Abstract
Primary cilia (PC) are solitary, sensory organelles that are critical for several signaling pathways. PC were detected by immunofluorescence of cultured cells and breast tissues. After growth for 7 days in vitro, PC were detected in ∼70% of breast fibroblasts and in 7–19% of epithelial cells derived from benign breast (184A1 and MCF10A). In 11 breast cancer cell lines, PC were present at a low frequency in four (from 0.3% to 4% of cells), but were absent in the remainder. The cancer cell lines with PC were all of the basal B subtype, which is analogous to the clinical triple-negative breast cancer subtype. Furthermore, the frequency of PC decreased with increasing degree of transformation/progression in the MCF10 and MDA-MB-435/LCC6 isogenic models of cancer progression. In histologically normal breast tissues, PC were frequent in fibroblasts and myoepithelial cells and less common in luminal epithelial cells. Of 26 breast cancers examined, rare PC were identified in cancer epithelial cells of only one cancer, which was of the triple-negative subtype. These data indicate a decrease or loss of PC in breast cancer and an association of PC with the basal B subtype. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 58:857–870, 2010)
Keywords: primary cilia, breast cancer, tubulin, carcinogenesis, Ki-67
Primary cilia (PC) are microtubule-based organelles, which were identified more than 100 years ago and were once considered to be vestigial organelles. However, in the last decade, the functional importance of PC has been established, and a cohort of ciliopathies, congenital diseases caused by dysfunction of PC resulting from mutations in cilia-related genes, has been identified (Quinlan et al. 2008; Sharma et al. 2008). PC project from the surfaces of cells and protrude into the extracellular milieu. They are studded with a variety of receptors, including receptors for Hedgehog, platelet-derived growth factor (PDGF), and Wnt signaling, and have been established as sensory organelles that are critical for cell signaling (Eggenschwiler and Anderson 2007). Cumulative evidence also indicates that PC are physical–chemical sensors, which monitor and respond to multiple stimuli, including changes in osmolarity, gravity, light, odorants, and flow stress (Yoder et al. 2002; Marshall and Nonaka 2006; Gradilone et al. 2007; Roepman and Wolfrum 2007; Haycraft and Serra 2008). The signals transmitted by PC are important for the control of cell growth, migration, and polarity during organogenesis and for the maintenance of tissue homeostasis (Christensen et al. 2007,2008).
PC are closely linked with the cell cycle. In postmitotic cells, the centrosome, which is composed of two centrioles surrounded by pericentriolar material, migrates to the cell surface, and one of the centrioles differentiates into a basal body that nucleates microtubules to give rise to the primary cilium (Plotnikova et al. 2008). During mitosis, the primary cilium is disassembled and the centrosome organizes the bipolar spindle. PC are typically present in cells that are in the G0 stage of the cell cycle, but occasionally can be identified in cells in stage G1 (Pedersen et al. 2008). Therefore, in most normal cell populations in culture, as cells become quiescent, an increasing proportion of the population is ciliated (Plotnikova et al. 2008). It is not yet clear whether PC have an active part in regulating cell division or are only passively linked with the cell cycle.
Many of the functions of PC, including their ability to sense various aspects of the cellular microenvironment, to regulate planar cell polarity, and to mediate Hedgehog, PDGF, and Wnt signaling, are also functions that play a role in or are altered during carcinogenesis (Schneider et al. 2005; McGlashan et al. 2006; Gradilone et al. 2007; Malone et al. 2007). In addition, several cilia-associated genes, including Gli1, RPGRIP1, and DNAH9, are commonly mutated in breast cancer (Sjoblom et al. 2006; Wood et al. 2007). These findings, plus the relationship between PC and proliferation, have led to speculation about the role of PC in cancer development or progression. Although PC have rarely been described by electron microscopy in epithelial cells of the normal human breast, the frequency of PC in specific cell types in the normal breast has not been previously defined, nor has the incidence of PC in human breast cancers (Stirling and Chandler 1976; Lingle and Salisbury 1999).
PC have been reported to be decreased in cells of several types of cancer, including cancers of the kidney, skin, brain, and pancreas in comparison with their normal cellular counterparts (Han et al. 2009; Moser et al. 2009; Schraml et al. 2009; Seeley et al. 2009; Wong et al. 2009). In cancers known to be driven by constitutive activation of Hedgehog signaling, specifically basal cell carcinomas of the skin and medulloblastomas, the PC can function as either enhancers or suppressors of tumorigenesis, depending on the molecular mechanism responsible for activation of the Hedgehog pathway (Han et al. 2009; Wong et al. 2009). PC act as enhancers of tumorigenesis when Hedgehog signaling is activated by overexpression of a pathway member that requires PC for optimal function, but PC are tumor suppressors in cancers initiated by overexpression of a pathway member that functions independently of PC.
In this study, we investigated the incidence of PC in a panel of breast cell lines and human breast tissues by immunofluorescence, a method that allows assessment of a larger number of cells and tissue area than the previously utilized transmission electron microscopy. Using this method, we provide new information on the relative frequency of PC in epithelial cell lines and fibroblast cultures derived from benign breast and breast cancer and in different cell types in histologically normal human breast and breast cancer tissues. In addition, we investigated the relationship between the presence of PC and rate of cellular proliferation in epithelial cells derived from benign breast and from breast cancer. Our findings indicate a general loss or inhibition of ciliagenesis in breast epithelium during carcinogenesis and cancer progression and a novel association between the presence of PC and the basal B subtype of human breast cell lines.
Materials and Methods
Cell Lines and Breast Tissues
The human breast cancer cell lines MDA-MB-468, MDA-MB-361, ZR75-1, BT474, SKBR3, T47D, MCF7, Hs578T, and MCF10A were obtained from the American Type Culture Collection (Manassas, VA). MCF10AT and MCF10AT3B cell lines were obtained from Karmanos Cancer Institute (Detroit, MI). MDA-MB-231, MDA-MB-435, MCF10DCIS.com, MCF10CA1, and SUM1315 cells were obtained from Dr. Danny Welch [University of Alabama at Birmingham (UAB), Birmingham, AL]. The 184A1 cell line, an immortalized human mammary epithelial cell line (Stampfer and Bartley 1985), was a gift of Dr. Mike Ruppert (University of West Virginia, Morgantown, WV). MDA435/LCC6 cells were provided by Dr. Donald Buchsbaum (UAB).
MDA-MB-231, MDA-MB-361, MDA-MB-468, Hs578T, MDA-MB-435, MDA435/LCC6, MCF10DCIS.com, MCF10CA1, and MCF7 cells were maintained in DMEM (Cellgro; Manassas, VA) supplemented with 10% FBS (Hyclone; Logan, UT). BT474 and SKBR3 cells were maintained in DMEM/Ham's F-12 (DMEM/F-12; Cellgro) supplemented with 5% FBS. T47D and ZR75-1 were maintained in RPMI-1640 (Cellgro) supplemented with 10% FBS and 10 μg/ml insulin (Sigma-Aldrich; St Louis, MO). SUM1315 was maintained in Ham's F-12 media supplemented with 5% FBS, 20 μg/ml insulin, and 10 ng/ml epidermal growth factor (EGF). 184A1 was maintained in mammary epithelial basal medium supplemented with MEGM SingleQuot Kit Supplement & Growth Factors (MEM; Lonza, Walkersville, MD). MCF10A, MCF10AT, and MCF10AT3B were maintained in DMEM/F-12 supplemented with 0.1 μg/ml cholera toxin (Calbiochem; San Diego, CA), 10 μg/ml insulin, 0.5 μg/ml hydrocortisone (Sigma-Aldrich), 0.02 μg/ml EGF (Upstate Biotechnology; Lake Placid, NY), and 5% horse serum (Invitrogen; Carlsbad, CA). Cultures of fibroblasts and breast epithelial cells were isolated from normal breast (breast reduction specimens) and breast cancer tissues using a standard dissociation protocol (Stampfer et al. 1980; Sadlonova et al. 2005), characterized by immunocytochemical staining for vimentin, epithelial membrane antigen, and cytokeratins, and maintained, as previously described (Sadlonova et al. 2005). The breast tissues for fibroblast and epithelial cell isolation were remnants of diagnostic surgical specimens obtained from UAB Tissue Procurement Facility after Institutional Review Board approval. All breast tissues were examined histologically to confirm the diagnosis of benignity or cancer. All the cell lines were maintained in 5% CO2 at 37C under humidified culture conditions.
