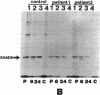
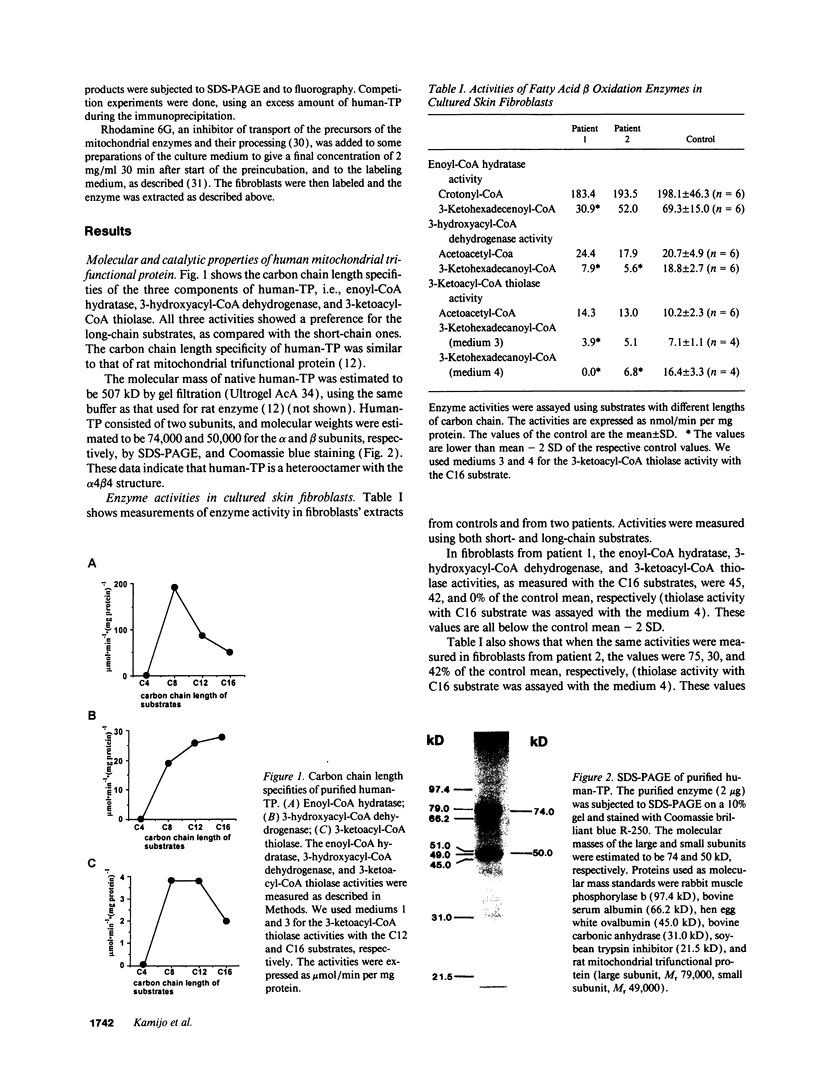
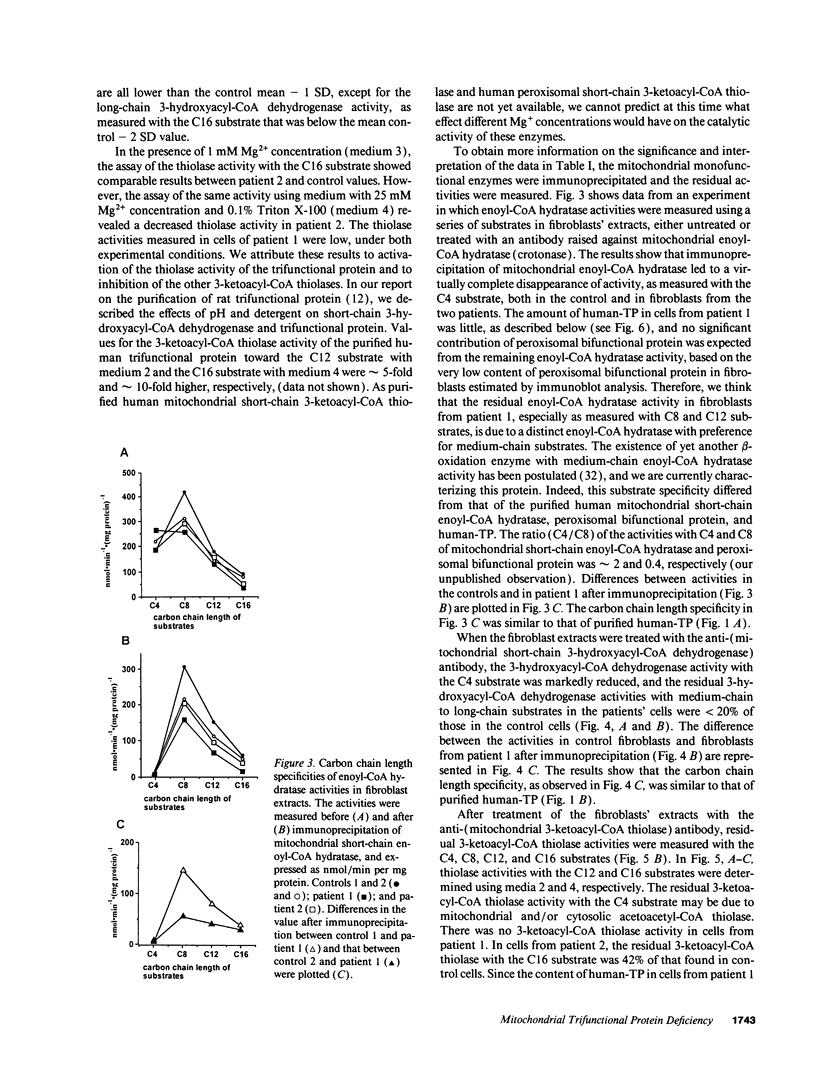
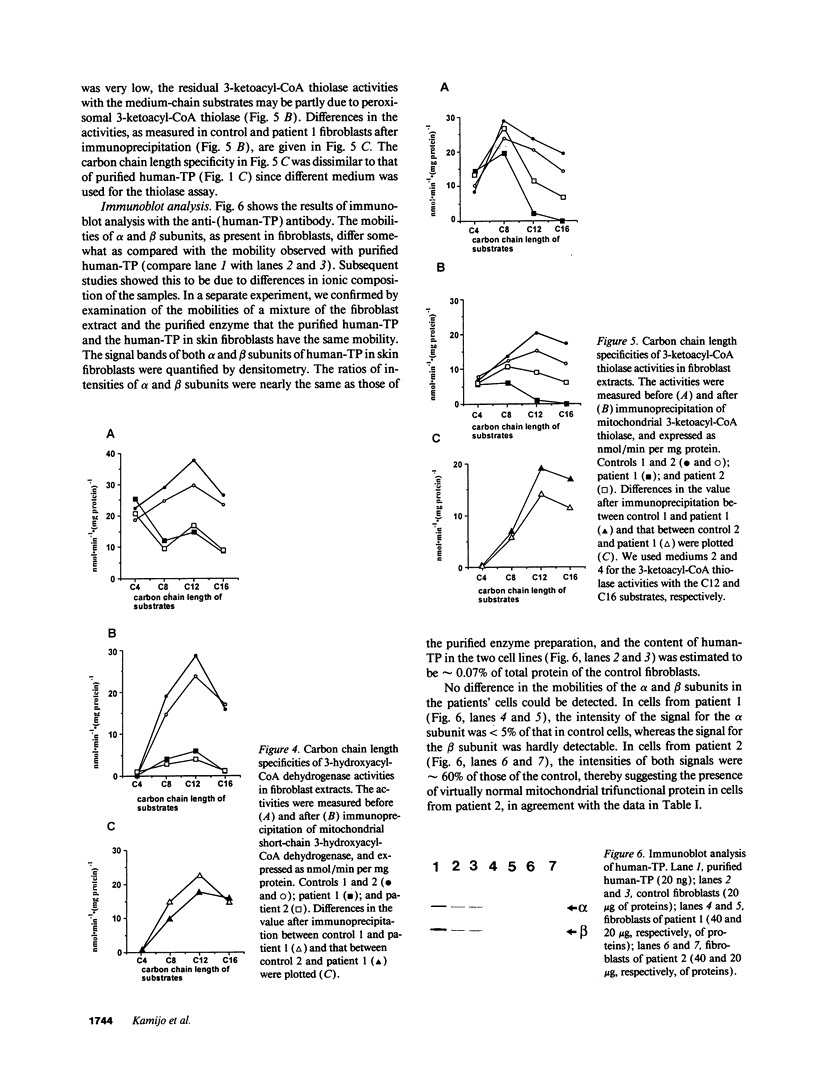
Abstract
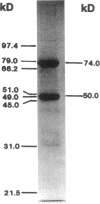
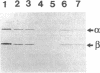
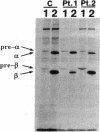
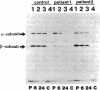
We examined the enzyme protein and biosynthesis of human trifunctional protein harboring enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase activity in cultured skin fibroblasts from two patients with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. The following results were obtained. (a) In cells from patient 1, immunoblot analysis and pulse-chase experiments indicated that the content of trifunctional protein was < 10% of that in control cells, due to a very rapid degradation of protein newly synthesized in the mitochondria. The diminution of trifunctional protein was associated with a decreased activity of enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase, when measured using medium-chain to long-chain substrates. (b) In cells from patient 2, the rate of degradation of newly synthesized trifunctional protein was faster than that in control cells, giving rise to a trifunctional protein amounting to 60% of the control levels. The 3-hydroxy-acyl-CoA dehydrogenase activity with medium-chain to long-chain substrates was decreased drastically, with minor changes in activities of the two other enzymes. These data suggest a subtle abnormality of trifunctional protein in cells from patient 2. Taken together, the results obtained show that in both patients, long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency is caused by an abnormality in the trifunctional protein, even though there is a heterogeneity in both patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter K., Pollitt R. J., Middleton B. Human liver long-chain 3-hydroxyacyl-coenzyme A dehydrogenase is a multifunctional membrane-bound beta-oxidation enzyme of mitochondria. Biochem Biophys Res Commun. 1992 Mar 16;183(2):443–448. doi: 10.1016/0006-291x(92)90501-b. [DOI] [PubMed] [Google Scholar]
