Abstract
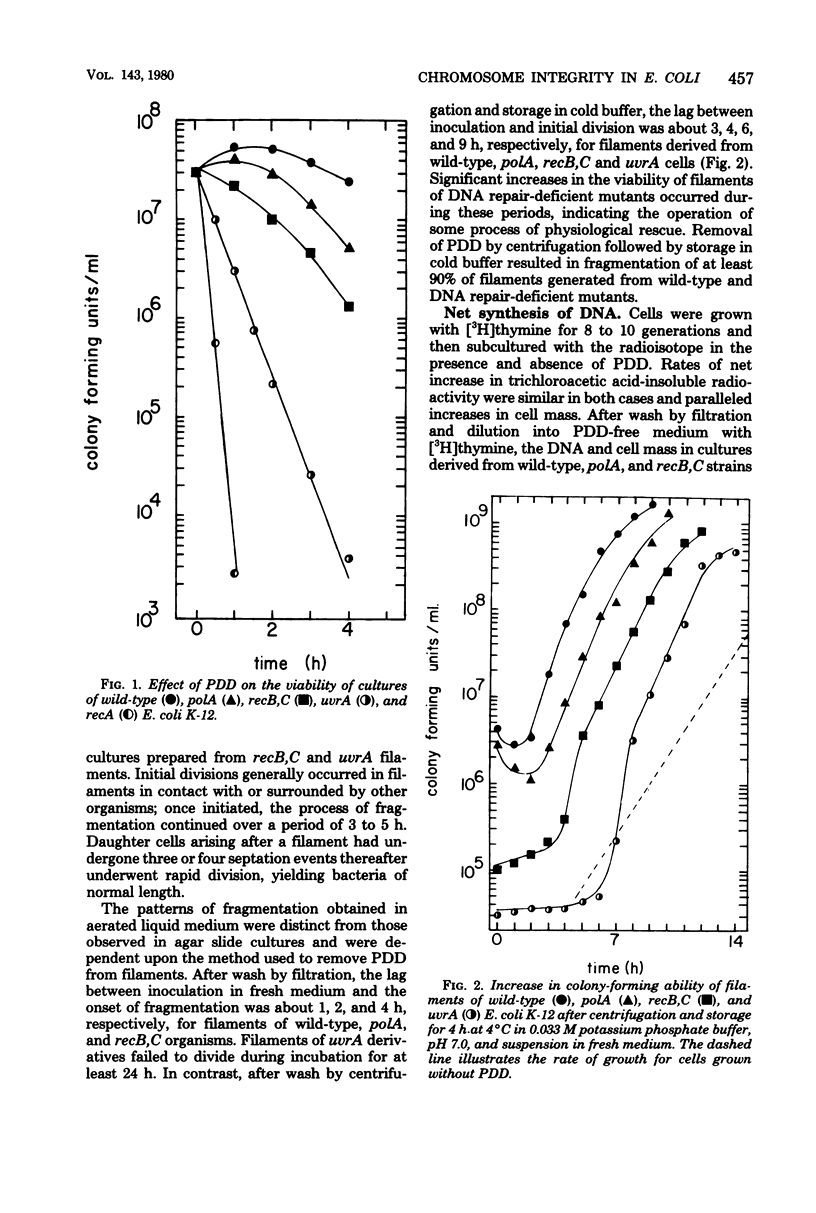
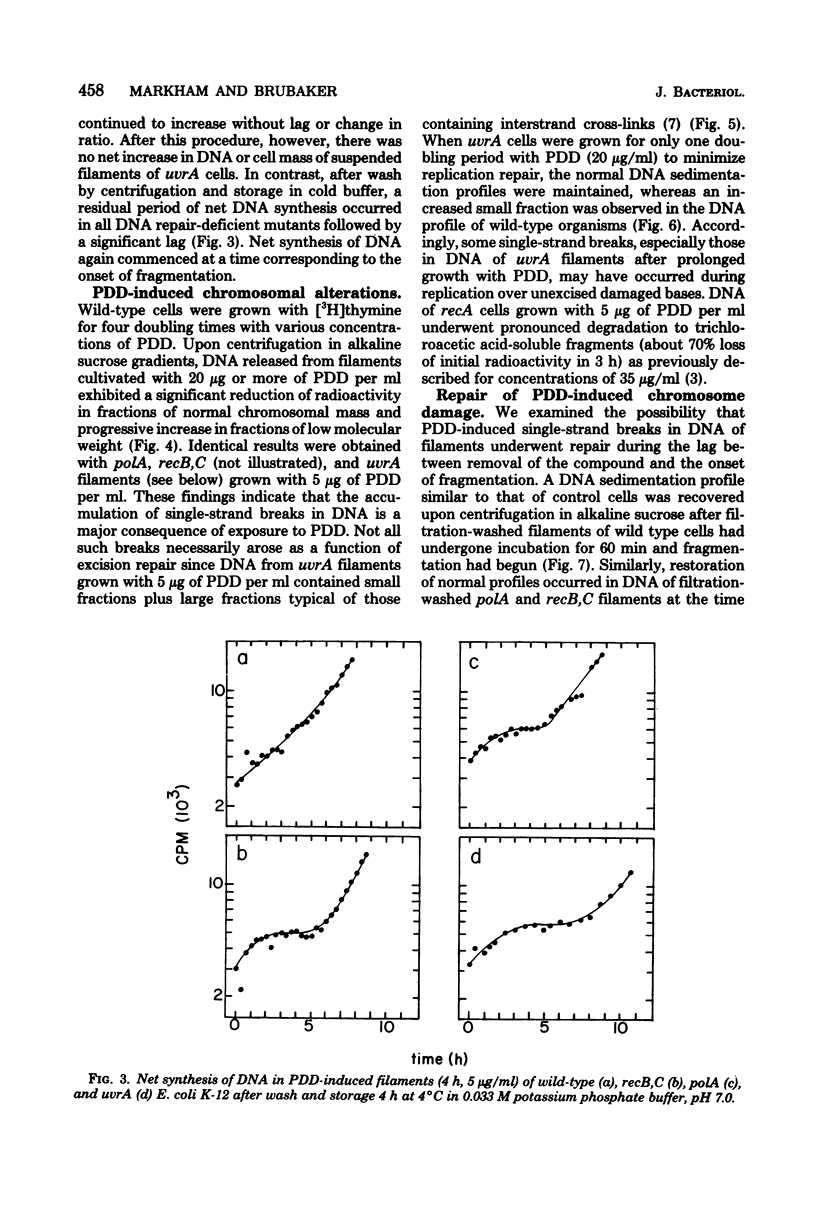
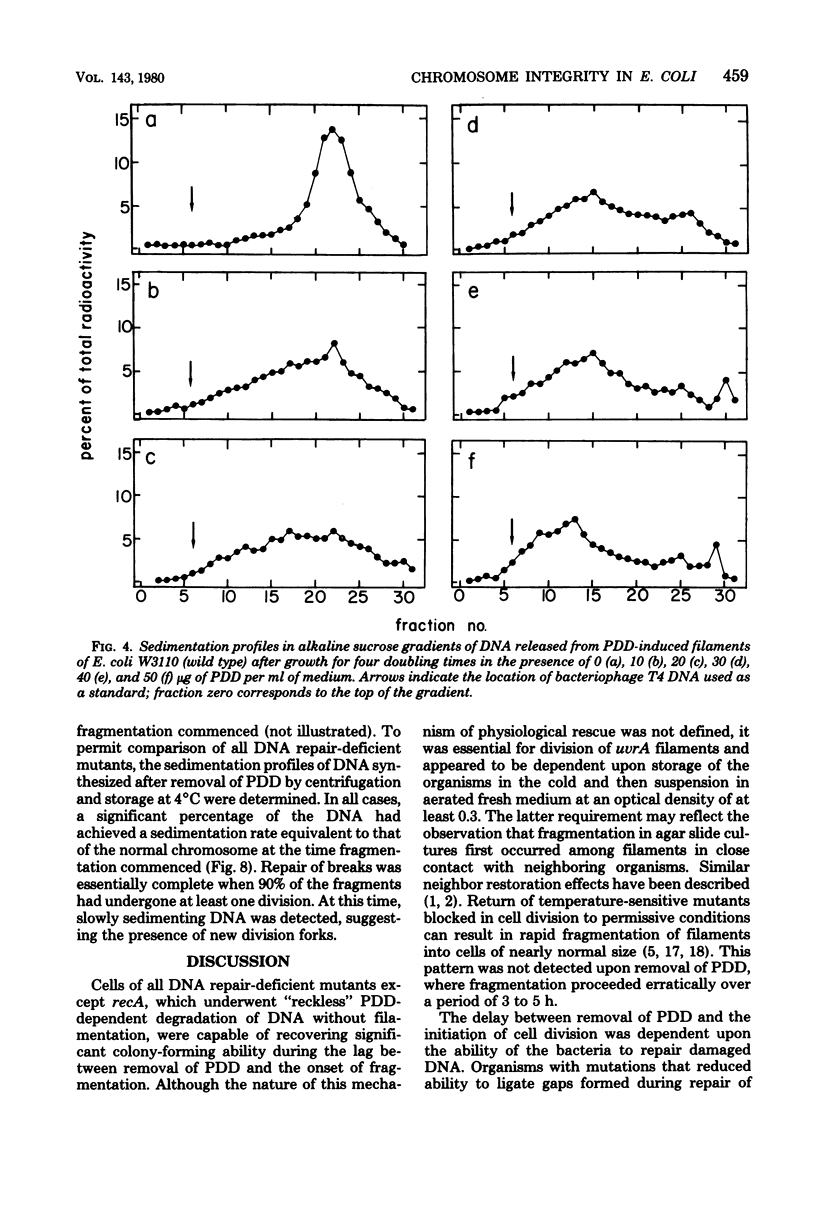
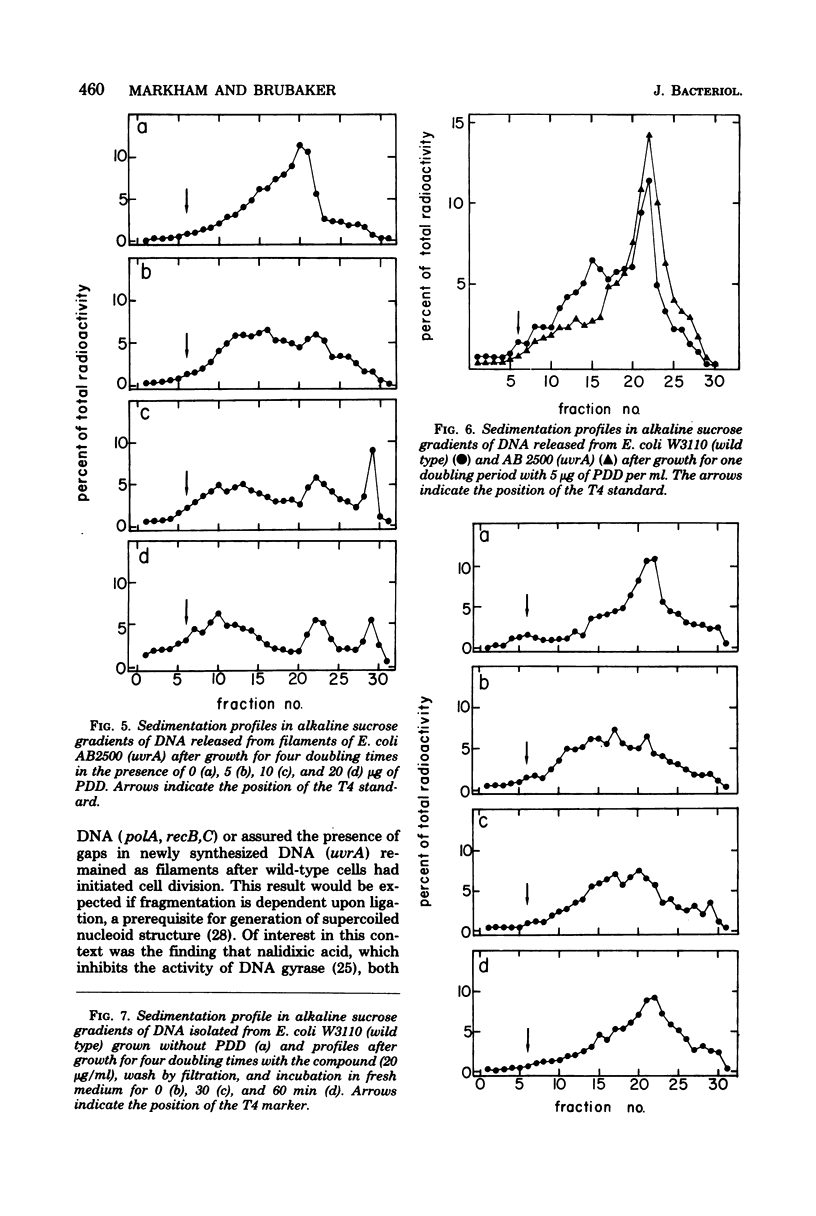
The antitumor agent cis-platinum(II)diamminodichloride (PDD) caused wild-type and recA+ deoxyribonucleic acid (DNA) repair-deficient mutant cells of Escherichia coli K-12 to grow as long, multinucleated filaments. At 5 micrograms/ml, the times required for reduction of viability to 37% for wild-type, polA, recB,C, uvrA, and recA organisms were > 200, 200, 120, 25, and 5 min, respectively. Only recA cells exhibited @reckless" degradation of DNA at this concentration of PDD. As shown by sedimentation in alkaline sucrose gradients, generation of single-strand breaks in DNA of the remaining organisms was a major consequence of growth in PDD. Upon incubation in fresh medium after removal of the compound and storage for 4 h at 4 degrees C, a respective lag of 3, 4, 6, and 9 h occurred before filaments of wild-type, polA, recB,C, and uvrA cells commenced cell division. Maintenance at 4 degrees C, which evidently delayed postshift initiation of chromosome replication, was only essential for fragmentation of uvrA filaments. In all cases, these periods of division delay corresponded to those required for restoration of normal chromosomal