Formalin-fixed, paraffin-embedded primary breast cancer tissues with corresponding non-cancerous, histologically normal breast tissue from the cancer resection specimens were obtained from the archives of the UAB Department of Pathology after Institutional Review Board approval. The breast cancer tissues were characterized for estrogen receptor (ER), progesterone receptor (PR), and Her2/neu expression by IHC, as described previously (Talley et al. 2002,2008).
Immunofluorescence
For immunofluorescence staining of cultured cells, 5 × 105 cells were seeded onto cover slips and cultured for the indicated time periods in maintenance medium with and without serum. Cells were washed with PBS, fixed in 3.9% paraformaldehyde for 10 min, and incubated in blocking solution (1% goat serum, 0.1% Triton X-100 in PBS) for 30 min. Cells were then incubated in primary antibodies for 1 hr at room temperature. Primary antibodies used were mouse anti-acetylated α-tubulin (1:4000 dilution, clone 6-11B-1; Sigma-Aldrich), rabbit anti-γ-tubulin (1:1000 dilution; Sigma-Aldrich), rabbit anti-Ki-67 (1:500 dilution; Zymed, San Francisco, CA), and a rabbit anti-cytokeratin cocktail (1:100 dilution, cytokeratins 10, 13, 14, 18, and 19; AnaSpec, San Jose, CA). After washing with PBS, cells were incubated with secondary antibodies (donkey anti-mouse Alexa 594 and donkey anti-rabbit Alexa 488; Invitrogen) for 1 hr at room temperature, followed by washing and incubation with 4′,6-diamidino-2-phenylindole (DAPI) (5 μg/ml; Sigma-Aldrich). Slides were mounted with Fluoromount-G (SouthernBiotech; Birmingham, AL). For staining with γ-tubulin, sections were pretreated with pepsin, as described below.
For the immunofluorescence detection of PC in formalin-fixed, paraffin-embedded tissues, histological sections (8 μm in thickness) were deparaffinized in xylene followed by rehydration. Immunostaining for pan-cytokeratin, keratin 14, and γ-tubulin required antigen retrieval consisting of incubation in 0.01% pepsin (Sigma-Aldrich) in PBS (pH adjusted to 2.0 by 0.01 M HCl) for 30 min at 37C in a humidified chamber. After gentle washing, sections were then treated with 0.05% Tween-20 for 5 min. The remainder of the immunofluorescence staining proceeded as described earlier. The rabbit anti-keratin 14 (AnaSpec) and anti-pan-cytokeratin (NCL-CKp; Novocastra, Newcastle, UK) were used at 1:150 dilution. Staining without the addition of primary antibodies served as negative controls. To identify the relative frequency of PC in the different cell types constituting the ductulobular units of normal breast, lobular acini, small ducts and terminal ducts and their immediate periductal stroma as well as the interlobular stroma were examined at 1000× magnification for the presence of PC. A minimum of 100 PC were identified per case, and the cell type in which each primary cilium was located was determined by a combination of co-staining for acetylated α-tubulin and pan-cytokeratin or keratin 14 and the location and morphology of the cell. In breast cancer, examination was limited to the confines of the cancer. A minimum of 20 high-power fields (×1000) per cancer was searched for the presence of PC, which were then localized to pan-cytokeratin-negative stromal cells or rarely to pan-cytokeratin-positive epithelial cells.
Computer-aided Quantification of PC and Image Processing
To improve the accuracy of determining the frequency of PC in cultured cells, a computer-aided approach was used. Because of the variable, three-dimensional (3D) morphology of PC, they were manually counted in each randomly selected ×400 microscopic field, which was subsequently photographed. ImageJ software [National Institutes of Health (NIH); Bethesda, MD] was used for the calculation of the number of cells (DAPI-stained nuclei) in each microscopic field. DAPI-stained nuclei in that field were captured at the corresponding blue channel. The number of nuclei in a total of 15 microscopic fields per slide (1000–2000 cells) was then used to calculate the percentage of cells with PC. The percentage of cells labeled by Ki-67 was quantified in a similar fashion.
Images were acquired from individual R-G-B channels by an Axioplan 2 microscope equipped with an AxioCam HRC Camera and AxioVision software (Carl Zeiss; Thornwood, NY). The same exposure time was applied during image acquisition for the same target antigen among different cells or time points. Entire images of each of the R-G-B channels were adjusted (i.e., background subtraction, brightness, and contrast) independently with ImageJ (NIH) before image merging using the “RGB merge” function of ImageJ. Identical settings were used for adjustments of images of each target antigen. To compile stacked images on different focal planes, Z-stack images were captured by multidimensional acquisition using the AxioVision imaging system. ImageSurfer (a non-commercial software developed by the University of North Carolina at Chapel Hill, Chapel Hill, NC) was used for the 3D reconstruction from Z-stack immunofluorescence images (Feng et al. 2007).
Statistical Analysis
Correlation between Ki-67 positivity and the incidence of PC was assessed by Spearman's rank correlation. Differences in percentages of cells with PC and Ki-67 labeling were compared between cell lines using Student's t-test or one-way ANOVA. Two-way ANOVA was used to compare the frequency of PC in the different cell types of the normal breast among different individuals. Significance was defined as p<0.05.
Results
PC Are More Frequent in Epithelial Cell Lines Derived From Normal Breast Than in Epithelial Cells Derived From Breast Cancer
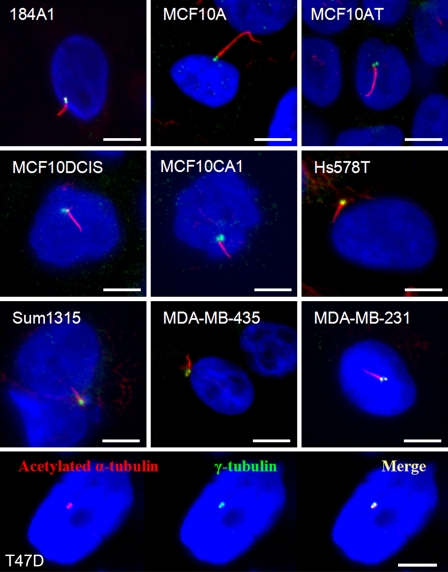
To compare the incidence of PC among breast cell lines, we detected PC by immunofluorescence. Antibodies that recognize stabilized acetylated α-tubulin in the axoneme are widely used for the identification of PC. The intensity of staining and morphology of PC stained with anti-acetylated α-tubulin alone are typically sufficient for their accurate identification. However, these antibodies may also bind to centrioles, mitotic spindles, midbodies, and, to a lesser extent, cytoplasmic microtubules (Piperno et al. 1987; Alieva et al. 1999). To increase the specificity of our immunofluorescence for PC, we used co-staining for γ-tubulin, a specific marker of centrosomes, and acetylated α-tubulin. A primary cilium extends from the mother centriole, which is one of the juxtaposed, paired centrioles of the centrosome, as shown in Figure 1. This specific co-localization of γ-tubulin and acetylated α-tubulin confirms the presence of a primary cilium.
Figure 1.
