Abstract
Acute salpingitis complicating cervical gonococcal infection is a significant cause of infertility. Relatively little data are available concerning the pathophysiologic mechanisms of this disease. A cohort of 243 prostitutes residing in Nairobi were followed between March 1985 and April 1988. Gonococcal cultures were performed at each visit, and acute salpingitis was diagnosed clinically. Serum at enrollment was tested by immunoblot for antibody to gonococcal outer membrane proteins. 8.6% (146/1689) of gonococcal infections were complicated by salpingitis. Increased risk of salpingitis was associated with younger age, shorter duration of prostitution, HIV infection, number of gonococcal infections, and episodes of nongonococcal salpingitis. Rmp antibody increased the risk of salpingitis. Antibody to Opa decreased the risk of salpingitis. By logistic regression analysis, antibody to Opa was independently associated with decreased risk of gonococcal salpingitis (adjusted odds ratio [OR], 0.35; 95% confidence interval [95%CI], 0.17-0.76); HIV infection (adjusted OR, 3.5; 95% CI, 0.96-12.8) and episodes of nongonococcal salpingitis (adjusted OR, 3.4; 95% CI, 1.8-6.4) were independently associated with an increased risk of salpingitis. Antibody to Opa appears to protect against ascending gonococcal infection, perhaps by interfering with Opa mediated adherence and endocytosis. The demonstration of natural immunity that protects against upper genital tract infection in women suggests that a vaccine to prevent gonococcal salpingitis is possible.
Full text
PDF

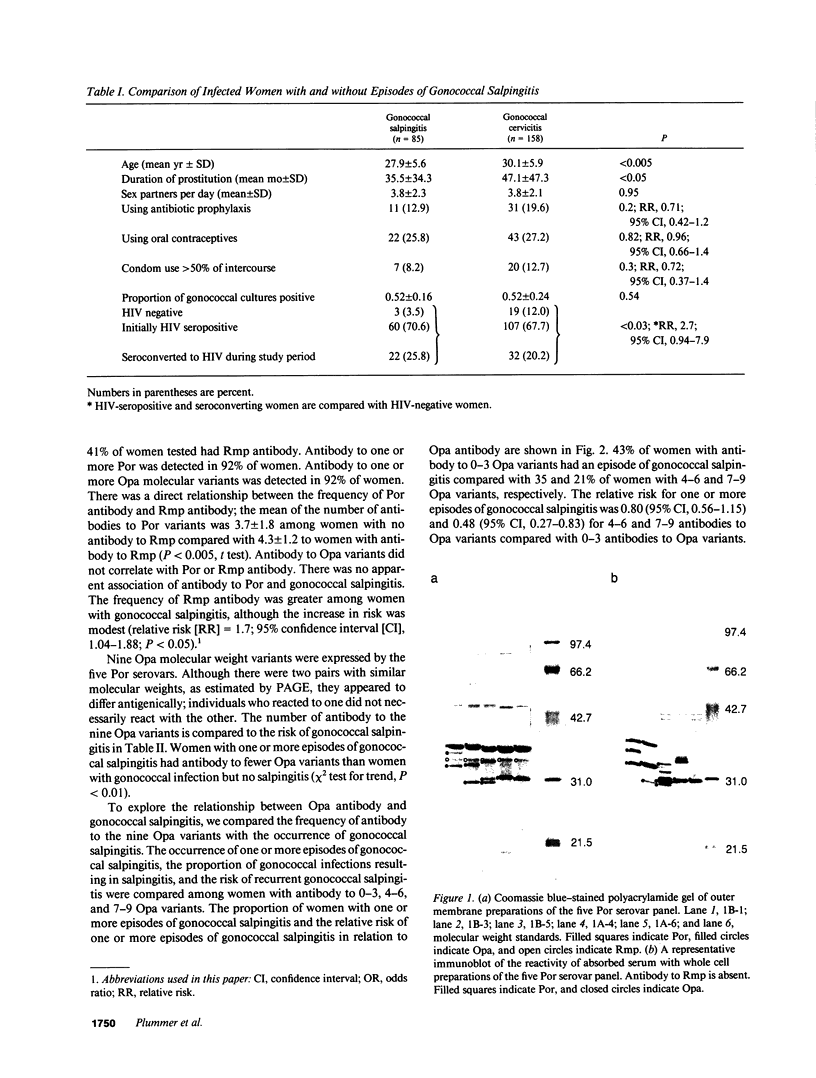
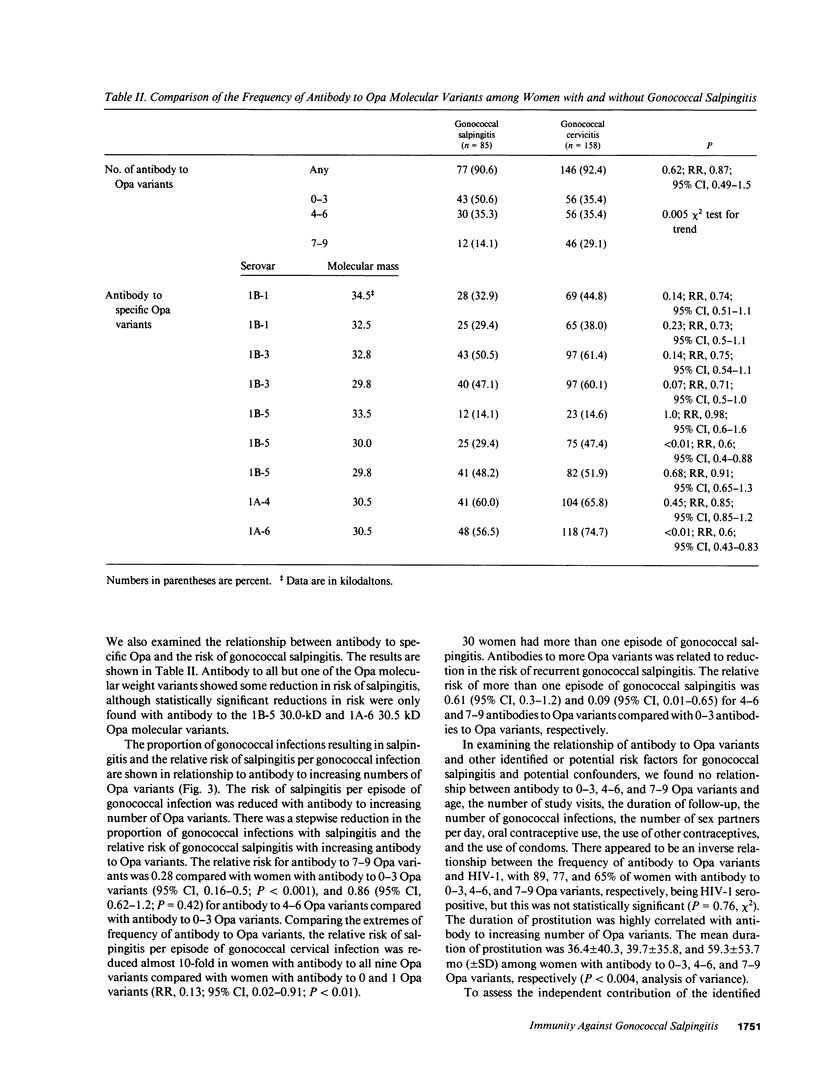



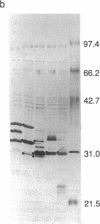
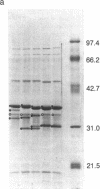
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aral S. O., Mosher W. D., Cates W., Jr Self-reported pelvic inflammatory disease in the United States, 1988. JAMA. 1991 Nov 13;266(18):2570–2573. [PubMed] [Google Scholar]
- Barritt D. S., Schwalbe R. S., Klapper D. G., Cannon J. G. Antigenic and structural differences among six proteins II expressed by a single strain of Neisseria gonorrhoeae. Infect Immun. 