- Coates P. M., Tanaka K. Molecular basis of mitochondrial fatty acid oxidation defects. J Lipid Res. 1992 Aug;33(8):1099–1110. [PubMed] [Google Scholar]
- El-Fakhri M., Middleton B. The existence of an inner-membrane-bound, long acyl-chain-specific 3-hydroxyacyl-CoA dehydrogenase in mammalian mitochondria. Biochim Biophys Acta. 1982 Nov 12;713(2):270–279. doi: 10.1016/0005-2760(82)90244-2. [DOI] [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Hashimoto T. Purification and properties of rat liver acyl-CoA dehydrogenases and electron transfer flavoprotein. J Biochem. 1981 Dec;90(6):1739–1750. doi: 10.1093/oxfordjournals.jbchem.a133651. [DOI] [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Osumi T., Hashimoto T., Ui N. Properties of mitochondria and peroxisomal enoyl-CoA hydratases from rat liver. J Biochem. 1980 Oct;88(4):1059–1070. doi: 10.1093/oxfordjournals.jbchem.a133057. [DOI] [PubMed] [Google Scholar]
- Gear A. R. Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3628–3637. [PubMed] [Google Scholar]
- Hale D. E., Thorpe C., Braat K., Wright J. H., Roe C. R., Coates P. M., Hashimoto T., Glasgow A. M. The L-3-hydroxyacyl-CoA dehydrogenase deficiency. Prog Clin Biol Res. 1990;321:503–510. [PubMed] [Google Scholar]
- Ikeda Y., Keese S. M., Tanaka K. Molecular heterogeneity of variant isovaleryl-CoA dehydrogenase from cultured isovaleric acidemia fibroblasts. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7081–7085. doi: 10.1073/pnas.82.20.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S., Ueda S., Mizugaki M., Kawaguchi A. Purification of the multienzyme complex for fatty acid oxidation from Pseudomonas fragi and reconstitution of the fatty acid oxidation system. J Biochem. 1990 Feb;107(2):184–189. doi: 10.1093/oxfordjournals.jbchem.a123023. [DOI] [PubMed] [Google Scholar]
- Izai K., Uchida Y., Orii T., Yamamoto S., Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. I. Purification and properties of very-long-chain acyl-coenzyme A dehydrogenase. J Biol Chem. 1992 Jan 15;267(2):1027–1033. [PubMed] [Google Scholar]
- Jackson S., Bartlett K., Land J., Moxon E. R., Pollitt R. J., Leonard J. V., Turnbull D. M. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Pediatr Res. 1991 Apr;29(4 Pt 1):406–411. doi: 10.1203/00006450-199104000-00016. [DOI] [PubMed] [Google Scholar]