Representative images of primary cilia (PC) in human breast cell lines. PC were identified by co-immunofluorescence for acetylated α-tubulin (red) and γ-tubulin (green), markers of the ciliary axoneme and centrosome, respectively. Acetylated α-tubulin antibody also recognizes the centrosome to a variable extent, and there was usually co-localization of α-tubulin and γ-tubulin at the centrosome (as shown in T47D cells). In 184A1 and MCF10A, the PC were relatively long and often curved (merged images). In the cancer cell lines, including Hs578T, SUM1315, MDA-MB-435, and MDA-MB-231, PC were shorter and straight (merged images). PC in MCF10AT were similar to MCF10A, but PC were shorter and straight in the more transformed MCF10DCIS.com and MCF10CA1. In T47D cells and in other breast cancer cell lines with no detectable PC, no ciliary axoneme could be observed, only co-localization of α-tubulin and γ-tubulin at the centrosome. In all images, nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Bar = 10 μm.
Cells were grown in routine culture conditions in maintenance media with or without serum, or in other medium as specifically indicated, for up to 7 days. Culture conditions that induce cell cycle arrest, including long duration and absence of serum (Wheatley et al. 1996), were used in an attempt to induce PC assembly. In multiple high-power microscopic fields, the number of PC per field was determined by manual examination. To count the total number of cells per microscopic field, we used a computer-aided approach, as described in Material and Methods. The percentage of cells containing PC was then calculated. Although the rate of proliferation was generally reduced after growth for 7 days, the majority of cells remained viable. Only adherent cells with intact nuclei were counted. Representative photomicrographs of cultures of MCF7 cells at day 7 are presented in the online Supplemental Figure SF1. PC were readily identified in 184A1 and MCF10A cells (Figure 1), both immortalized cell lines derived from benign breast (Stampfer and Bartley 1985; Soule et al. 1990). We assessed the incidence of PC in primary breast epithelial cells which we isolated from the benign breast of a 62-year-old woman with a history of breast cancer and also found a relatively high incidence of PC (12.7% of epithelial cells were ciliated) (online Supplemental Figure SF2). However, PC were found at a lower frequency in breast cancer cell lines. In several breast cancer cell lines, specifically MDA-MB-468, MDA-MB-361, T47D, MCF7, BT474, ZR75-1, and SKBR3, PC were absent. In these cancer cell lines without PC, only co-localization of γ-tubulin and acetylated α-tubulin at the centrosome was identified with no ciliary axoneme discernable, as depicted in T47D cells in Figure 1. PC were identified in Hs578T, SUM1315, MDA-MB-435, and MDA-MB-231 cancer cells (Figure 1), but at a lower maximum frequency than in 184A1 or MCF10A cells. Table 1 lists the percentages of cells with PC after culture in maintenance media, with the exception of 184A1, which was grown in DMEM, with and without serum for 7 days. Morphologically, the length and shape of PC differed among the epithelial cell lines, from being long and smoothly curved in MCF10A and 184A1 to straight and short in the breast cancer cell lines (Figure 1). Morphological disparity in the length and curvature of PC among different cell lines may reflect an intrinsic difference in the cilia assembly machinery, as well as factors that control and maintain ciliagenesis. PC were most frequently found on fibroblasts which we isolated from normal breast [normal breast-associated fibroblasts (NAF)] or from breast cancer [cancer-associated fibroblasts (CAF)], and their morphology was long and slender, similar to 184A1.
Table 1.
Percentages of cells with primary cilium in human breast cell lines
| Percentages of cells with primary cilium on day 7 |
|||
|---|---|---|---|
| Cell line | Subtypea | With serum | Without serum |
| Epithelial cells derived from benign breast | |||
| 184A1 | Basal Bb | 7.6 | 19.0 |
| MCF10A | Basal B | 10.5 | 6.6 |
| Epithelial cells derived from cancer | |||
| Hs578T | Basal B | 3.3 | 4 |
| SUM1315 | Basal B | 1.3 | 1.6 |
| MDA-MB-435 | Basal B | 0.9 | 0.7 |
| MDA-MB-231 | Basal B | 1.7 | 0.3 |
| MDA-MB-468 | Basal A | 0 | 0 |
| MDA-MB-361 | Luminal | 0 | 0 |
| T47D | Luminal | 0 | 0 |
| MCF7 | Luminal | 0 | 0 |
| BT474 | Luminal | 0 | 0 |
| ZR75-1 | Luminal | 0 | 0 |
| SKBR3 | Luminal | 0 | 0 |
| Fibroblasts | |||
| Normal breast-associated fibroblast | NA | 68 | ND |
| Breast cancer-associated fibroblast | NA | 73 | ND |
According to the classification of Neve et al. (2006).
Data for subclassification of 184A1 provided in Blick et al. (2008).
NA, not applicable; ND, not determined.
While most of the cell lines were grown in DMEM with and without serum, several cancer cell lines were grown in DMEM/F-12 or RPMI-1640 with insulin for optimal viability. To assure that the use of different culture media was not significantly influencing the frequency of PC among cancer cell lines, we cultured T47D, which lack PC, and Hs578T, the cancer cell line with the highest incidence of PC, in three different media—DMEM, DMEM/F-12, and RPMI-1640 + insulin—each with and without serum. T47D failed to assemble PC in all three media with and without serum. The frequency of PC in Hs578T varied little among the three media (online Supplemental Figure SF3A).
Interestingly, the breast cancer cell lines that possess PC all belong to the basal B subtype of breast cell lines. Human breast cell lines can be divided into two major groups, luminal and basal, based on clustering of gene expression profiles, with the basal subtype cell lines being further classified into two groups, basal A and basal B (Neve et al. 2006). Basal B cell lines, which include MCF10A and 184A1, are epithelial, but are negative for ER, PR, and Her2/neu. Basal B cell lines also possess some mesenchymal features, including expression of vimentin and a spindled or stellate morphology. Basal B subtype cell lines are typically more highly invasive and migratory in vitro compared with basal A and luminal subtype cell lines (Neve et al. 2006; Blick et al. 2008). Therefore, PC were found to be present only on those cell lines with a more mesenchymal phenotype, including fibroblasts and the basal B subtype of epithelial cell lines. Of the basal B subtype of breast cancer cell lines, Hs578T has the highest incidence of PC. The Hs578T cell line is unusual in that it was isolated from a carcinosarcoma of the breast, which is a biphasic cancer with both mesenchymal (sarcoma) and epithelial (carcinoma) components (Hackett et al. 1977; Charafe-Jauffret et al. 2006). Therefore, of the breast cancer cell lines, Hs578T has the most mesenchymal/fibroblastic features and also the highest incidence of PC. In addition, Hs578T is non-tumorigenic in immunocompromised mice (Lacroix and Leclercq 2004).
