Abstract
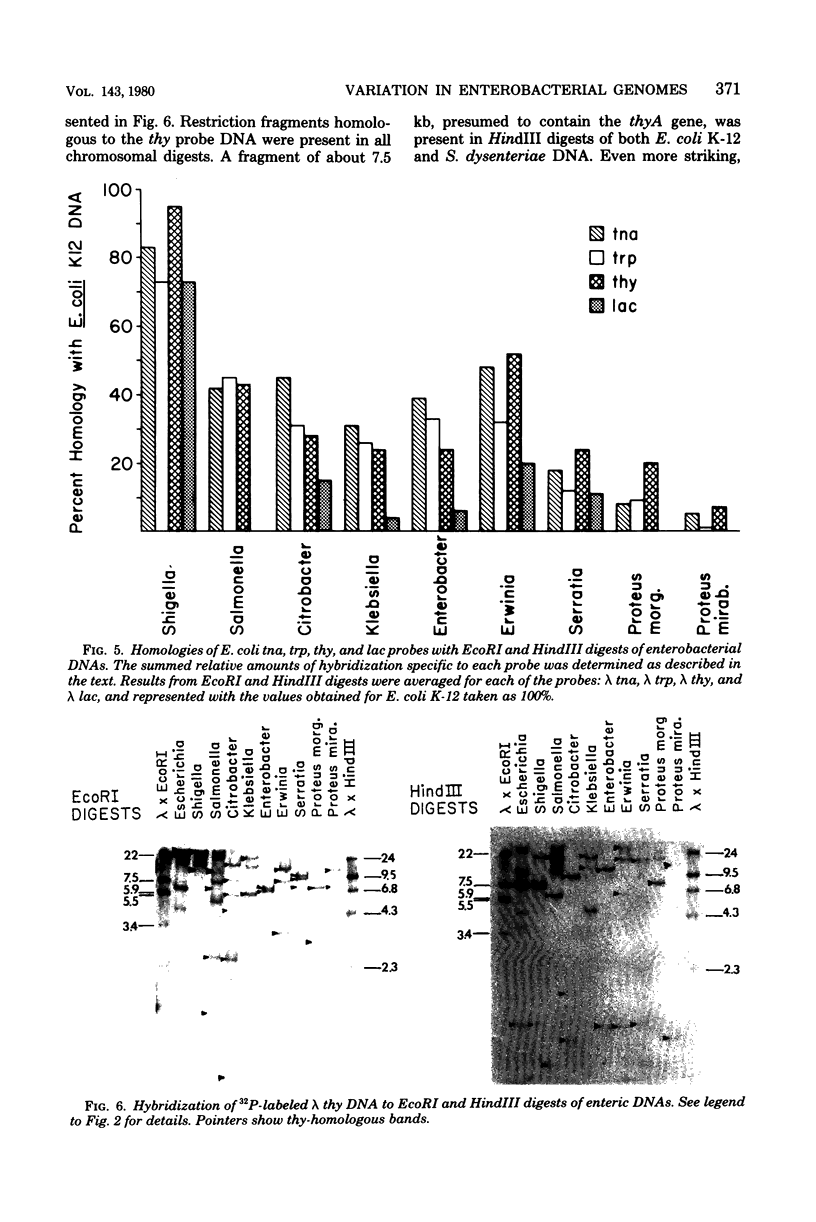
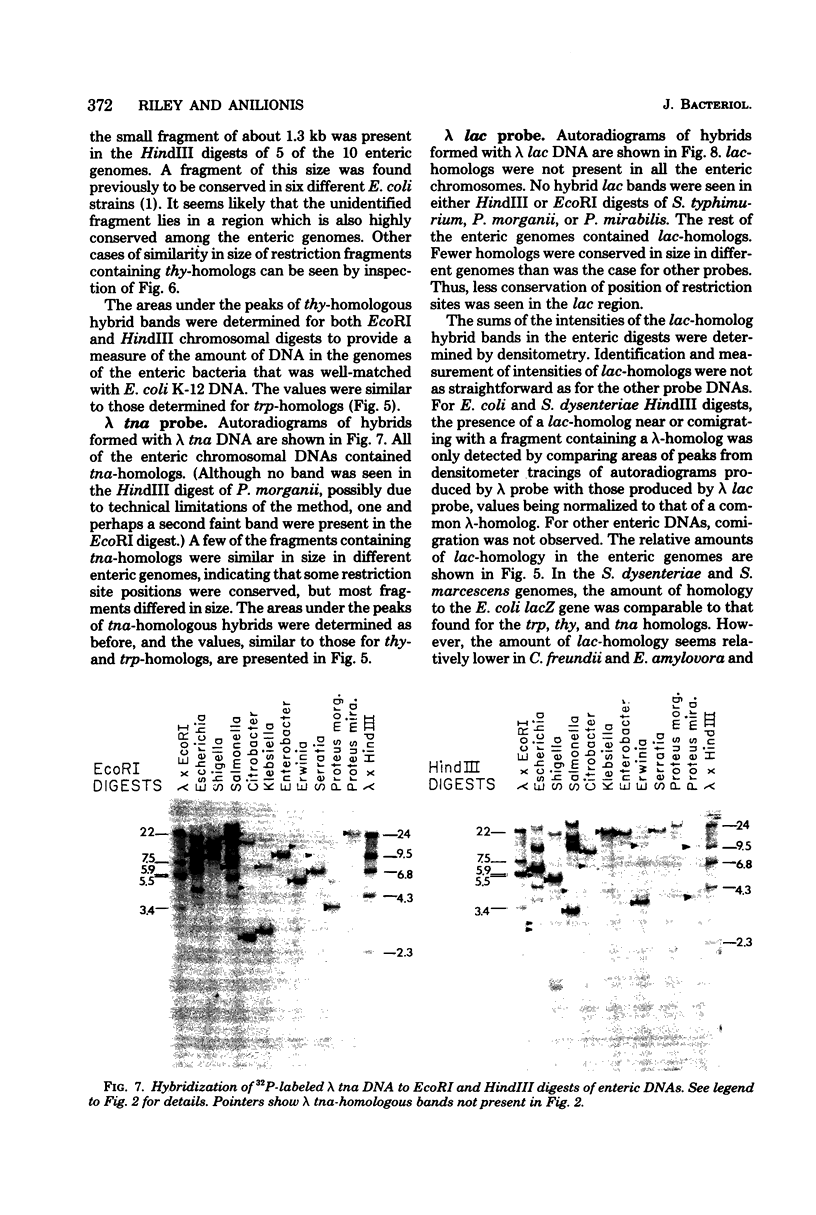
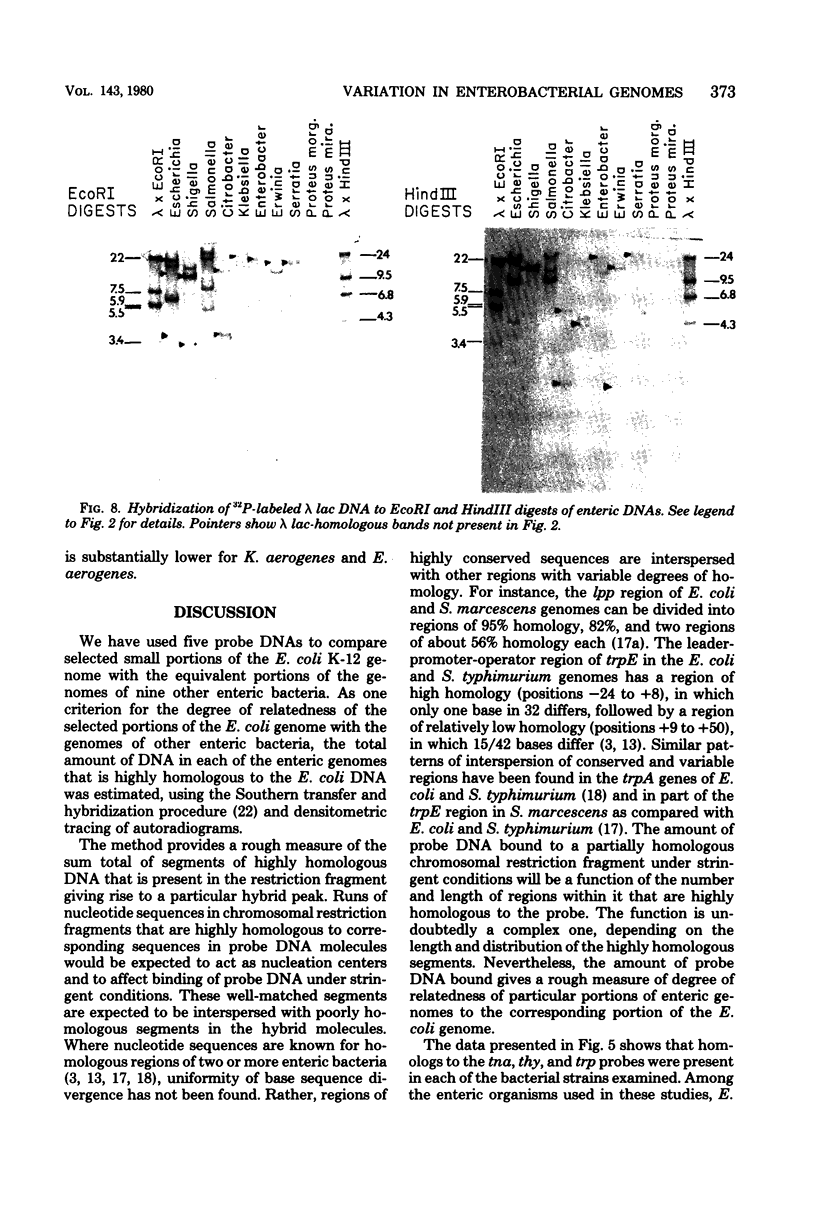
We have assessed the degree of relatedness of several portions of the Escherichia coli genome to the corresponding portions of the genomes of representative enteric bacteria, using the Southern transfer and hybridization technique (E. Southern, J. Mol. Biol. 98:503-517, 1975). The degree of relatedness varied among the regions examined. Judging both by the relative amounts of deoxyribonucleic acid in the various enteric genomes that are highly homologous and by the conservation of positions of restriction enzyme cleavage sites in these regions, the enteric genomes have diverged to greater extents in some parts of the genomes than in others. Portions of the genomes (including the tnaA and thyA genes, the trp operon, and one other unassigned segment) appear to have evolved in concert with the genome as a whole. By contrast, the lacZ gene and portions of the genome that are homologous to phage lambda vary more widely, perhaps reflecting a separate evolutionary origin for these segments of deoxyribonucleic acid.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Riley M. Conservation and variation of nucleotide sequences within related bacterial genomes: Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):355–365. doi: 10.1128/jb.143.1.355-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. N., Brown K. D., Yanofsky C. Nucleotide sequence of the promoter--operator region of the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):139–152. doi: 10.1016/s0022-2836(78)80002-3. [DOI] [PubMed] [Google Scholar]
