Abstract
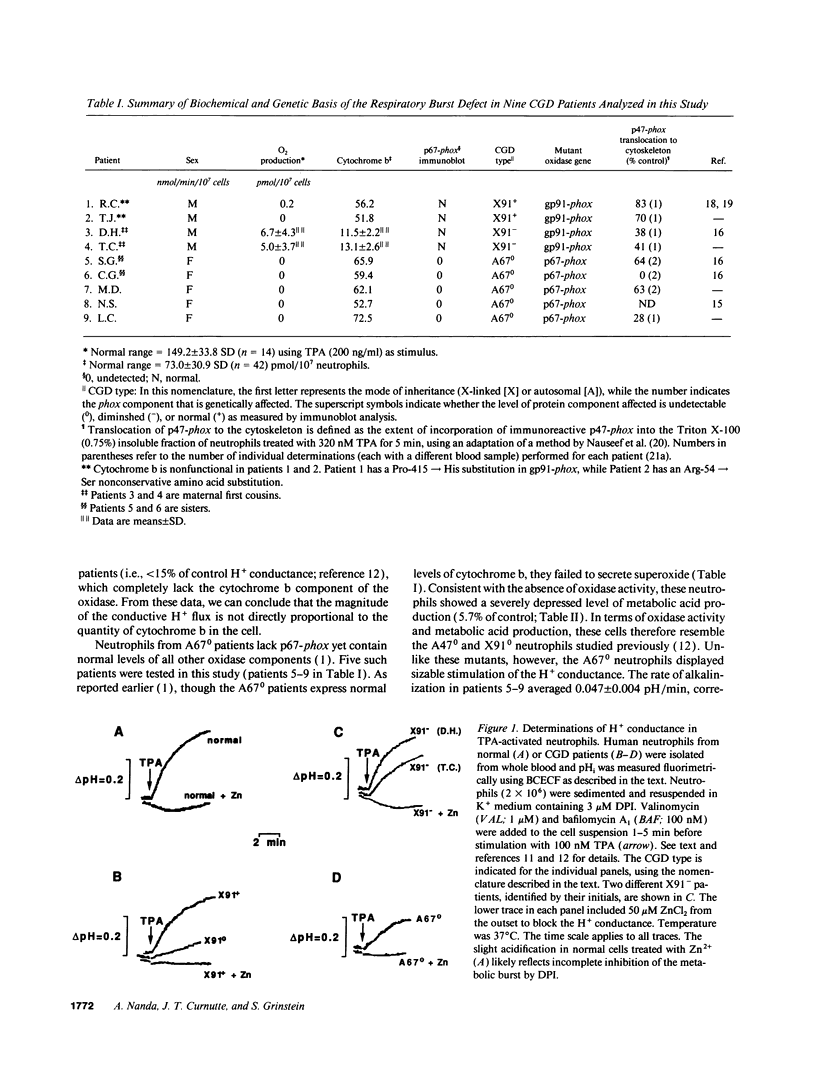
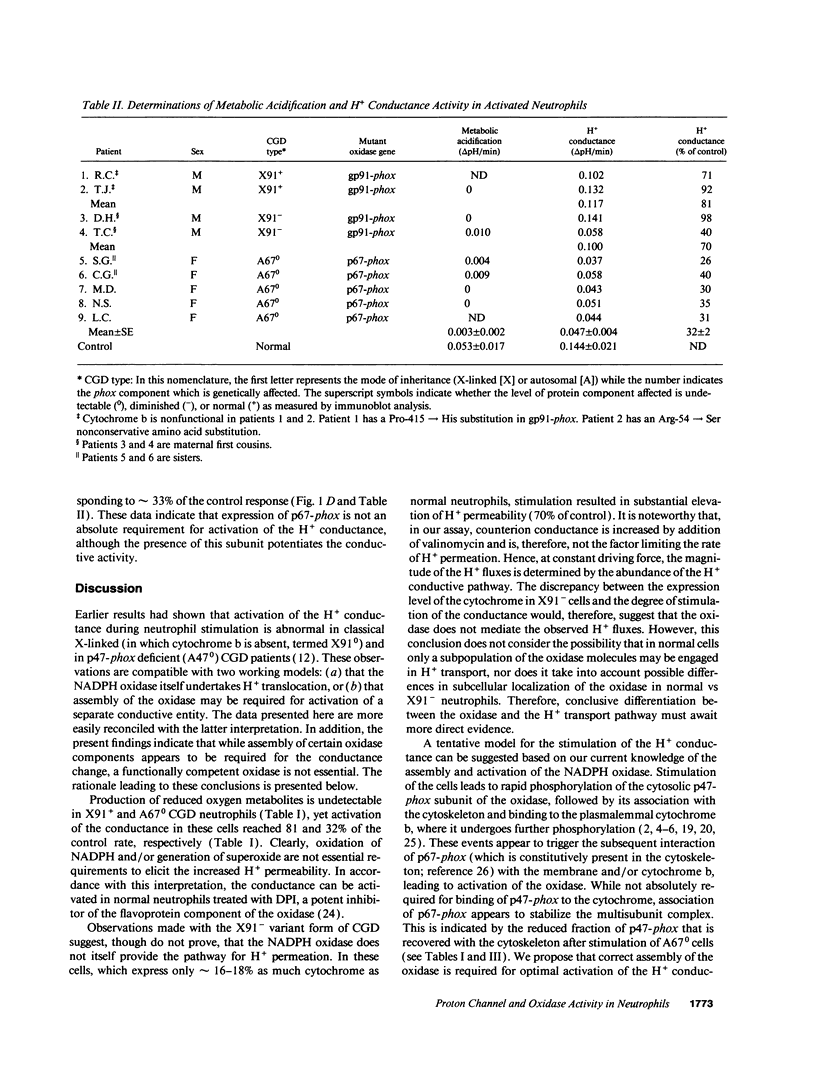
In phagocytes, superoxide generation by the NADPH oxidase is accompanied by metabolic acid production. Cytoplasmic acidification during this metabolic burst is prevented by a combination of H+ extrusion mechanisms, including a unique H+ conductance. NADPH oxidase is deficient in chronic granulomatous disease (CGD) patients. The burst of acid production is absent in CGD patients lacking the 47-kD (p47-phox) or the 91-kD (gp91-phox) subunits of the oxidase. Activation of the H+ conductance is also defective in these patients suggesting that (a) the oxidase itself undertakes H+ translocation or (b) oxidase assembly is required to stimulate a separate H+ conducting entity. To discern between these possibilities, three rare forms of CGD were studied. In neutrophils expressing nonfunctional cytochrome b, the conductance was activated to near-normal levels, implying that functional oxidase is not required to activate H+ extrusion. CGD cells expressing diminished amounts of cytochrome displayed H+ conductance approaching normal levels, suggesting that the oxidase itself does not translocate H+. Finally, the conductance was only partially inhibited in patients lacking the 67-kD subunit, indicating that this component is not essential for stimulation of H+ transport. We propose that normal assembly of the oxidase subunits is required for optimal activation of a closely associated but distinct H+ conducting entity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. The respiratory burst oxidase. Adv Enzymol Relat Areas Mol Biol. 1992;65:49–95. doi: 10.1002/9780470123119.ch2. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Schwartz J. H., Tauber A. I. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J Clin Invest. 1984 Aug;74(2):455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990 Jun;161(6):1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. Enzymic mechanisms of superoxide production. Biochim Biophys Acta. 1991 May 6;1057(3):281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986 Jul 1;237(1):111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Berkow R. L., Roberts R. L., Shurin S. B., Scott P. J. Chronic granulomatous disease due to a defect in the cytosolic factor required for nicotinamide adenine dinucleotide phosphate oxidase activation. J Clin Invest. 