The Low Incidence of PC in Breast Cancer Cell Lines Is not a Result of a High Rate of Proliferation
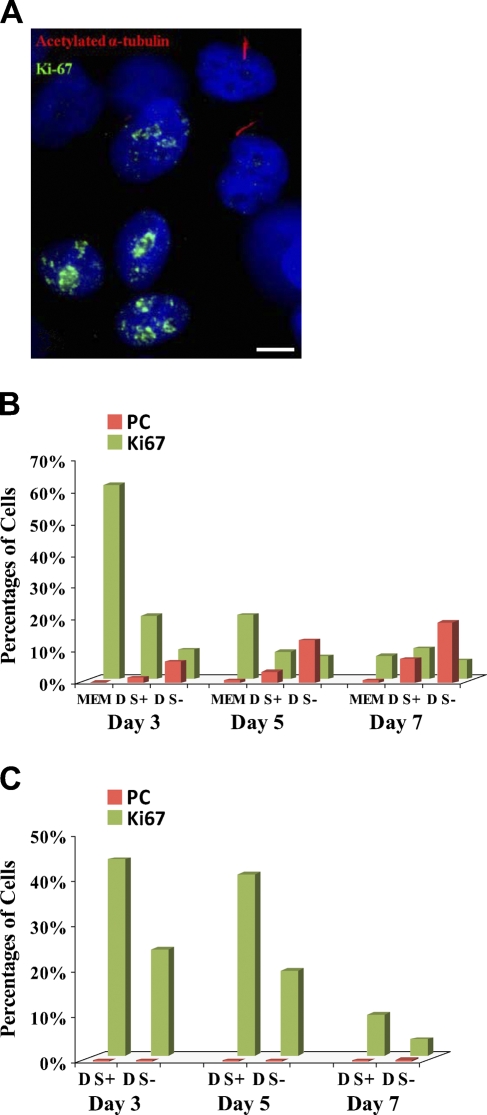
The presence of PC is closely related to the cell cycle, and they are most commonly found in quiescent cells in G0 phase (Molla-Herman et al. 2008). In many cell types, the incidence of PC increases in culture conditions that induce growth arrest, including serum starvation, long culture duration, and high confluency (Wheatley et al. 1996; Alieva et al. 1999). Therefore, we speculated that the low frequency or absence of PC in breast cancer epithelial cells in vitro may result from a high rate of proliferation. To address this, we explored the relationship between proliferation and the incidence of PC in breast epithelial cell lines by immunofluorescence staining for Ki-67 and PC. Ki-67 is strongly expressed in stages S, M, and G2 of the cell cycle and weakly expressed in stage G1, but is absent in stage G0 (Gerdes et al. 1984; Braun et al. 1988; Lopez et al. 1991). For 184A1, cells were grown for 3–7 days in several conditions: (1) their complete medium (MEM), which is optimized for growth of mammary epithelial cells and contains a variety of growth factors and supplements, (2) DMEM with serum, or (3) DMEM without serum. As expected, PC were localized only to cells that were negative for Ki-67 and, therefore, to cells in G0 stage (Figure 2A). In each growth condition, the percentages of cells containing PC or labeled with Ki-67 were determined. The number of PC was higher and proliferation was lower in 184A1 grown in DMEM without serum in comparison to cells grown in either complete medium or DMEM with serum (Figure 2B). There was a statistically significant inverse correlation between Ki-67 labeling and the incidence of PC among all the culture conditions and time points (Spearman's rank correlation coefficient r = −0.77, p=0.02).
Figure 2.
Relationship between the incidence of PC and rate of proliferation in 184A1 and MDA-MB-231. (A,B) 184A1 was cultured in three different media: DMEM without FBS (D S−), DMEM with 10% FBS (D S+), and optimal basal medium with supplements and growth factors (MEM). PC were labeled by anti-acetylated α-tubulin (red) and proliferating cells by anti-Ki-67 (green). (A) PC were located only in non-proliferating Ki-67-negative cells. (B) The incidence of PC (red bars) in 184A1 cells was higher in serum-free conditions (D S−) and with longer duration of culture. Data are the mean and standard error of two independent experiments performed in triplicate. (C) MDA-MB-231 were grown in DMEM with (D S+) and without (D S−) serum. Proliferation (Ki-67 labeling, green bars) was lower in serum-free conditions; however, there was no corresponding statistically significant increase in PC (red bars). Data are the mean of two independent experiments performed in triplicate. Bar: A = 10 μm.
Similar experiments were performed with MDA-MB-231 breast cancer cell lines in DMEM with and without serum. Although proliferation was lower in serum-free medium (for all three time points combined, t-test, p<0.05) and with increasing duration of culture (for both culture conditions, single-factor ANOVA, p<0.01), there was no statistically significant difference in the occurrence of PC in MDA-MB-231 cells cultured in medium with or without serum or with longer duration of culture (Figure 2C). Unlike 184A1, there was no significant correlation between Ki-67 staining and the frequency of PC.
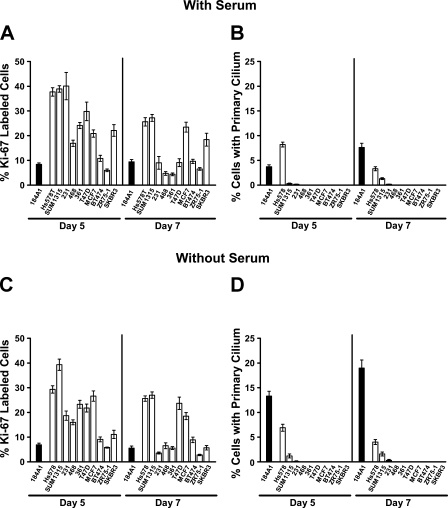
Additional breast cancer cell lines were also cultured in their maintenance medium with and without serum for 5 and 7 days to determine whether growth limiting conditions and a decrease in proliferation would increase the incidence of PC to the level seen in 184A1 or MCF10A. In the cancer cell lines, in general, the absence of serum had less effect on the rates of proliferation than did the duration of culture. Interestingly, Hs578T cancer cells exhibited the highest frequency of PC at day 5 in the presence of serum when the rate of proliferation was greatest, further underscoring the poor correlation between the rate of proliferation and the incidence of PC in some cancer cell lines. SUM1315 and 231 showed only a modest increase or no change in the frequency of PC with lower rates of proliferation. Furthermore, some of the cancer cell lines (MDA-MB-231, MDA-MB-468, MDA-MB-361, T47D, BT474, ZR75-1, and SKBR3) achieved low rates of proliferation, similar to 184A1, yet the incidence of PC in those cancer cell lines did not reach the relatively high frequency of PC found in 184A1 (Figures 3A and 3B). Treatment with the DNA crosslinking agent mitomycin C to produce cell cycle arrest (Lu et al. 2009) also failed to induce the presence of PC in T47D cells and had little effect on the incidence of PC in MDA-MB-435 cells (online Supplemental Figure SF3B). These results suggest that the general low level or absence of PC in cancer cell lines is not caused solely by a higher proliferative activity, but that other, as yet unknown, factors or conditions are also preventing the assembly or promoting the disassembly of PC in the cancer cell lines.
Figure 3.
Proliferation and frequency of PC in 184A1 and breast cancer cell lines. Ki-67 labeling and the frequency of PC were determined for each cell line after 5 and 7 days of culture in media with and without serum. (A,B) In growth conditions with serum, the rates of proliferation, i.e., Ki-67-labeling (A), decreased in most cancer cell lines with duration of culture in growth-limiting conditions, whereas the incidence of PC (B) increased only in SUM1315 cancer cells. However, the incidence of PC increased in 184A1 cells with culture duration. (C,D) In growth conditions without serum, the rates of proliferation of the cancer cells was only marginally, if at all, lower than when grown with serum (C) and the incidence of PC changes little with time (D). In 184A1, the rate of proliferation was lower in serum-free medium, particularly at day 7, and the incidence of PC was highest in serum-free medium at day 7. The incidence of PC in the cancer cells never approached the maximum incidence of PC found in 184A1 (at day 7 without serum), even in cell lines with similar rates of proliferation as 184A1 cells. The data presented are the mean and standard error of two independent experiments performed in triplicate.