molecular weight as determined in alkaline sucrose gradients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Hardigree A. A., Stapleton G. E. Repair of radiation-induced damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1966 Feb;91(2):737–742. doi: 10.1128/jb.91.2.737-742.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. S., Filip C. C., Gustafson R. A., Allen R. G., Walker J. R. Regulation of bacterial cell division: genetic and phenotypic analysis of temperature-sensitive, multinucleate, filament-forming mutants of Escherichia. J Bacteriol. 1974 Mar;117(3):978–986. doi: 10.1128/jb.117.3.978-986.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. J., Brubaker R. R. Effect of cis-platinum(II)diamminodichloride on wild type and deoxyribonucleic acid repair deficient mutants of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1247–1252. doi: 10.1128/jb.116.3.1247-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D. J., Brubaker R. R. Mutagenic properties of cis-plantinum(II)diammino-dichloride in Escherichia coli. Mutat Res. 1975 Feb;27(2):181–189. doi: 10.1016/0027-5107(75)90077-9. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Cohen G. L., Bauer W. R., Barton J. K., Lippard S. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to DNA: evidence for unwinding and shortening of the double helix. Science. 1979 Mar 9;203(4384):1014–1016. doi: 10.1126/science.370979. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Bullock M. L. DNA ligase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1580–1587. doi: 10.1073/pnas.67.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Lecointe P., Macquet J. P., Butour J. L., Paoletti C. Relative efficiencies of a series of square-planar plantinum(II) compounds on Salmonella mutagenesis. Mutat Res. 1977 Apr;48(2):139–143. doi: 10.1016/0027-5107(77)90153-1. [DOI] [PubMed] [Google Scholar]
- Leopold W. R., Miller E. C., Miller J. A. Carcinogenicity of antitumor cis-platinum(II) coordination complexes in the mouse and rat. Cancer Res. 1979 Mar;39(3):913–918. [PubMed] [Google Scholar]
- Little J. W., Kleid D. G. Escherichia coli protein X is the recA gene product. J Biol Chem. 1977 Sep 25;252(18):6251–6252. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. An endonuclease from Escherichia coli that introduces single polynucleotide chain scissions in ultraviolet-irradiated DNA. J Biol Chem. 1976 Mar 10;251(5):1438–1445. [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. J., Pascoe J. M. Cross-linking of complementary strands of DNA in mammalian cells by antitumour platinum compounds. Nature. 1972 Feb 4;235(5336):282–284. doi: 10.1038/235282a0. [DOI] [PubMed] [Google Scholar]
- Robins A. B. The reaction of 14 C-labelled platinum ethylenediamine dichloride with nucleic acid constituents. Chem Biol Interact. 1973 Jan;6(1):35–45. doi: 10.1016/0009-2797(73)90084-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg B., Renshaw E., Vancamp L., Hartwick J., Drobnik J. Platinum-induced filamentous growth in Escherichia coli. J Bacteriol. 1967 Feb;93(2):716–721. doi: 10.1128/jb.93.2.716-721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B., VanCamp L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970 Jun;30(6):1799–1802. [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G. Genetic and kinetic evidence for different types of postreplication repair in Escherichia coli B. J Bacteriol. 1975 Jul;123(1):154–161. doi: 10.1128/jb.123.1.154-161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Carver J. H., Hanna M. L., Wandres D. L. Platinum-induced mutations to 8-azaguanine resistance in Chinese hamster ovary cells. Mutat Res. 1979 May;67(1):65–80. doi: 10.1016/0165-1218(79)90100-9. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. On the structure of the folded chromosome of Escherichia coli. J Mol Biol. 1972 Nov 14;71(2):127–147. doi: 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]