- Jackson S., Kler R. S., Bartlett K., Briggs H., Bindoff L. A., Pourfarzam M., Gardner-Medwin D., Turnbull D. M. Combined enzyme defect of mitochondrial fatty acid oxidation. J Clin Invest. 1992 Oct;90(4):1219–1225. doi: 10.1172/JCI115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo T., Aoyama T., Miyazaki J., Hashimoto T. Molecular cloning of the cDNAs for the subunits of rat mitochondrial fatty acid beta-oxidation multienzyme complex. Structural and functional relationships to other mitochondrial and peroxisomal beta-oxidation enzymes. J Biol Chem. 1993 Dec 15;268(35):26452–26460. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYNEN F., HENNING U., BUBLITZ C., SORBO B., KROPLIN-RUEFF L. Der chemische Mechanismus der Acetessigsäurebildung in der Leber. Biochem Z. 1958;330(4):269–295. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T. The presence of a new 3-oxoacyl-CoA thiolase in rat liver peroxisomes. Eur J Biochem. 1980 Feb;103(3):589–596. doi: 10.1111/j.1432-1033.1980.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Occurrence of two 3-hydroxyacyl-CoA dehydrogenases in rat liver. Biochim Biophys Acta. 1979 Aug 30;574(2):258–267. [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Peroxisomal beta oxidation system of rat liver. Copurification of enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase. Biochem Biophys Res Commun. 1979 Jul 27;89(2):580–584. doi: 10.1016/0006-291x(79)90669-7. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Purification and properties of mitochondrial and peroxisomal 3-hydroxyacyl-CoA dehydrogenase from rat liver. Arch Biochem Biophys. 1980 Aug;203(1):372–383. doi: 10.1016/0003-9861(80)90189-7. [DOI] [PubMed] [Google Scholar]
- Przyrembel H., Jakobs C., IJlst L., de Klerk J. B., Wanders R. J. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 1991;14(5):674–680. doi: 10.1007/BF01799932. [DOI] [PubMed] [Google Scholar]
- Rhead W. J. Inborn errors of fatty acid oxidation in man. Clin Biochem. 1991 Aug;24(4):319–329. doi: 10.1016/0009-9120(91)80006-o. [DOI] [PubMed] [Google Scholar]