The Incidence of PC Is Lowest in the Most Transformed/Tumorigenic Cells in Isogenic Models of Cancer Progression
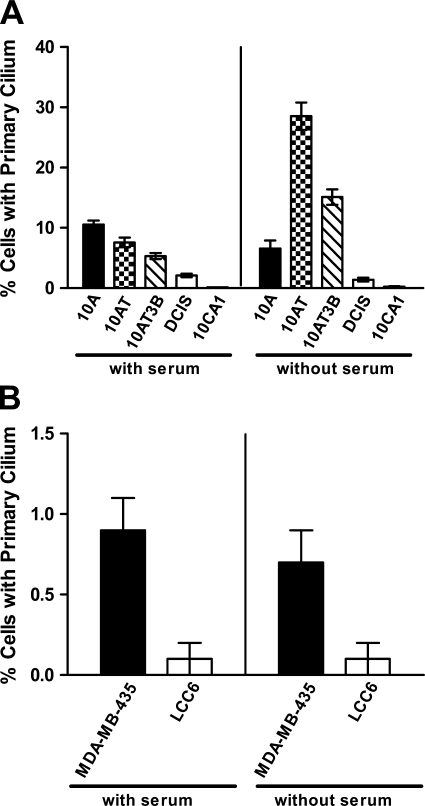
The development and progression of malignancies has been modeled by selecting and establishing isogenic cell lines with escalating degrees of transformation and tumorigenicity. The MCF10 series of cell lines is one such model system. These cell lines are genetically related, all being originally derived from MCF10A cells, and represent the continuum from normal/hyperplastic breast (MCF10A), to premalignant breast disease (MCF10AT and MCF10AT3B), to comedo ductal carcinoma in situ (MCF10DCIS.com), and to invasive and metastatic carcinoma (MCF10CA1) (Dawson et al. 1996; Strickland et al. 2000; Hurst et al. 2009). We assessed the presence of PC in this series of cell lines by immunofluorescence staining after growing the cells in maintenance medium with serum and without serum for 7 days. In culture conditions with serum, the incidence of PC gradually decreased with increasing degree of transformation of the cell lines. In serum-free conditions, the incidence of PC was higher in the less transformed MCF10A, MCF10AT, and MCF10AT3B cells (14.5% of cells in all three cell lines combined at day 7 in serum-free conditions), but was significantly lower in MCF10DCIS.com and MCF10CA1 cells (1.9% of cells in both cell lines combined, p<0.001, t-test) (Figure 4A). The incidence of PC was higher in serum-free, growth inhibitory conditions than in culture conditions with serum in the MCF10AT and MCF10AT3B cells (p < 0.001 for both cell lines, t-test). However, serum-free culture conditions did not increase the frequency of PC in the more transformed MCF10DCIS.com and MCF10CA1 cancer cell lines (Figure 4A), even with lower rates of proliferation in serum-free conditions (1% and 0.6% Ki-67 labeling, respectively) than with serum (8% and 10% Ki-67 labeling, respectively). Interestingly, the serum-free conditions did not increase the frequency of PC in MCF10A, as was the case in 184A1 cells. The reason for this is not clear, but the finding suggests that even in “benign” or non-tumorigenic cells, there are factors other than the absence of proliferation that control the assembly or disassembly of PC. To assure that differences in the two media used to culture the cell lines (i.e., MCF10A maintenance media for MCF10A, MCF10AT, and MCF10AT3B and DMEM for MCF10DCIS.com and MCF10CA1) were not responsible for the differences in the incidence of PC, MCF10CA1 was cultured in the MCF10A maintenance medium or DMEM, each with serum. The incidence of PC was similar in the two media (online Supplemental Figure SF3C). In general, the morphology of the PC also differed between the more and less transformed cell lines. In MCF10AT and MCF10AT3B, the PC were long and curved, similar to those in MCF10A, whereas in MCF10DCIS.com and MCF10CA1, the cilia were shorter and straight, similar to those in other breast cancer cell lines (Figure 1).
Figure 4.
Incidence of PC in the MCF10A series of cell lines and in MDA-MB-435, MDA435/LCC6. (A) MCF10A (10A), MCF10AT (10AT), MCF10AT3B (10AT3B), MCF10DCIS.com (DCIS), and MCF10CA1 (10CA1) were grown in their respective maintenance media with or without serum for 7 days. The frequencies of PC were determined by co-immunofluorescence staining for acetylated α-tubulin and γ-tubulin. In culture conditions with serum, the incidence of PC gradually decreased with increasing transformation. Without serum, the incidence of PC was higher in the less transformed MCF10A, MCF10AT, and MCF10AT3B cells (14.5% of cells in all three cell lines combined), but was significantly lower in MCF10DCIS.com and MCF10CA1 cells (1.9% of cells in both cell lines combined, p < 0.001, t-test) Data represent the mean and standard error of two independent experiments performed in triplicate. (B) MDA-MB-435 and its metastatic derivative MDA435/LCC6 (LCC6) were grown in culture conditions with and without serum for 7 days, and the incidence of PC was determined as described earlier. MDA435/LCC6 cells displayed a significantly lower incidence of PC compared with MDA-MB-435 cells in culture conditions with and without serum (p < 0.05, t-test). Data represent the mean and standard error of two separate experiments performed in triplicate.
Another progression model of breast cancer is the pair of cell lines MDA-MB-435 and MDA435/LCC6. The origin of MDA-MB-435 cells has been questioned, with gene expression profiling suggesting that it is of melanocytic rather than breast epithelial origin (Ross et al. 2000). It has been argued that MDA-MB-435 is actually the M14 melanoma cell line (Rae et al. 2007). However, MDA-MB-435 cells secrete milk proteins (Sellappan et al. 2004), and, importantly, they were shown to have two X chromosomes, whereas the M14 cell line was derived from a man (Chambers 2009). Therefore, MDA-MB-435 is not the M14 cell line and has mammary epithelial features. Consequently, we have included MDA-MB-435 in our analysis of breast cancer cell lines. The MDA435/LCC6 cell line was established from spontaneous metastases to the peritoneal cavity of parental MDA-MB-435 cells after they had been inoculated in the mammary fat pads of nude mice (Leonessa et al. 1996). Similar to the results from the MCF10 series of cell lines, the metastatic derivative of MDA-MB-435, MDA435/LCC6 cells displayed a significantly lower incidence of PC compared with the parental cell line in culture conditions with and without serum after growth for 7 days (p<0.05, t-test) (Figure 4B). Similar to other breast cancer cell lines, the percentages of cells with PC did not change significantly in culture conditions with serum vs those without serum, even with lower rates of proliferation for both MDA-MB-435 and MDA435/LCC6 (12% and 15% Ki-67 labeling, respectively, with serum vs 4% and 6%, respectively, without serum). Because the more transformed tumorigenic or metastatic cell lines in these two series of cell lines are isogenic to their respective parental cell lines, these results further support a loss of PC during breast carcinogenesis and cancer progression.
PC Are Readily Identified in Epithelial Cells in Normal Breast Tissue
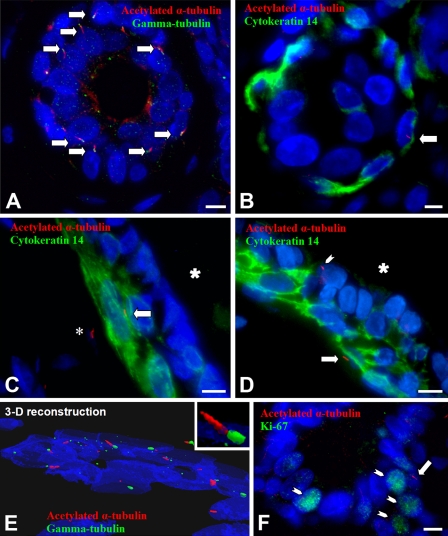
To determine whether our in vitro results were relevant to human breast tissue and breast cancers in vivo, we similarly analyzed histologically normal human breast and breast cancers for the presence of PC by immunofluorescence. We examined the histologically normal breast tissue from five women with breast cancer, who ranged in age from 37 to 73 years. By co-staining the cells with γ-tubulin and acetylated α-tubulin, we first confirmed the presence of PC in morphologically normal ducts and lobular units (Figures 5A and 5E). To confirm the presence of PC in lobular and ductal epithelium, we used co-immunofluorescence for acetylated α-tubulin and pan-cytokeratin (a marker of both luminal cells and myoepithelial cells) or cytokeratin 14 [a marker specific for myoepithelial cells (Figures 5B–5D)]. Two layers of epithelial cells are present in the normal breast: an inner, cuboidal, luminal epithelial layer and an outer layer of cytokeratin 14-positive myoepithelial cells. PC were identified in luminal and myoepithelial cells of ducts and lobules (Figures 5B–5D) and were also present in stromal cells (Figure 5C). These stromal cells were identified as intralobular and periductal stromal fibroblasts based on their location, morphology, and absence of cytokeratin staining. Ki-67 staining was also performed on these tissues, and PC were located in Ki-67-negative non-proliferating cells (Figure 5F).