1987 Sep;55(9):2026–2031. doi: 10.1128/iai.55.9.2026-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat K. S., Gibbs C. P., Barrera O., Morrison S. G., Jähnig F., Stern A., Kupsch E. M., Meyer T. F., Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991 Aug;5(8):1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Brooks G. F., Olinger L., Lammel C. J., Bhat K. S., Calvello C. A., Palmer M. L., Knapp J. S., Stephens R. S. Prevalence of gene sequences coding for hypervariable regions of Opa (protein II) in Neisseria gonorrhoeae. Mol Microbiol. 1991 Dec;5(12):3063–3072. doi: 10.1111/j.1365-2958.1991.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Garnett G. P., Swinton J., Anderson R. M. Gonococcal infection and human fertility in sub-Saharan Africa. Proc Biol Sci. 1991 Nov 22;246(1316):173–177. doi: 10.1098/rspb.1991.0141. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Eschenbach D. A., Knapp J. S., Holmes K. K. Gonococcal salpingitis is less likely to recur with Neisseria gonorrhoeae of the same principal outer membrane protein antigenic type. Am J Obstet Gynecol. 1980 Dec 1;138(7 Pt 2):978–980. doi: 10.1016/0002-9378(80)91091-1. [DOI] [PubMed] [Google Scholar]
- Connell T. D., Shaffer D., Cannon J. G. Characterization of the repertoire of hypervariable regions in the Protein II (opa) gene family of Neisseria gonorrhoeae. Mol Microbiol. 1990 Mar;4(3):439–449. doi: 10.1111/j.1365-2958.1990.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Dekker N. P., Lammel C. J., Mandrell R. E., Brooks G. F. Opa (protein II) influences gonococcal organization in colonies, surface appearance, size and attachment to human fallopian tube tissues. Microb Pathog. 1990 Jul;9(1):19–31. doi: 10.1016/0882-4010(90)90037-q. [DOI] [PubMed] [Google Scholar]
- Draper D. L., James J. F., Brooks G. F., Sweet R. L. Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical Neisseria gonorrhoeae isolates from women with acute salpingitis. Infect Immun. 1980 Mar;27(3):882–888. doi: 10.1128/iai.27.3.882-888.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach D. A., Harnisch J. P., Holmes K. K. Pathogenesis of acute pelvic inflammatory disease: role of contraception and other risk factors. Am J Obstet Gynecol. 1977 Aug 15;128(8):838–850. doi: 10.1016/0002-9378(77)90051-5. [DOI] [PubMed] [Google Scholar]
- Fischer S. H., Rest R. F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988 Jun;56(6):1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes D. A. Deaths due to sexually transmitted diseases. The forgotten component of reproductive mortality. JAMA. 1986 Apr 4;255(13):1727–1729. [PubMed] [Google Scholar]