- Saudubray J. M., Mitchell G., Bonnefont J. P., Schwartz G., Nuttin C., Munnich A., Brivet M., Vassault A., Demaugre F., Rabier D. Approach to the patient with a fatty acid oxidation disorder. Prog Clin Biol Res. 1992;375:271–288. [PubMed] [Google Scholar]
- Steinman H. M., Hill R. L. Bovine liver crotonase (enoyl coenzyme A hydratase). EC 4.2.1.17 L-3-hydroxyacyl-CoA hydrolyase. Methods Enzymol. 1975;35:136–151. doi: 10.1016/0076-6879(75)35149-5. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Shimozawa N., Yajima S., Yamaguchi S., Orii T., Hashimoto T. Effects of sodium 2-[5-(4-chlorophenyl)pentyl]-oxirane-2-carboxylate (POCA) on fatty acid oxidation in fibroblasts from patients with peroxisomal diseases. Biochem Pharmacol. 1991 Feb 1;41(3):453–456. doi: 10.1016/0006-2952(91)90544-f. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y., Izai K., Orii T., Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J Biol Chem. 1992 Jan 15;267(2):1034–1041. [PubMed] [Google Scholar]
- Wanders R. J., Duran M., Ijlst L., de Jager J. P., van Gennip A. H., Jakobs C., Dorland L., van Sprang F. J. Sudden infant death and long-chain 3-hydroxyacyl-CoA dehydrogenase. Lancet. 1989 Jul 1;2(8653):52–53. doi: 10.1016/s0140-6736(89)90300-0. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., IJlst L., Poggi F., Bonnefont J. P., Munnich A., Brivet M., Rabier D., Saudubray J. M. Human trifunctional protein deficiency: a new disorder of mitochondrial fatty acid beta-oxidation. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1139–1145. doi: 10.1016/0006-291x(92)91350-y. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., IJlst L., van Gennip A. H., Jakobs C., de Jager J. P., Dorland L., van Sprang F. J., Duran M. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency: identification of a new inborn error of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis. 1990;13(3):311–314. doi: 10.1007/BF01799383. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Orii T., Sakura N., Miyazawa S., Hashimoto T. Defect in biosynthesis of mitochondrial acetoacetyl-coenzyme A thiolase in cultured fibroblasts from a boy with 3-ketothiolase deficiency. J Clin Invest. 1988 Mar;81(3):813–817. doi: 10.1172/JCI113388. [DOI] [PMC free article] [PubMed] [Google Scholar]