Figure 5.
Representative images of PC in histologically normal breast. (A) Co-immunofluorescence for acetylated α-tubulin (red, axoneme) and γ-tubulin (green, centrosome) in histologically normal breast tissue reveals multiple PC (arrows) in an acinus of a lobule (merged image). (B) Co-immunofluorescence for acetylated α-tubulin (red, axoneme, arrow) and cytokeratin 14 (diffuse green cytoplasmic staining) allows distinction of lobular myoepithelial cells (green cytoplasm) from stroma and from luminal cells (inner cell layer). The arrow marks a primary cilium on a myoepithelial cell within a lobular acinus. (C) Anti-cytokeratin 14 (green) labels myoepithelial cells of a duct. The large asterisk marks the duct lumen. PC, labeled by anti-acetylated α-tubulin (red, axoneme), are present in a myoepithelial cell (arrow) and in a stromal cell located outside the duct (small asterisk). (D) Anti-cytokeratin 14 (green) labels myoepithelial cells of a duct. The asterisk marks the duct lumen. PC, labeled by anti-acetylated α-tubulin (red, axoneme), are present in a myoepithelial cell (large arrow) and luminal epithelial cell (small arrowhead). (E) A three-dimensional reconstruction was prepared using ImageSurfer software and shows the relationship of the centrosome (green) and ciliary axoneme (red) in a lobular acinus. The inset is a higher magnification of one primary cilium with its centrosome. (F) Co-immunofluorescence of a histologically normal lobular unit for acetylated α-tubulin (red) and Ki-67 (green, small arrows) demonstrates that PC (large arrow) were located in Ki-67-negative, non-proliferating cells. Bar = 10 μm.
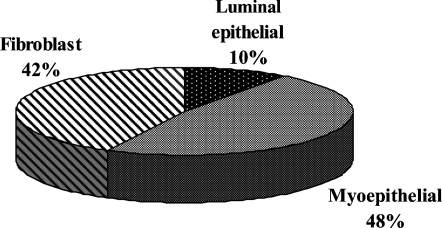
To determine the relative frequency of PC in each of these cell types, we quantified the cellular location of 100 PC within ductolobular units, including smaller ducts extending from lobular units, per case. After a primary cilium was identified, its cellular location was determined based on co-immunostaining with cytokeratin 14 and the location of the cell containing the primary cilium. We chose this approach, rather than quantifying the number of PC per each cell type, because the histological sections, although relatively thick (8 μM), contain only portions, rather than the entirety, of some cells. The inability to examine the entirety of each cell would have likely underestimated the incidence of PC. However, our approach does bias the results toward those cell types that are more numerous. The distribution of cell types containing PC was similar among each of the five individuals (two-way ANOVA, p=0.989, data not shown). The incidence of PC was higher in fibroblasts and myoepithelial cells than in luminal epithelial cells (Figure 6).
Figure 6.
Location of PC in histologically normal human breast. A pie chart demonstrates the cellular location of 500 PC identified in ductolobular units of the histologically normal breast from five different women (100 PC per breast).
PC Are Very Rare in Breast Cancer Epithelial Cells and Are Present Primarily in Stromal Cells in Breast Cancer
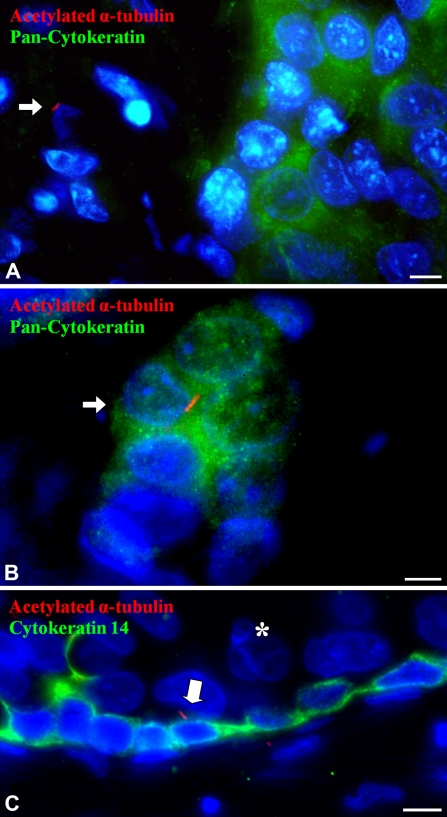
Twenty-six breast cancers were examined for the presence of PC by co-immunofluorescence staining for acetylated α-tubulin and pan-cytokeratin. These cancers consisted of infiltrating ductal carcinomas from women ranging in age from 26 to 82 years. Because PC were found only in the basal B subgroup of breast cancer cell lines, we enriched the breast cancers for “triple-negative” breast cancers, which are negative for ER, PR, and Her2/neu (Rakha and Ellis 2009). The basal B subtype of cell lines is believed to reflect the clinical triple-negative breast cancer subtype (Neve et al. 2006). The 26 breast cancers consisted of 8 ER-positive breast cancers, 3 ER-negative and Her2/neu-positive breast cancers, and 15 triple-negative breast cancers. In these cancers, the epithelial cell component was marked by staining for pan-cytokeratin. In 25 of the 26 cases, PC were absent in cancerous epithelial cells and were present only in intermingled, cytokeratin-negative stromal cells, identified as stromal fibroblasts by location and cell morphology (Figure 7A). However, in one triple-negative invasive breast cancer, rare PC were identified in cytokeratin-positive cancer cells [two PC in 25 high-power fields, ∼8000 cancer epithelial cells (0.03%)] in addition to PC located within stromal cells (Figure 7B). DCIS was also found in association with three of the invasive carcinomas. These cancers were also immunofluorescently stained for cytokeratin 14 to mark residual myoepithelial cells in DCIS. In each of these DCIS, PC were found in cytokeratin 14–positive myoepithelial cells at the periphery of the DCIS lesions, but not in the neoplastic luminal epithelial cells (Figure 7C). Therefore, in breast cancer tissues, PC are rare or absent in cancer epithelial cells, but are frequent only in cancer-associated stromal fibroblasts. The only breast cancer in which we found PC in cytokeratin-positive cancer cells was a triple-negative carcinoma, reflecting the association that we observed between the presence of PC and basal B cell lines.
Figure 7.
Location of PC in human breast cancer. (A) Co-immunofluorescence for acetylated α-tubulin (red, axoneme) and pan-cytokeratin (diffuse green cytoplasmic staining) of an invasive breast cancer. The green cytoplasmic staining marks the cancer epithelial cells. In most invasive breast cancers examined, PC (arrow) were only identified on stromal cells without cytokeratin positivity, which were most often solitary and with an elongate nucleus typical of a stromal fibroblast. (B) Co-immunofluorescence for acetylated α-tubulin (red, axoneme) and pan-cytokeratin (diffuse green cytoplasmic staining) of one invasive breast cancer of the triple-negative subtype exhibited rare PC (arrow) in pan-cytokeratin-labeled cancer epithelial cells. (C) Co-immunofluorescence for acetylated α-tubulin (red, axoneme) and keratin 14 (green cytoplasm, myoepithelium) of ductal carcinoma in situ (DCIS) shows a primary cilium (arrow) on residual myoepithelial cells ringing the DCIS. No PC were identified on the luminal epithelial cells (asterisk) in DCIS. Bar = 10 μm.