- Hoegsberg B., Abulafia O., Sedlis A., Feldman J., DesJalais D., Landesman S., Minkoff H. Sexually transmitted diseases and human immunodeficiency virus infection among women with pelvic inflammatory disease. Am J Obstet Gynecol. 1990 Oct;163(4 Pt 1):1135–1139. doi: 10.1016/0002-9378(90)90671-s. [DOI] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Zurlinden E., Lammel C. J., Brooks G. F. Relation of protein I and colony opacity to serum killing of Neisseria gonorrhoeae. J Infect Dis. 1982 Jan;145(1):37–44. doi: 10.1093/infdis/145.1.37. [DOI] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Lammel C. J., Sweet R. L., Rice P. A., Knapp J. S., Schoolnik G. K., Heilbron D. C., Brooks G. F. Antibody-antigen specificity in the immune response to infection with Neisseria gonorrhoeae. J Infect Dis. 1985 Nov;152(5):990–1001. doi: 10.1093/infdis/152.5.990. [DOI] [PubMed] [Google Scholar]
- Mayer L. W. Rates in vitro changes of gonococcal colony opacity phenotypes. Infect Immun. 1982 Aug;37(2):481–485. doi: 10.1128/iai.37.2.481-485.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981 Mar;143(3):413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., van Putten J. P. Genetic mechanisms and biological implications of phase variation in pathogenic neisseriae. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S139–S145. doi: 10.1128/cmr.2.suppl.s139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngugi E. N., Plummer F. A., Simonsen J. N., Cameron D. W., Bosire M., Waiyaki P., Ronald A. R., Ndinya-Achola J. O. Prevention of transmission of human immunodeficiency virus in Africa: effectiveness of condom promotion and health education among prostitutes. Lancet. 1988 Oct 15;2(8616):887–890. doi: 10.1016/s0140-6736(88)92480-4. [DOI] [PubMed] [Google Scholar]
- Plummer F. A., Chubb H., Simonsen J. N., Bosire M., Slaney L., Maclean I., Ndinya-Achola J. O., Waiyaki P., Brunham R. C. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J Clin Invest. 1993 Jan;91(1):339–343. doi: 10.1172/JCI116190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer F. A., Simonsen J. N., Chubb H., Slaney L., Kimata J., Bosire M., Ndinya-Achola J. O., Ngugi E. N. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989 May;83(5):1472–1476. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., McCormack W. M., Kasper D. L. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J Immunol. 1980 May;124(5):2105–2109. [PubMed] [Google Scholar]
