(88)80424-8. [DOI] [PubMed] [Google Scholar]
- Smith J. D., Plump A. S., Hayek T., Walsh A., Breslow J. L. Accumulation of human apolipoprotein E in the plasma of transgenic mice. J Biol Chem. 1990 Sep 5;265(25):14709–14712. [PubMed] [Google Scholar]
- Walsh A., Ito Y., Breslow J. L. High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem. 1989 Apr 15;264(11):6488–6494. [PubMed] [Google Scholar]
- Wang C. S. Structure and functional properties of apolipoprotein C-II. Prog Lipid Res. 1991;30(2-3):253–258. doi: 10.1016/0163-7827(91)90022-w. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W., Kowal R. C., Herz J., Goldstein J. L., Brown M. S. Apolipoprotein C-I modulates the interaction of apolipoprotein E with beta-migrating very low density lipoproteins (beta-VLDL) and inhibits binding of beta-VLDL to low density lipoprotein receptor-related protein. J Biol Chem. 1990 Dec 25;265(36):22453–22459. [PubMed] [Google Scholar]
- Whitlock J. P., Jr The regulation of cytochrome P-450 gene expression. Annu Rev Pharmacol Toxicol. 1986;26:333–369. doi: 10.1146/annurev.pa.26.040186.002001. [DOI] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J Biol Chem. 1980 Jun 10;255(11):5475–5480. [PubMed] [Google Scholar]
- Windler E., Havel R. J. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985 May;26(5):556–565. [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]