1988 Feb;81(2):606–610. doi: 10.1172/JCI113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T. Molecular basis of the autosomal recessive forms of chronic granulomatous disease. Immunodefic Rev. 1992;3(2):149–172. [PubMed] [Google Scholar]
- Dinauer M. C., Curnutte J. T., Rosen H., Orkin S. H. A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J Clin Invest. 1989 Dec;84(6):2012–2016. doi: 10.1172/JCI114393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. W., Malawista S. E., Garrett M. C., Van Blaricom G., Leto T. L., Curnutte J. T. Identification of a thermolabile component of the human neutrophil NADPH oxidase. A model for chronic granulomatous disease caused by deficiency of the p67-phox cytosolic component. J Clin Invest. 1992 May;89(5):1587–1595. doi: 10.1172/JCI115753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Biggar W. D. Cytoplasmic pH regulation in normal and abnormal neutrophils. Role of superoxide generation and Na+/H+ exchange. J Biol Chem. 1986 Jan 15;261(2):512–514. [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Characterization of the amiloride-sensitive Na+-H+ antiport of human neutrophils. Am J Physiol. 1986 Feb;250(2 Pt 1):C283–C291. doi: 10.1152/ajpcell.1986.250.2.C283. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Curnutte J. T., Nauseef W. M., Volpp B. D., Pearson D. W., Rosen H., Clark R. A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J Clin Invest. 1991 Jan;87(1):352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. S., Saad A. H., Koong A. C., Hahn G. M., Giaccia A. J. Potassium-channel activation in response to low doses of gamma-irradiation involves reactive oxygen intermediates in nonexcitatory cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):908–912. doi: 10.1073/pnas.90.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel F., Doussiere J., Vignais P. V. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991 Nov 1;201(3):523–546. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- Nanda A., Grinstein S., Curnutte J. T. Abnormal activation of H+ conductance in NADPH oxidase-defective neutrophils. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):760–764. doi: 10.1073/pnas.90.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Grinstein S. Protein kinase C activates an H+ (equivalent) conductance in the plasma membrane of human neutrophils. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10816–10820. doi: 10.1073/pnas.88.23.10816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Gukovskaya A., Tseng J., Grinstein S. Activation of vacuolar-type proton pumps by protein kinase C. Role in neutrophil pH regulation. J Biol Chem. 1992 Nov 15;267(32):22740–22746. [PubMed] [Google Scholar]
- Nauseef W. M., Volpp B. D., McCormick S., Leidal K. G., Clark R. A. Assembly of the neutrophil respiratory burst oxidase. Protein kinase C promotes cytoskeletal and membrane association of cytosolic oxidase components. J Biol Chem. 1991 Mar 25;266(9):5911–5917. [PubMed] [Google Scholar]
- Okamura N., Malawista S. E., Roberts R. L., Rosen H., Ochs H. D., Babior B. M., Curnutte J. T. Phosphorylation of the oxidase-related 48K phosphoprotein family in the unusual autosomal cytochrome-negative and X-linked cytochrome-positive types of chronic granulomatous disease. Blood. 1988 Aug;72(2):811–816. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Simchowitz L. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. II. Intracellular pH changes. J Biol Chem. 1985 Oct 25;260(24):13248–13255. [PubMed] [Google Scholar]
- Wilson M. T., Bickar D. Cytochrome oxidase as a proton pump. J Bioenerg Biomembr. 1991 Oct;23(5):755–771. doi: 10.1007/BF00786000. [DOI] [PubMed] [Google Scholar]
- Woodman R. C., Erickson R. W., Rae J., Jaffe H. S., Curnutte J. T. Prolonged recombinant interferon-gamma therapy in chronic granulomatous disease: evidence against enhanced neutrophil oxidase activity. Blood. 1992 Mar 15;79(6):1558–1562. [PubMed] [Google Scholar]
- Woodman R. C., Ruedi J. M., Jesaitis A. J., Okamura N., Quinn M. T., Smith R. M., Curnutte J. T., Babior B. M. Respiratory burst oxidase and three of four oxidase-related polypeptides are associated with the cytoskeleton of human neutrophils. J Clin Invest. 1991 Apr;87(4):1345–1351. doi: 10.1172/JCI115138. [DOI] [PMC free article] [PubMed] [Google Scholar]