Discussion
Our data indicate a marked decrease or absence of PC in breast cancer epithelial cells compared with normal breast epithelial cells, both in cultured cell lines and in breast tissues. In histologically normal breast tissues, PC were most common in stromal fibroblasts followed by myoepithelial cells [also referred to as basal cells (Perou et al. 2000)] and were less frequent in luminal epithelial cells. Breast cancers and breast cell lines can be classified by gene expression profiling into two major subtypes that reflect the luminal and myoepithelial (or basal) cell gene expression patterns, referred to as luminal and basal subtypes. Basal subtype breast cell lines can be further divided into basal A and basal B subtypes, with the basal B subtype exhibiting vimentin expression and a stem cell line expression profile (Neve et al. 2006). In breast cancer cell lines, PC were identified (although at a relatively low frequency) only in basal B subtype epithelial cell lines, whereas they were absent in luminal and basal A subtype cell lines. MCF10A and 184A1 epithelial cells, which possess relatively abundant PC, also cluster with the basal B subtype and express vimentin (Neve et al. 2006; Blick et al. 2008). In breast cancer tissues, rare PC were identified in cancer epithelial cells only in triple-negative cancer, which possesses a basal phenotype (Rakha and Ellis 2009). Therefore, these data indicate that the presence of PC is associated with a basal phenotype in the normal breast and breast cancer. The reason that PC were exclusively found in the basal B subtype cancer cell lines is not known. One possible explanation is that the basal B cancer cell lines may have arisen from the neoplastic transformation of a progenitor cell with a basal phenotype and frequent ciliation, perhaps similar to MCF10A or 184A1, and with malignant progression, the incidence of PC decreased. Luminal or basal A subtype cancer cells may have originated from a subset of epithelial progenitor cells with a very low level of PC or without PC. It is also possible that the constellation of molecular features that characterize the basal B phenotype is particularly conducive to PC formation, and, therefore, PC are more frequently assembled in epithelial cells with this phenotype, whether benign or malignant. Whether or not the presence of PC promotes breast cancer development or progression by participating in a particular signaling pathway, such as the Hedgehog pathway, remains to be determined.
Our data also demonstrate a lower incidence of PC in the more transformed and aggressive derivatives of MCF10A (i.e., MCF10DCIS.com and MCF10CA1) and MDA-MB-435 (i.e., MDA435/LCC6) compared with the parental cell lines or less transformed derivatives. These data further indicate that PC are lost during carcinogenesis and cancer progression. Additionally, our data show that the relative absence of PC in breast cancer cell lines is not simply a result of a high rate of proliferation of breast cancer epithelial cells, but is presumably due to an inability, or reduced ability, to assemble or maintain PC. These findings raise the possibility that those breast cancer epithelial cells without PC may lack certain intrinsic mechanisms for ciliogenesis. Our results are similar to findings in renal cell and pancreatic carcinomas, where there was no correlation between the rates of proliferation and the frequencies of PC (Schraml et al. 2009; Seeley et al. 2009).
At present, it is not known whether the loss of PC during breast carcinogenesis is causative or simply a consequence of cancer development. Their loss in cancer cells does suggest that PC are important in the maintenance of normal breast tissue homeostasis and raises the possibility that PC have a tumor suppressor function in the breast. In the normal breast, PC were most frequent in fibroblasts and myoepithelial cells, which constitute a cilia-rich fibroblast–epithelial cell interface where there is likely to be active crosstalk between epithelial cells and stroma. The mechanosensory and chemosensory functions of PC may be important in mediating this crosstalk. In addition, PC were found to extend from the apical membranes of some luminal cells into the ductal/acinar lumen. This localization is similar to the presence of PC on the epithelial cells of the renal tubules. In renal tubular cells, bending of the PC by the force of fluid flow within the renal tubule results in an intracellular influx of calcium (Nauli et al. 2003), and the absence or disruption of PC function in renal tubular cells causes cyst formation, resulting in polycystic kidney disease (Uhlenhaut and Treier 2008). This has led to the belief that PC are sensors of fluid flow or sheer forces in renal epithelial cells, and this sensory ability is important to the normal development of renal tubules. The PC of cholangiocytes lining intrahepatic bile ducts also project into the ductal lumen and are able to detect osmotic pressure via TRPV4, a [Ca2+]-permeable ion channel, and biliary nucleotides via P2Y12 purinergic receptors located in PC (Gradilone et al. 2007; Masyuk et al. 2008). PC may play similar roles in the breast by sensing mechanical forces or chemical constituents of breast epithelial secretions in acini or ducts, particularly during lactation. Therefore, loss of PC during breast carcinogenesis may prevent breast epithelial cells from sensing and responding to environmental signals, such as secretory products from stromal cells or mechanical forces emanating from ductal contents or the extracellular matrix (Plotnikova et al. 2008), possibly resulting in changes in the regulation of growth and differentiation that promote cancer development.
In established breast cancers, the presence of PC in CAF may be important for those fibroblast–epithelial interactions that promote cancer progression. In comparison to NAF, CAF exhibit an increased production of type-specific collagens and a variety of growth factors and proteases (Tlsty 2001). As a consequence, CAF enhance tumor growth, facilitate metastasis, mediate resistance to chemotherapy and hormonal therapies, and promote neoangiogenesis (Horgan et al. 1987; Noel et al. 1993; Muerkoster et al. 2004; Orimo et al. 2005; Shekhar et al. 2007; Ostman and Augsten 2009). The CAF phenotype is stimulated by paracrine signaling between cancer cells and resident fibroblasts or bone marrow-derived mesenchymal stem cells and is known to be induced by PDGF and Hedgehog ligand, both of which require PC for regulated signaling (Shao et al. 2000; Ishii et al. 2003; Kalluri and Zeisberg 2006; Zeisberg et al. 2007; Bailey et al. 2008; Ostman and Augsten 2009). Therefore, PC in established breast cancers may have a role in regulating cancer behavior by their presence on CAF.
In summary, our systematic examination of PC in human breast cell lines, as well as in histologically normal or malignant human breast tissues, indicates that a loss of PC occurs during breast carcinogenesis. Considering the versatility and complexity of this unique organelle, there are likely multiple functional consequences for the absence of PC in breast epithelial cells. With accumulating data demonstrating a decrease in the occurrence of PC in several carcinoma types, the spectrum of ciliopathies may also include epithelial malignancies.
Acknowledgments
We thank Dr. Brad Yoder for his technical assistance in the identification of PC by immunofluorescence and Dr. Lusheng Xu for helpful suggestions and discussion. We also thank AnaSpec, San Jose, CA, for its gift of several cytokeratin antibodies.
This article is a JHC article of the month. This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication with the exception of the JHC articles of the month which are immediately released for public access.
This work was funded by the Department of Defense Breast Cancer Research Program (BC083907), the American Cancer Society (RSG-05-207-01-TBE), the Susan G. Komen Foundation (BCTR0707453), and NIH/National Cancer Institute (R03-CA130057).
