Abstract
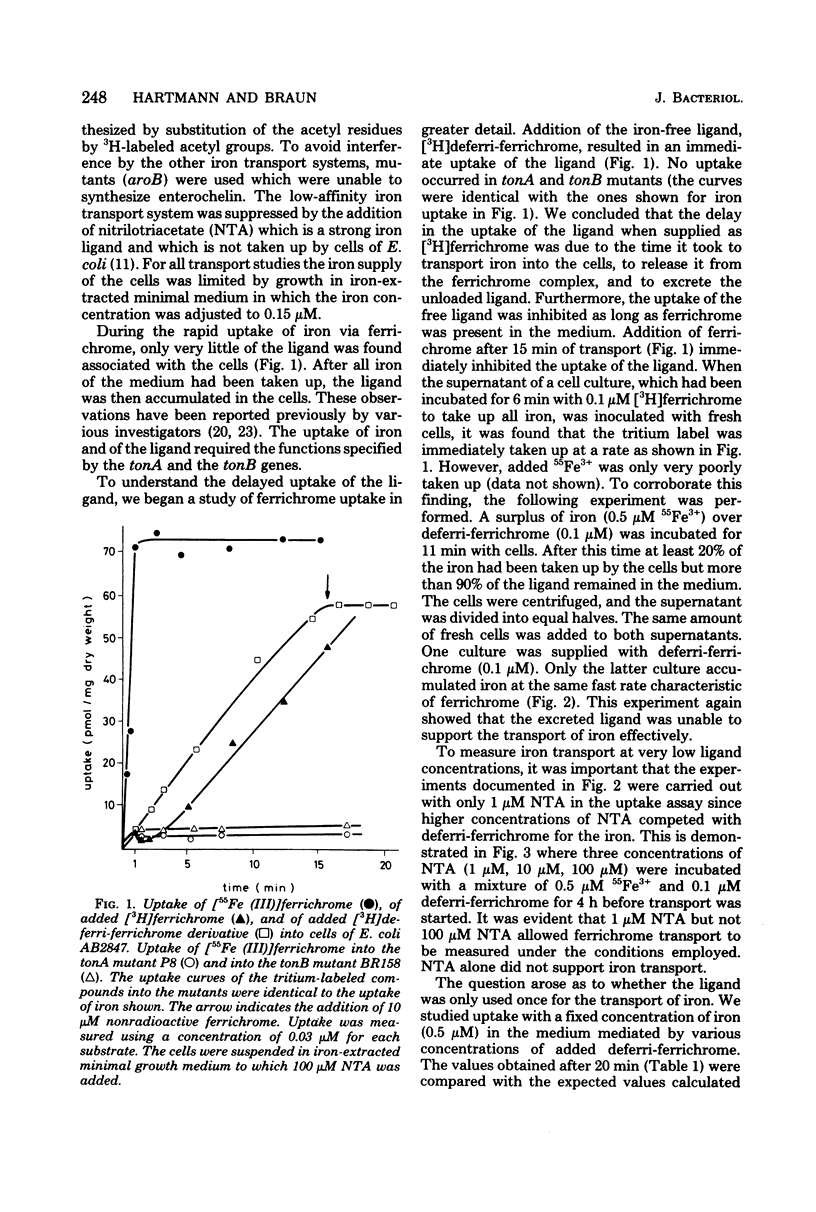
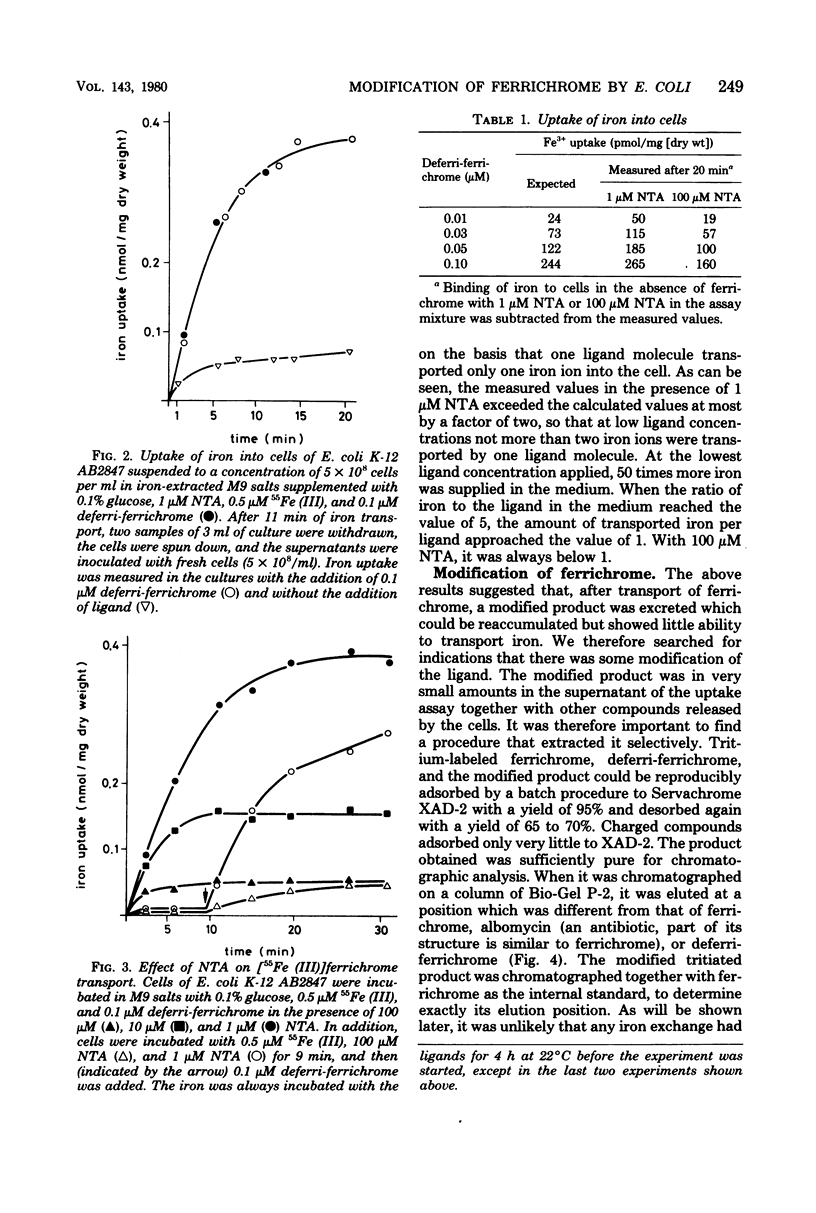
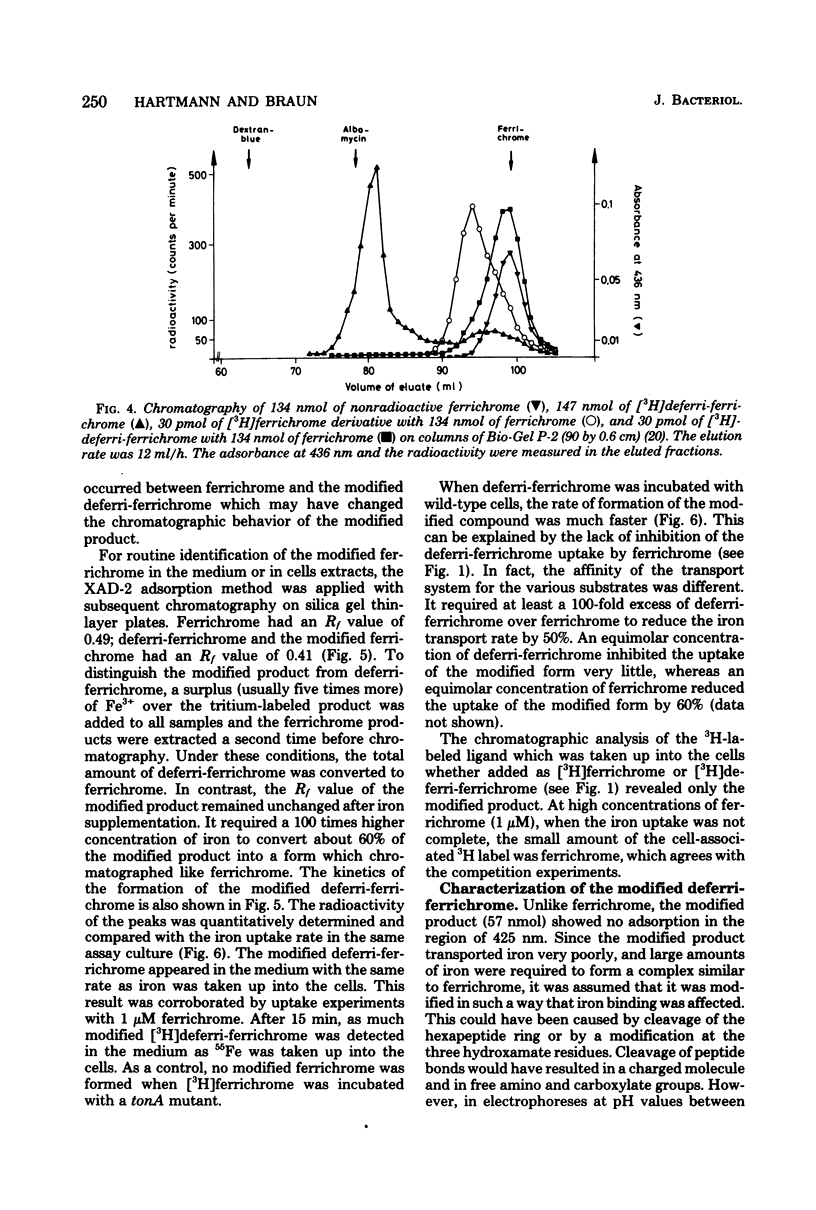
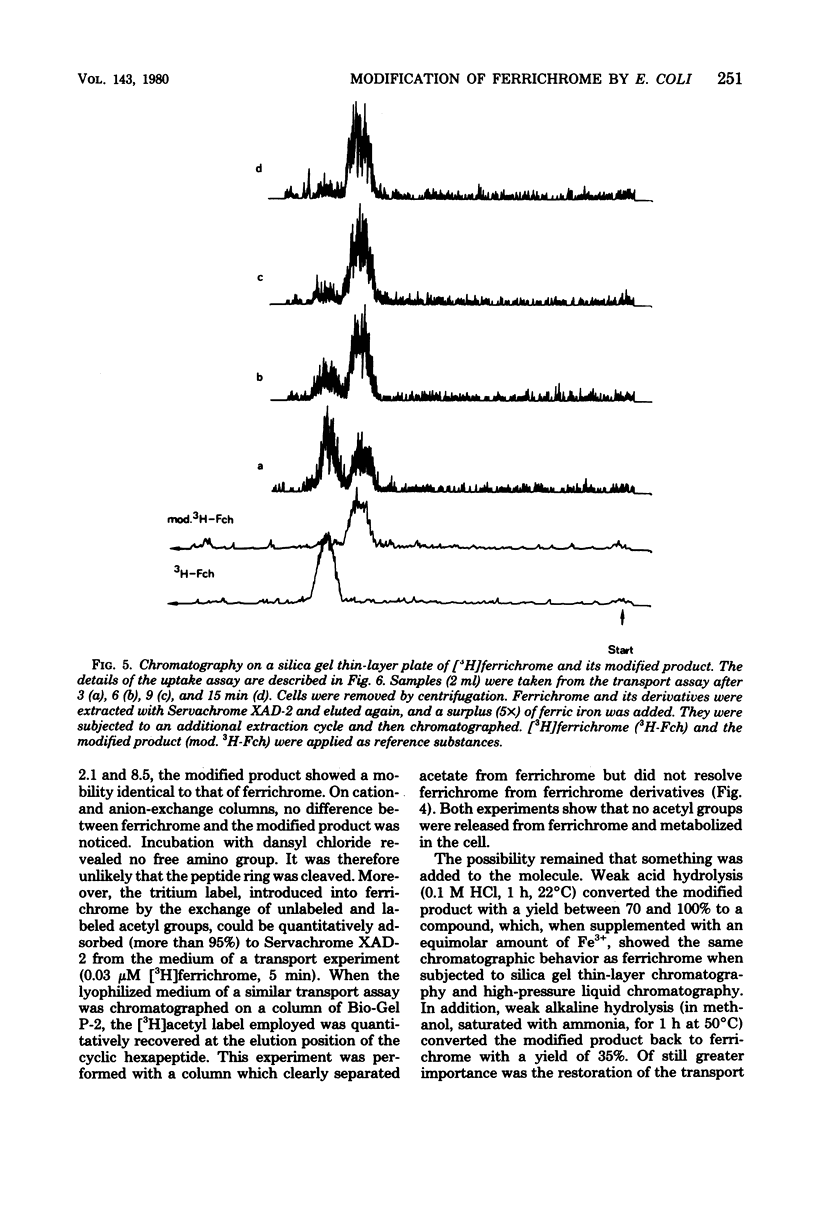
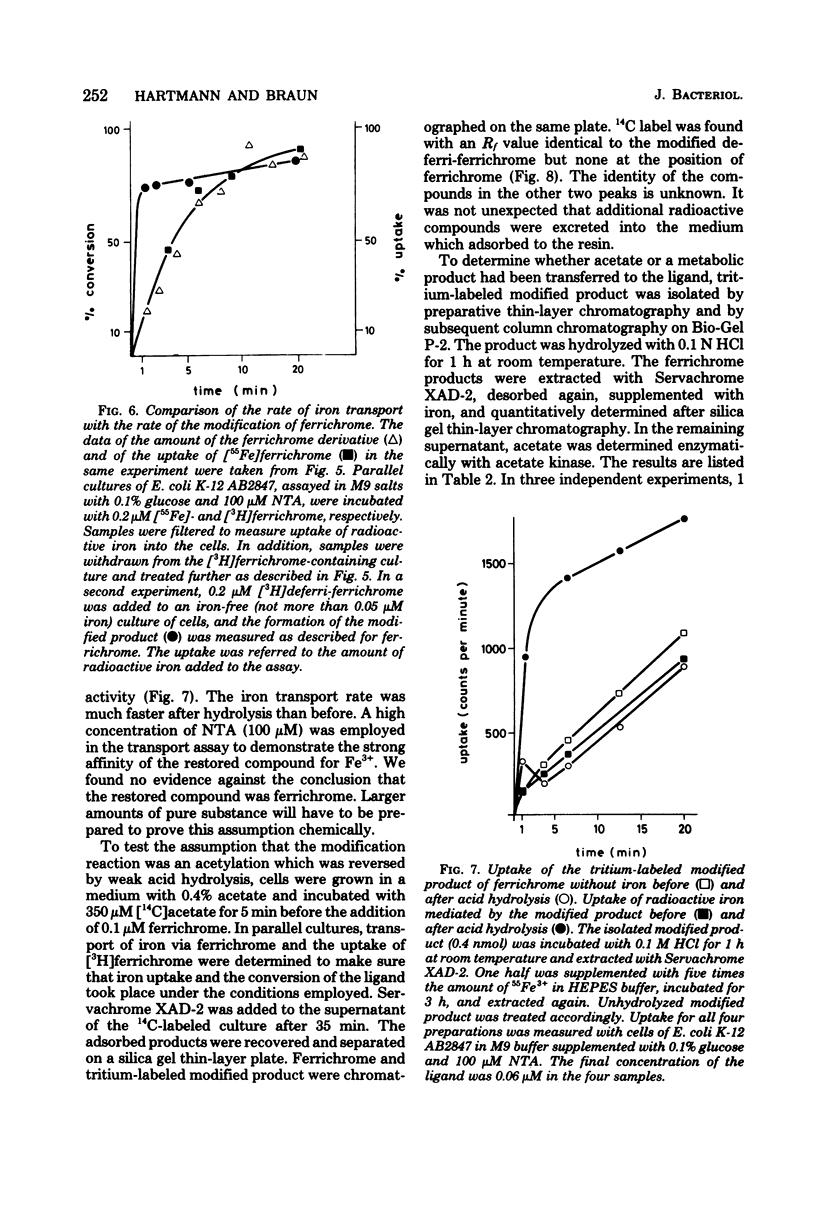
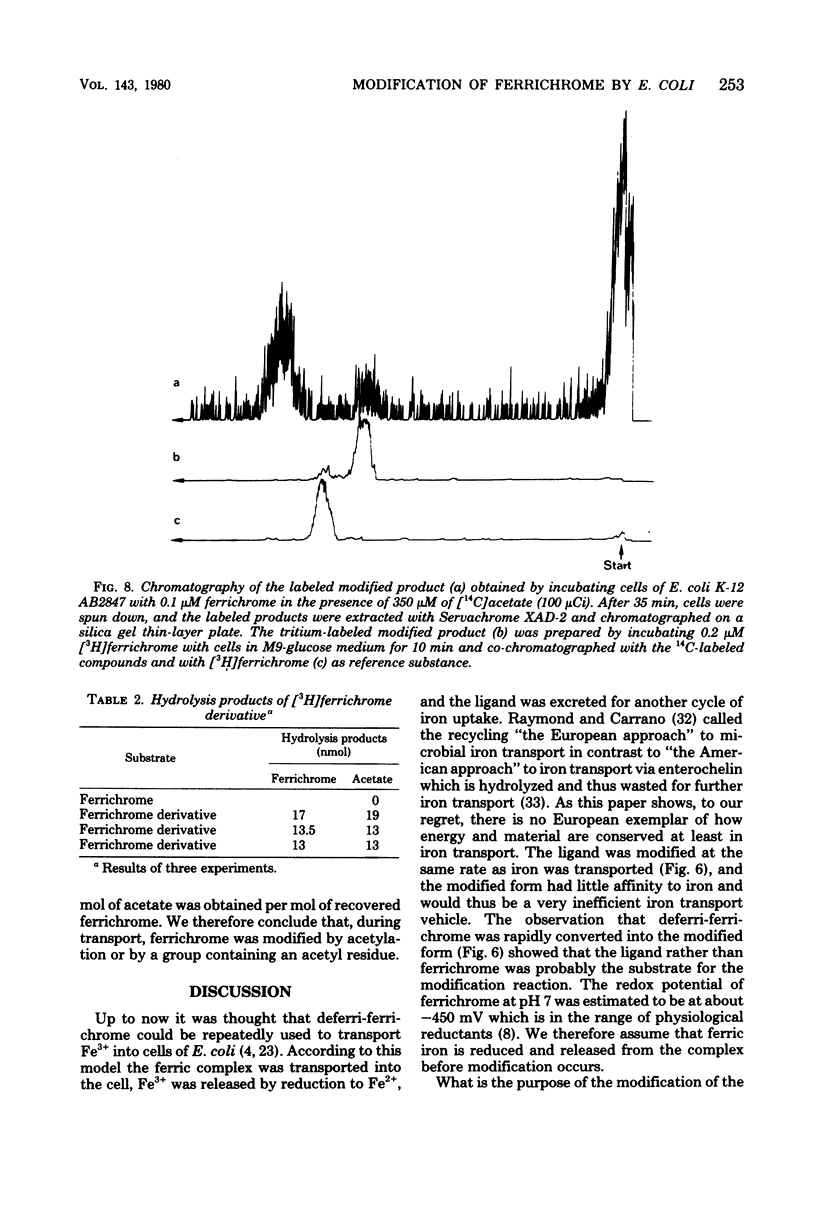
During the transport of iron as ferrichrome complex into cells of Escherichia coli K-12, the ligand was modified and excreted into the medium. The rate of the formation of the modified product corresponded with the rate of iron transport. The modified product showed a decreased affinity for ferric iron and did not serve as an effective iron ionophore. After all of the ferrichrome had been converted, the modified product was taken up into the cell in an iron-free form. The uptake of ferrichrome and of the modified product depended on the transport system specified by the tonA and tonB genes. The modified product could be converted back into ferrichrome by mild acid or alkaline hydrolysis. One mole of acetate was released per mole of ferrichrome. It is proposed