Abstract
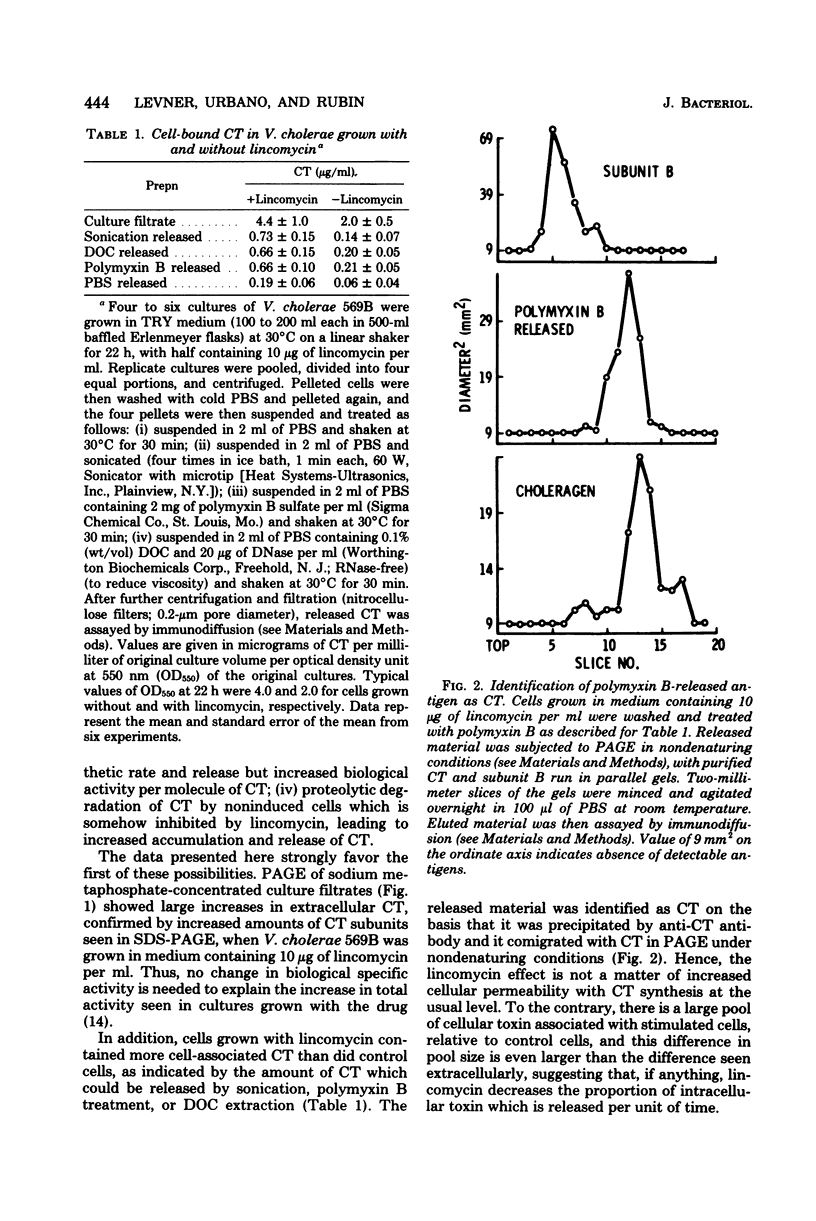
Increased enterotoxigenicity of Vibrio cholerae 569B grown with low concentrations of lincomycin, previously described in terms of increased extracellular biological activity (capillary permeability factor and fluid accumulation in ligated rabbit ileal loops), was further characterized. Polyacrylamide gel electrophoresis and single radial immunodiffusion showed that lincomycin-stimulated cells produced increased molar quantities of cholera toxin (CT) both extra- and intracellularly. The intracellular CT was released in comparable amounts by sonication, deoxycholate extraction, and polymyxin B treatment. Polymyxin B release of CT was nearly complete under conditions wherein only 6% of total cellular beta-galactosidase was released, implying a periplasmic pool of CT in stimulated cells. No intracellular choleragenoid (CT subunit B) was found in stimulated cells by polymyxin B release. No proteolysis of 14C-labeled CT was detected after prolonged incubation with sonicated nonstimulated cultures or sonicated concentrated cells. These data support the conclusion that the stimulatory effect of lincomycin involves an increase in the rate of synthesis of the CT molecule, and argue against alternative models involving inhibition of putative normal degradation of CT, increased release of otherwise cell-bound CT, or activation of inactive, or less active, forms of CT.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. The molecular nature of heat-labile enterotoxin (LT) of escherichia coli. Nature. 1979 Feb 1;277(5695):406–407. doi: 10.1038/277406a0. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P. B., Smith H. L., Jr The effect of anaerobiosis and bile salts on the growth and toxin production by Vibrio cholerae. J Gen Microbiol. 1977 Jan;98(1):77–86. doi: 10.1099/00221287-98-1-77. [DOI] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Rappaport R. S. Origin of the enzymatically active A1 fragment of cholera toxin. J Infect Dis. 1979 Jun;139(6):674–680. doi: 10.1093/infdis/139.6.674. [DOI] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Childs G., Inouye M. Differential inhibitory effects of antibiotics on the biosynthesis of envelope proteins of Escherichia coli. J Mol Biol. 1973 Sep 15;79(2):373–389. doi: 10.1016/0022-2836(73)90012-0. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNELL D., MAGASANIK B. THE CONTROL OF THE RATE OF ENZYME SYNTHESIS IN AEROBACTER AEROGENES. Biochim Biophys Acta. 1964 Mar 9;81:418–434. doi: 10.1016/0926-6569(64)90127-0. [DOI] [PubMed] [Google Scholar]
- Levner M., Wiener F. P., Rubin B. A. Induction of Escherichia coli and Vibrio cholerae enterotoxins by an inhibitor of protein synthesis. Infect Immun. 1977 Jan;15(1):132–137. doi: 10.1128/iai.15.1.132-137.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. C., Richardson S. H., Sheridan B. Biochemistry of Vibrio cholerae virulence: purification of cholera enterotoxin by preparative disc electrophoresis. Appl Environ Microbiol. 1976 Aug;32(2):288–293. doi: 10.1128/aem.32.2.288-293.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. The use of inhibitors in studies on protein synthesis. Methods Enzymol. 1974;30:261–282. doi: 10.1016/0076-6879(74)30030-4. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Hejtmancik K. E., Markel D. E., Craig J. P., Kurosky A. Antigenic specificity of neutralizing antibody to cholera toxin. Infect Immun. 1979 Jun;24(3):774–779. doi: 10.1128/iai.24.3.774-779.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Synthesis of exported proteins by membrane-bound polysomes from Escherichia coli. Eur J Biochem. 1977 May 2;75(1):43–53. doi: 10.1111/j.1432-1033.1977.tb11502.x. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Rubin B. A., Tint H. Development of a purified cholera toxoid. I. Purification of toxin. Infect Immun. 1974 Feb;9(2):294–303. doi: 10.1128/iai.9.2.294-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. H. Factors influencing in vitro skin permeability factor production by Vibrio cholerae. J Bacteriol. 1969 Oct;100(1):27–34. doi: 10.1128/jb.100.1.27-34.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]