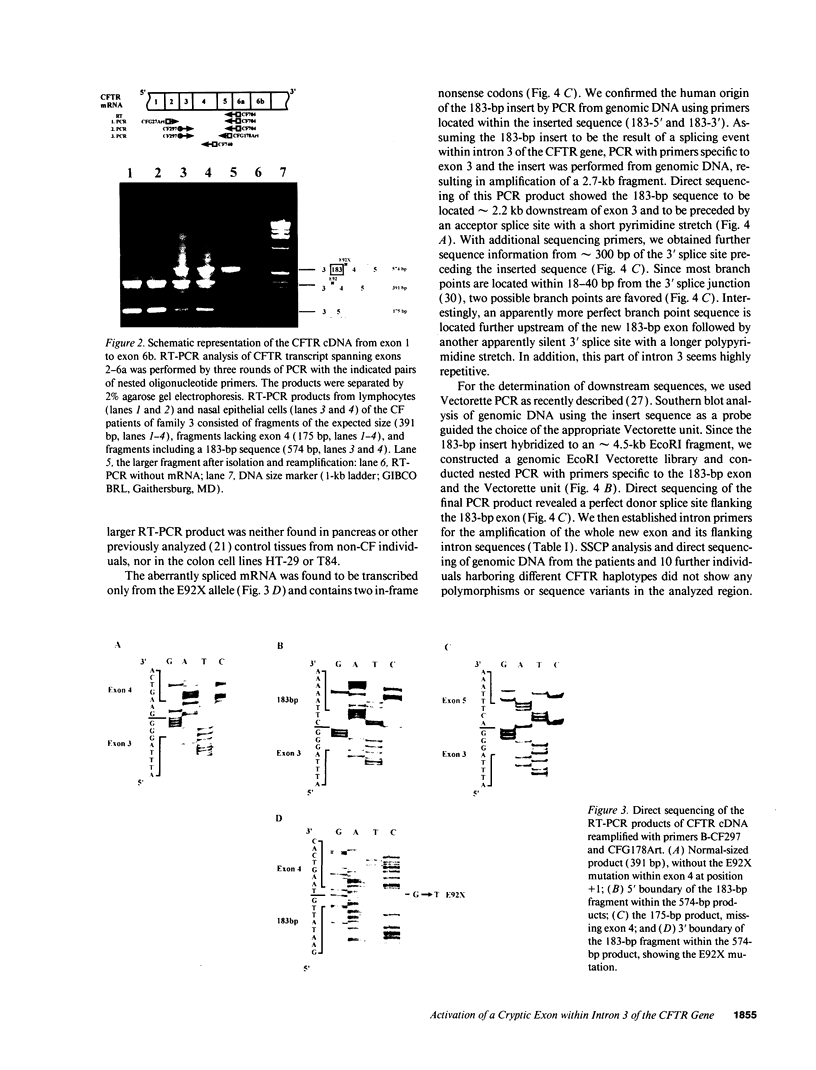
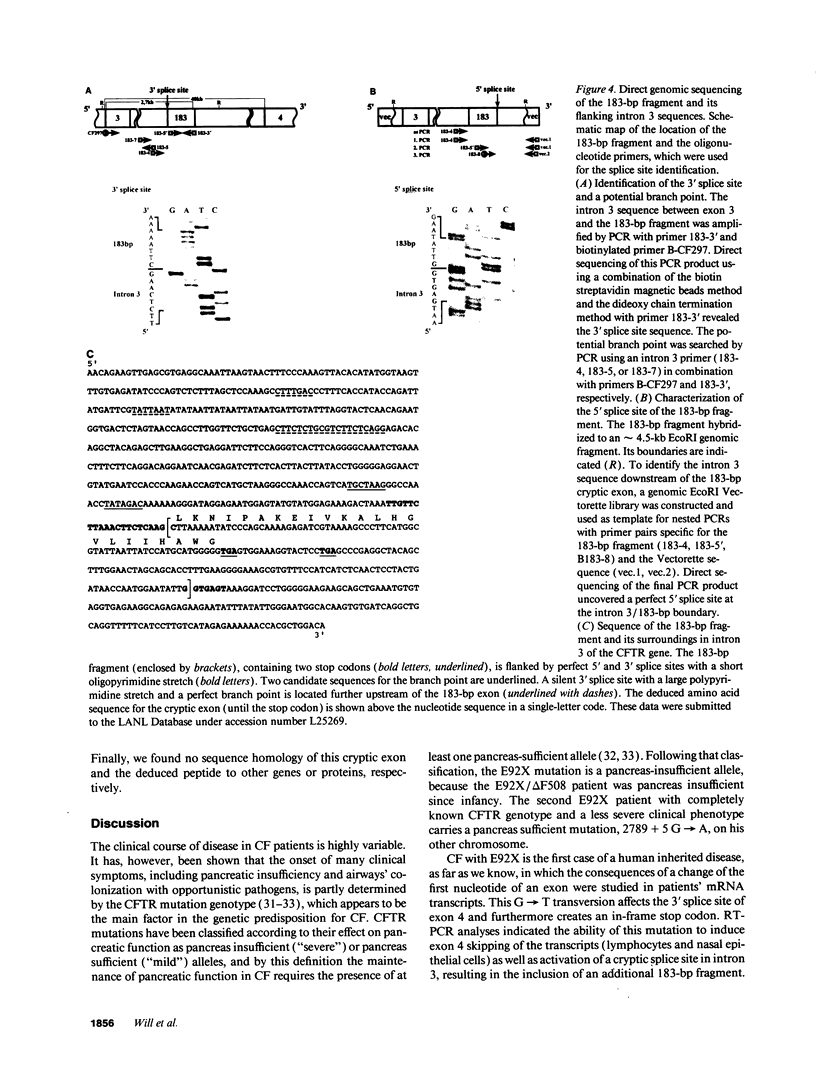
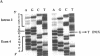Abstract
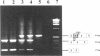
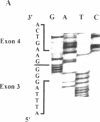
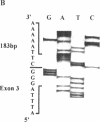
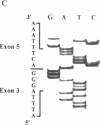
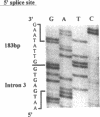
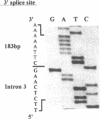
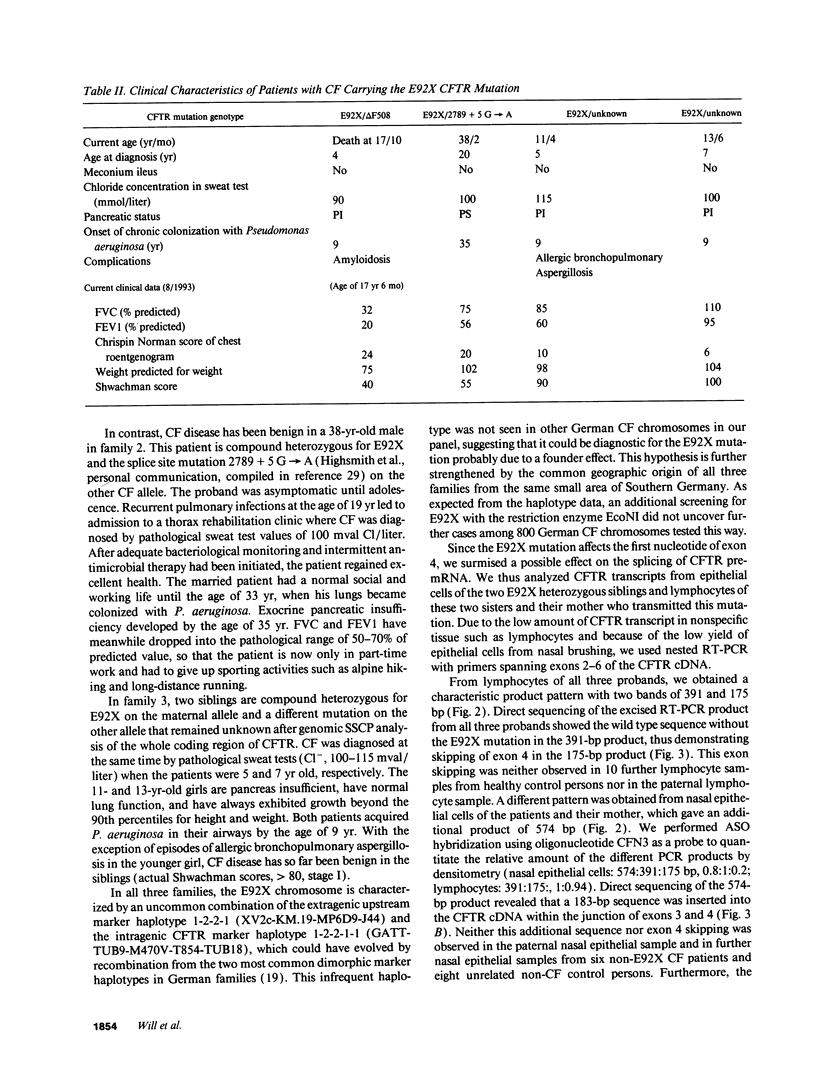
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. We report on a novel nonsense mutation that leads to exon skipping and the activation of a cryptic exon. Screening of genomic DNA from 700 German patients with CF uncovered four cases with the nonsense mutation E92X, a G-->T transversion that creates a termination codon and affects the first base of exon 4 of the CFTR gene. Lymphocyte RNA of two CF patients heterozygous for E92X was found to contain the wild type sequence and a differentially spliced isoform lacking exon 4. In RNA derived from nasal epithelial cells of E92X patients, a third fragment of longer size was observed. Sequencing revealed the presence of E92X and an additional 183-bp fragment, inserted between exons 3 and 4. The 183-bp sequence was mapped to intron 3 of the CFTR gene. It is flanked by acceptor and donor splice sites. We conclude that the 183-bp fragment in intron 3 is a cryptic CFTR exon that can be activated in epithelial cells by the presence of the E92X mutation. E92X abolishes correctly spliced CFTR mRNA and leads to severe cystic fibrosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold C., Hodgson I. J. Vectorette PCR: a novel approach to genomic walking. PCR Methods Appl. 1991 Aug;1(1):39–42. doi: 10.1101/gr.1.1.39. [DOI] [PubMed] [Google Scholar]
- Augarten A., Kerem B. S., Yahav Y., Noiman S., Rivlin Y., Tal A., Blau H., Ben-Tur L., Szeinberg A., Kerem E. Mild cystic fibrosis and normal or borderline sweat test in patients with the 3849 + 10 kb C-->T mutation. Lancet. 1993 Jul 3;342(8862):25–26. doi: 10.1016/0140-6736(93)91885-p. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Chou T. B., Mims I., Zachar Z. On/off regulation of gene expression at the level of splicing. Trends Genet. 1988 May;4(5):134–138. doi: 10.1016/0168-9525(88)90136-9. [DOI] [PubMed] [Google Scholar]