that one N-hydroxyl group of ferrichrome is acetylated to explain the low affinity for iron as the N-hydroxyl groups form the ligands for iron (III). A weak ester linkage by which the acetyl group is covalently bonded would account for the easy hydrolysis. The iron-free form of ferrichrome, deferri-ferrichrome, was also rapidly converted when incubated with cells with a functional transport system. It is therefore likely that iron is released from ferrichrome by reduction before modification takes place. The conversion of the ligand could be a mechanism by which cells rid themselves of a potentially deleterious ligand for iron in the cytoplasm. A possible role in ferrichrome transport is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Schnaitman C. A., Kadner R. J. Functional stability of the bfe and tonB gene products in Escherichia coli. J Bacteriol. 1977 May;130(2):750–758. doi: 10.1128/jb.130.2.750-758.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Braun V., Schaller K., Wolff H. A common receptor protein for phage T5 and colicin M in the outer membrane of Escherichia coli B. Biochim Biophys Acta. 1973 Sep 27;323(1):87–97. doi: 10.1016/0005-2736(73)90433-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. Characterization of the receptor protein for phage T5 and colicin M in the outer membrane of E. coli B. FEBS Lett. 1973 Aug 1;34(1):77–80. doi: 10.1016/0014-5793(73)80707-0. [DOI] [PubMed] [Google Scholar]
- Cooper S. R., McArdle J. V., Raymond K. N. Siderophore electrochemistry: relation to intracellular iron release mechanism. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3551–3554. doi: 10.1073/pnas.75.8.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Naegeli H. U., Braun V. Iron supply of Escherichia coli with polymer-bound ferricrocin. Eur J Biochem. 1979 Aug 15;99(1):39–47. doi: 10.1111/j.1432-1033.1979.tb13228.x. [DOI] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- Graham A. C., Stocker B. A. Genetics of sensitivity of Salmonella species to colicin M and bacteriophages T5, T1, and ES18. J Bacteriol. 