- Botstein D., Herskowitz I. Properties of hybrids between Salmonella phage P22 and coliphage lambda. Nature. 1974 Oct 18;251(5476):584–589. doi: 10.1038/251584a0. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Falkow S. Genetics of the Enterobacteriaceae. C. Molecular relationships among members of the Enterobacteriaceae. Adv Genet. 1971;16:81–118. doi: 10.1016/s0065-2660(08)60355-7. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Skerman F. J., Falkow S. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J Bacteriol. 1972 Mar;109(3):953–965. doi: 10.1128/jb.109.3.953-965.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney R. M., Yanofsky C. Detection of tryptophan messenger RNA in several bacterial species and examination of the properties of heterologous DNA-RNA hybrids. J Mol Biol. 1972 Mar 14;64(2):319–339. doi: 10.1016/0022-2836(72)90501-3. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Lee F., Bertrand K., Bennett G., Yanofsky C. Comparison of the nucleotide sequences of the initial transcribed regions of the tryptophan operons of Escherichia coli and Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):193–217. doi: 10.1016/s0022-2836(78)80005-9. [DOI] [PubMed] [Google Scholar]
- Li S. L., Denney R. M., Yanofsky C. Nucleotide sequence divergence in the -chain-structural genes of tryptophan synthetase from Escherichia coli, Salmonella typhimurium, and Aerobacter aerogenes. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1112–1116. doi: 10.1073/pnas.70.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Miozzari G. F., Yanofsky C. The regulatory region of the trp operon of Serratia marcescens. Nature. 1978 Dec 14;276(5689):684–689. doi: 10.1038/276684a0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the Serratia marcescens lipoprotein gene. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1369–1373. doi: 10.1073/pnas.77.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., Yanofsky C. Nucleotide sequences of trpA of Salmonella typhimurium and Escherichia coli: an evolutionary comparison. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5244–5248. doi: 10.1073/pnas.76.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Sarkar S. Properties and regulation of the beta-D-galactosidase in Shigella dysenteriae and in Escherichia coli-Shigella dysenteriae hybrids. J Bacteriol. 1966 Apr;91(4):1477–1488. doi: 10.1128/jb.91.4.1477-1488.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Starlinger P. DNA rearrangements in procaryotes. Annu Rev Genet. 1977;11:103–126. doi: 10.1146/annurev.ge.11.120177.000535. [DOI] [PubMed] [Google Scholar]
- Thomas P. S., Farquhar M. N. Specific measurement of DNA in nuclei and nucleic acids using diaminobenzoic acid. Anal Biochem. 1978 Aug 15;89(1):35–44. doi: 10.1016/0003-2697(78)90724-8. [DOI] [PubMed] [Google Scholar]