- Bremer S., Hoof T., Wilke M., Busche R., Scholte B., Riordan J. R., Maass G., Tümmler B. Quantitative expression patterns of multidrug-resistance P-glycoprotein (MDR1) and differentially spliced cystic-fibrosis transmembrane-conductance regulator mRNA transcripts in human epithelia. Eur J Biochem. 1992 May 15;206(1):137–149. doi: 10.1111/j.1432-1033.1992.tb16911.x. [DOI] [PubMed] [Google Scholar]
- Carothers A. M., Urlaub G., Grunberger D., Chasin L. A. Splicing mutants and their second-site suppressors at the dihydrofolate reductase locus in Chinese hamster ovary cells. Mol Cell Biol. 1993 Aug;13(8):5085–5098. doi: 10.1128/mcb.13.8.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. T., Chasin L. A. Direct selection for mutations affecting specific splice sites in a hamster dihydrofolate reductase minigene. Mol Cell Biol. 1993 Jan;13(1):289–300. doi: 10.1128/mcb.13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dalemans W., Barbry P., Champigny G., Jallat S., Dott K., Dreyer D., Crystal R. G., Pavirani A., Lecocq J. P., Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991 Dec 19;354(6354):526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Delaney S. J., Rich D. P., Thomson S. A., Hargrave M. R., Lovelock P. K., Welsh M. J., Wainwright B. J. Cystic fibrosis transmembrane conductance regulator splice variants are not conserved and fail to produce chloride channels. Nat Genet. 1993 Aug;4(4):426–431. doi: 10.1038/ng0893-426. [DOI] [PubMed] [Google Scholar]
- Dörk T., Neumann T., Wulbrand U., Wulf B., Kälin N., Maass G., Krawczak M., Guillermit H., Ferec C., Horn G. Intra- and extragenic marker haplotypes of CFTR mutations in cystic fibrosis families. Hum Genet. 1992 Feb;88(4):417–425. doi: 10.1007/BF00215676. [DOI] [PubMed] [Google Scholar]
- Dörk T., Will K., Demmer A., Tümmler B. A donor splice mutation (405 + 1 G-->A) in cystic fibrosis associated with exon skipping in epithelial CFTR mRNA. Hum Mol Genet. 1993 Nov;2(11):1965–1966. doi: 10.1093/hmg/2.11.1965. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferrie R. M., Schwarz M. J., Robertson N. H., Vaudin S., Super M., Malone G., Little S. Development, multiplexing, and application of ARMS tests for common mutations in the CFTR gene. Am J Hum Genet. 1992 Aug;51(2):251–262. [PMC free article] [PubMed] [Google Scholar]