- Safrin S., Dattel B. J., Hauer L., Sweet R. L. Seroprevalence and epidemiologic correlates of human immunodeficiency virus infection in women with acute pelvic inflammatory disease. Obstet Gynecol. 1990 Apr;75(4):666–670. [PubMed] [Google Scholar]
- Shafer W. M., Rest R. F. Interactions of gonococci with phagocytic cells. Annu Rev Microbiol. 1989;43:121–145. doi: 10.1146/annurev.mi.43.100189.001005. [DOI] [PubMed] [Google Scholar]
- Simon D., Rest R. F. Escherichia coli expressing a Neisseria gonorrhoeae opacity-associated outer membrane protein invade human cervical and endometrial epithelial cell lines. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5512–5516. doi: 10.1073/pnas.89.12.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Swanson J., Barrera O., Sola J., Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988 Dec 1;168(6):2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986 Feb;132(2):503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- Weel J. F., Hopman C. T., van Putten J. P. In situ expression and localization of Neisseria gonorrhoeae opacity proteins in infected epithelial cells: apparent role of Opa proteins in cellular invasion. J Exp Med. 1991 Jun 1;173(6):1395–1405. doi: 10.1084/jem.173.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weström L. Effect of acute pelvic inflammatory disease on fertility. Am J Obstet Gynecol. 1975 Mar 1;121(5):707–713. doi: 10.1016/0002-9378(75)90477-9. [DOI] [PubMed] [Google Scholar]
- Wølner-Hanssen P., Eschenbach D. A., Paavonen J., Stevens C. E., Kiviat N. B., Critchlow C., DeRouen T., Koutsky L., Holmes K. K. Association between vaginal douching and acute pelvic inflammatory disease. JAMA. 1990 Apr 11;263(14):1936–1941. doi: 10.1001/jama.1990.03440140062032. [DOI] [PubMed] [Google Scholar]
- Zak K., Diaz J. L., Jackson D., Heckels J. E. Antigenic variation during infection with Neisseria gonorrhoeae: detection of antibodies to surface proteins in sera of patients with gonorrhea. J Infect Dis. 1984 Feb;149(2):166–174. doi: 10.1093/infdis/149.2.166. [DOI] [PubMed] [Google Scholar]