1977 Jun;130(3):1214–1223. doi: 10.1128/jb.130.3.1214-1223.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport in Escherichia coli K-12. 2,3-Dihydroxybenzoate-promoted iron uptake. Arch Microbiol. 1977 Sep 28;114(3):231–239. doi: 10.1007/BF00446867. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport of Escherichia coli K-12: involvement of the colicin B receptor and of a citrate-inducible protein. J Bacteriol. 1976 Sep;127(3):1370–1375. doi: 10.1128/jb.127.3.1370-1375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. A function common to iron-enterochelin transport and action of colicins B, I, V in Escherichia coli. FEBS Lett. 1975 Nov 15;59(2):277–281. doi: 10.1016/0014-5793(75)80392-9. [DOI] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Fiedler H. P., Braun V. Uptake and conversion of the antibiotic albomycin by Escherichia coli K-12. Eur J Biochem. 1979 Sep;99(3):517–524. doi: 10.1111/j.1432-1033.1979.tb13283.x. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Hunt A. G., Masters P. S., Lieberman M. A. Requirements of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1213–1217. doi: 10.1073/pnas.76.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Involvement of outer membrane proteins in enterochelin-mediated iron uptake in Escherichia coli. J Biochem. 1977 Mar;81(3):749–756. doi: 10.1093/oxfordjournals.jbchem.a131513. [DOI] [PubMed] [Google Scholar]
- Leong J., Neilands J. B. Mechanisms of siderophore iron transport in enteric bacteria. J Bacteriol. 1976 May;126(2):823–830. doi: 10.1128/jb.126.2.823-830.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Wayne R., Neilands J. B. In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun. 1975 May 19;64(2):687–693. doi: 10.1016/0006-291x(75)90375-7. [DOI] [PubMed] [Google Scholar]
- McIntosh M. A., Chenault S. S., Earhart C. F. Genetic and physiological studies on the relationship between colicin B resistance and ferrienterochelin uptake in Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):653–657. doi: 10.1128/jb.137.1.653-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plastow G. S., Holland I. B. Identification of an Escherichia coli inner membrane polypeptide specified by a lambda-tonB transducing. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1007–1014. doi: 10.1016/0006-291x(79)91927-2. [DOI] [PubMed] [Google Scholar]
- Postle K., Reznikoff W. S. Identification of the Escherichia coli tonB gene product in minicells containing tonB hybrid plasmids. J Mol Biol. 1979 Jul 5;131(3):619–636. doi: 10.1016/0022-2836(79)90011-1. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. The role of colicin receptors in the uptake of ferrienterochelin by Escherichia coli K-12. Biochem Biophys Res Commun. 1977 Feb 7;74(3):903–911. doi: 10.1016/0006-291x(77)91604-7. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Uptake of ferrienterochelin by Escherichia coli: energy dependent stage of uptake. J Bacteriol. 1977 Apr;130(1):26–36. doi: 10.1128/jb.130.1.26-36.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