- Fonknechten N., Chomel J. C., Kitzis A., Kahn A., Kaplan J. C. Skipping of exon 5 as a consequence of the 711 + 1 G-->T mutation in the CFTR gene. Hum Mol Genet. 1992 Jul;1(4):281–282. doi: 10.1093/hmg/1.4.281. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Gregory R. J., Rich D. P., Cheng S. H., Souza D. W., Paul S., Manavalan P., Anderson M. P., Welsh M. J., Smith A. E. Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol Cell Biol. 1991 Aug;11(8):3886–3893. doi: 10.1128/mcb.11.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamosh A., Rosenstein B. J., Cutting G. R. CFTR nonsense mutations G542X and W1282X associated with severe reduction of CFTR mRNA in nasal epithelial cells. Hum Mol Genet. 1992 Oct;1(7):542–544. doi: 10.1093/hmg/1.7.542. [DOI] [PubMed] [Google Scholar]
- Hamosh A., Trapnell B. C., Zeitlin P. L., Montrose-Rafizadeh C., Rosenstein B. J., Crystal R. G., Cutting G. R. Severe deficiency of cystic fibrosis transmembrane conductance regulator messenger RNA carrying nonsense mutations R553X and W1316X in respiratory epithelial cells of patients with cystic fibrosis. J Clin Invest. 1991 Dec;88(6):1880–1885. doi: 10.1172/JCI115510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull J., Shackleton S., Harris A. Abnormal mRNA splicing resulting from three different mutations in the CFTR gene. Hum Mol Genet. 1993 Jun;2(6):689–692. doi: 10.1093/hmg/2.6.689. [DOI] [PubMed] [Google Scholar]
- Jones C. T., McIntosh I., Keston M., Ferguson A., Brock D. J. Three novel mutations in the cystic fibrosis gene detected by chemical cleavage: analysis of variant splicing and a nonsense mutation. Hum Mol Genet. 1992 Apr;1(1):11–17. doi: 10.1093/hmg/1.1.11. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kerem E., Corey M., Kerem B. S., Rommens J., Markiewicz D., Levison H., Tsui L. C., Durie P. The relation between genotype and phenotype in cystic fibrosis--analysis of the most common mutation (delta F508). N Engl J Med. 1990 Nov 29;323(22):1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- Koh J., Sferra T. J., Collins F. S. Characterization of the cystic fibrosis transmembrane conductance regulator promoter region. Chromatin context and tissue-specificity. J Biol Chem. 1993 Jul 25;268(21):15912–15921. [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Kristidis P., Bozon D., Corey M., Markiewicz D., Rommens J., Tsui L. C., Durie P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet. 1992 Jun;50(6):1178–1184. [PMC free article] [PubMed] [Google Scholar]
- Kubesch P., Dörk T., Wulbrand U., Kälin N., Neumann T., Wulf B., Geerlings H., Weissbrodt H., von der Hardt H., Tümmler B. Genetic determinants of airways' colonisation with Pseudomonas aeruginosa in cystic fibrosis. Lancet. 1993 Jan 23;341(8839):189–193. doi: 10.1016/0140-6736(93)90062-l. [DOI] [PubMed] [Google Scholar]
- Libri D., Balvay L., Fiszman M. Y. In vivo splicing of the beta tropomyosin pre-mRNA: a role for branch point and donor site competition. Mol Cell Biol. 1992 Jul;12(7):3204–3215. doi: 10.1128/mcb.12.7.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda A., Helfman D. M., Krainer A. R. Modulation of exon skipping and inclusion by heterogeneous nuclear ribonucleoprotein A1 and pre-mRNA splicing factor SF2/ASF. Mol Cell Biol. 1993 May;13(5):2993–3001. doi: 10.1128/mcb.13.5.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki H., Morisaki T., Newby L. K., Holmes E. W. Alternative splicing: a mechanism for phenotypic rescue of a common inherited defect. J Clin Invest. 1993 May;91(5):2275–2280. doi: 10.1172/JCI116455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes V., Chillón M., Dörk T., Tümmler B., Casals T., Estivill X. A new missense mutation (E92K) in the first transmembrane domain of the CFTR gene causes a benign cystic fibrosis phenotype. Hum Mol Genet. 1993 Jan;2(1):79–80. doi: 10.1093/hmg/2.1.79. [DOI] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. The organization of 3' splice-site sequences in mammalian introns. Genes Dev. 1989 Dec;3(12B):2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. N., Rich D. P., Ostedgaard L. S., Gregory R. J., Smith A. E., Welsh M. J. Mutations in CFTR associated with mild-disease-form Cl- channels with altered pore properties. Nature. 1993 Mar 11;362(6416):160–164. doi: 10.1038/362160a0. [DOI] [PubMed] [Google Scholar]
- Smit L. S., Nasr S. Z., Iannuzzi M. C., Collins F. S. An African-American cystic fibrosis patient homozygous for a novel frameshift mutation associated with reduced CFTR mRNA levels. Hum Mutat. 1993;2(2):148–151. doi: 10.1002/humu.1380020217. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Talerico M., Berget S. M. Effect of 5' splice site mutations on splicing of the preceding intron. Mol Cell Biol. 1990 Dec;10(12):6299–6305. doi: 10.1128/mcb.10.12.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui L. C. Mutations and sequence variations detected in the cystic fibrosis transmembrane conductance regulator (CFTR) gene: a report from the Cystic Fibrosis Genetic Analysis Consortium. Hum Mutat. 1992;1(3):197–203. doi: 10.1002/humu.1380010304. [DOI] [PubMed] [Google Scholar]
- Valcárcel J., Singh R., Zamore P. D., Green M. R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature. 1993 Mar 11;362(6416):171–175. doi: 10.1038/362171a0. [DOI] [PubMed] [Google Scholar]
- Will K., Reiss J., Dean M., Schlösser M., Slomski R., Schmidtke J., Stuhrmann M. CFTR transcripts are undetectable in lymphocytes and respiratory epithelial cells of a CF patient homozygous for the nonsense mutation R553X. J Med Genet. 1993 Oct;30(10):833–837. doi: 10.1136/jmg.30.10.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will K., Stuhrmann M., Dean M., Schmidtke J. Alternative splicing in the first nucleotide binding fold of CFTR. Hum Mol Genet. 1993 Mar;2(3):231–235. doi: 10.1093/hmg/2.3.231. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Chu C. S., Crystal R. G. Alternative splicing of intron 23 of the human cystic fibrosis transmembrane conductance regulator gene resulting in a novel exon and transcript coding for a shortened intracytoplasmic C terminus. J Biol Chem. 1993 Jan 5;268(1):686–690. [PubMed] [Google Scholar]
- Zielenski J., Bozon D., Markiewicz D., Aubin G., Simard F., Rommens J. M., Tsui L. C. Analysis of CFTR transcripts in nasal epithelial cells and lymphoblasts of a cystic fibrosis patient with 621 + 1G-->T and 711 + 1G-->T mutations. Hum Mol Genet. 1993 Jun;2(6):683–687. doi: 10.1093/hmg/2.6.683. [DOI] [PubMed] [Google Scholar]