References
- Alieva IB, Gorgidze LA, Komarova YA, Chernobelskaya OA, Vorobjev IA (1999) Experimental model for studying the primary cilia in tissue culture cells. Membr Cell Biol 12:895–905 [PubMed] [Google Scholar]
- Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, et al. (2008) Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 14:5995–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW (2008) Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis 25:629–642 [DOI] [PubMed] [Google Scholar]
- Braun N, Papadopoulos T, Muller-Hermelink HK (1988) Cell cycle dependent distribution of the proliferation-associated Ki-67 antigen in human embryonic lung cells. Virchows Arch B Cell Pathol Incl Mol Pathol 56:25–33 [DOI] [PubMed] [Google Scholar]
- Chambers AF (2009) MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res 69:5292–5293 [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adelaide J, Cervera N, Fekairi S, et al. (2006) Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene 25:2273–2284 [DOI] [PubMed] [Google Scholar]
- Christensen ST, Pedersen LB, Schneider L, Satir P (2007) Sensory cilia and integration of signal transduction in human health and disease. Traffic 8:97–109 [DOI] [PubMed] [Google Scholar]
- Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L (2008) The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol 85:261–301 [DOI] [PubMed] [Google Scholar]
- Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR (1996) MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol 148:313–319 [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV (2007) Cilia and developmental signaling. Annu Rev Cell Dev Biol 23:345–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Marshburn D, Jen D, Weinberg RJ, Taylor RM II, Burette A (2007) Stepping into the third dimension. J Neurosci 27:12757–12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715 [PubMed] [Google Scholar]
- Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, et al. (2007) Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci USA 104:19138–19143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett AJ, Smith HS, Springer EL, Owens RB, Nelson-Rees WA, Riggs JL, Gardner MB (1977) Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J Natl Cancer Inst 58:1795–1806 [DOI] [PubMed] [Google Scholar]
- Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A (2009) Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med 15:1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Serra R (2008) Cilia involvement in patterning and maintenance of the skeleton. Curr Top Dev Biol 85:303–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan K, Jones DL, Mansel RE (1987) Mitogenicity of human fibroblasts in vivo for human breast cancer cells. Br J Surg 74:227–229 [DOI] [PubMed] [Google Scholar]
- Hurst DR, Edmonds MD, Scott GK, Benz CC, Vaidya KS, Welch DR (2009) Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res 69:1279–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, et al. (2003) Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun 309:232–240 [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401 [DOI] [PubMed] [Google Scholar]
- Lacroix M, Leclercq G (2004) Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat 83:249–289 [DOI] [PubMed] [Google Scholar]
- Leonessa F, Green D, Licht T, Wright A, Wingate-Legette K, Lippman J, Gottesman MM, et al. (1996) MDA435/LCC6 and MDA435/LCC6MDR1: ascites models of human breast cancer. Br J Cancer 73:154–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle WL, Salisbury JL (1999) Altered centrosome structure is associated with abnormal mitoses in human breast tumors. Am J Pathol 155:1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P, Boisseau MR (1991) Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry 12:42–49 [DOI] [PubMed] [Google Scholar]
- Lu X, Liu J, Legerski RJ (2009) Cyclin E is stabilized in response to replication fork barriers leading to prolonged S phase arrest. J Biol Chem 284:35325–35337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR (2007) Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci USA 104:13325–13330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Nonaka S (2006) Cilia: tuning in to the cell's antenna. Curr Biol 16:R604–614 [DOI] [PubMed] [Google Scholar]
- Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, et al. (2008) Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol 295:G725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan SR, Jensen CG, Poole CA (2006) Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem 54:1005–1014 [DOI] [PubMed] [Google Scholar]
- Molla-Herman A, Boularan C, Ghossoub R, Scott MG, Burtey A, Zarka M, Saunier S, et al. (2008) Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS One 3:e3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JJ, Fritzler MJ, Rattner JB (2009) Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer 9:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muerkoster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, Sebens T, et al. (2004) Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res 64:1331–1337 [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, et al. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33:129–137 [DOI] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, et al. (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10:515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel A, De Pauw-Gillet MC, Purnell G, Nusgens B, Lapiere CM, Foidart JM (1993) Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. Br J Cancer 68:909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, et al. (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348 [DOI] [PubMed] [Google Scholar]
- Ostman A, Augsten M (2009) Cancer-associated fibroblasts and tumor growth—bystanders turning into key players. Curr Opin Genet Dev 19:67–73 [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Veland IR, Schroder JM, Christensen ST (2008) Assembly of primary cilia. Dev Dyn 237:1993–2006 [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, et al. (2000) Molecular portraits of human breast tumours. Nature 406:747–752 [DOI] [PubMed] [Google Scholar]
- Piperno G, LeDizet M, Chang XJ (1987) Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol 104:289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Golemis EA, Pugacheva EN (2008) Cell cycle-dependent ciliogenesis and cancer. Cancer Res 68:2058–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RJ, Tobin JL, Beales PL (2008) Modeling ciliopathies: primary cilia in development and disease. Curr Top Dev Biol 84:249–310 [DOI] [PubMed] [Google Scholar]
- Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD (2007) MDA-MB-435 cells are derived from M14 melanoma cells—a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat 104:13–19 [DOI] [PubMed] [Google Scholar]
- Rakha EA, Ellis IO (2009) Triple-negative/basal-like breast cancer: review. Pathology 41:40–47 [DOI] [PubMed] [Google Scholar]
- Roepman R, Wolfrum U (2007) Protein networks and complexes in photoreceptor cilia. Subcell Biochem 43:209–235 [DOI] [PubMed] [Google Scholar]
- Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, et al. (2000) Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 24:227–235 [DOI] [PubMed] [Google Scholar]
- Sadlonova A, Novak Z, Johnson MR, Bowe DB, Gault SR, Page GP, Thottassery JV, et al. (2005) Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture. Breast Cancer Res 7:R46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST (2005) PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 15:1861–1866 [DOI] [PubMed] [Google Scholar]
- Schraml P, Frew IJ, Thoma CR, Boysen G, Struckmann K, Krek W, Moch H (2009) Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Mod Pathol 22:31–36 [DOI] [PubMed] [Google Scholar]
- Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M (2009) Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res 69:422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellappan S, Grijalva R, Zhou X, Yang W, Eli MB, Mills GB, Yu D (2004) Lineage infidelity of MDA-MB-435 cells: expression of melanocyte proteins in a breast cancer cell line. Cancer Res 64:3479–3485 [DOI] [PubMed] [Google Scholar]
- Shao ZM, Nguyen M, Barsky SH (2000) Human breast carcinoma desmoplasia is PDGF initiated. Oncogene 19:4337–4345 [DOI] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK (2008) Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol 85:371–427 [DOI] [PubMed] [Google Scholar]
- Shekhar MP, Santner S, Carolin KA, Tait L (2007) Direct involvement of breast tumor fibroblasts in the modulation of tamoxifen sensitivity. Am J Pathol 170:1546–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, et al. (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314:268–274 [DOI] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD Jr, Brenz R, McGrath CM, Russo J, et al. (1990) Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res 50:6075–6086 [PubMed] [Google Scholar]
- Stampfer M, Hallowes RC, Hackett AJ (1980) Growth of normal human mammary cells in culture. In Vitro 16:415–425 [DOI] [PubMed] [Google Scholar]
- Stampfer MR, Bartley JC (1985) Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci USA 82:2394–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling JW, Chandler JA (1976) Ultrastructural studies of the female breast. I. 9 + 0 Cilia in myoepithelial cells. Anat Rec 186:413–416 [DOI] [PubMed] [Google Scholar]
- Strickland LB, Dawson PJ, Santner SJ, Miller FR (2000) Progression of premalignant MCF10AT generates heterogeneous malignant variants with characteristic histologic types and immunohistochemical markers. Breast Cancer Res Treat 64:235–240 [DOI] [PubMed] [Google Scholar]
- Talley L, Chhieng DC, Bell WC, Grizzle WE, Frost AR (2008) Immunohistochemical detection of EGFR, p185(erbB-2), Bcl-2 and p53 in breast carcinomas in pre-menopausal and post-menopausal women. Biotech Histochem 83:5–14 [DOI] [PubMed] [Google Scholar]
- Talley LI, Grizzle WE, Waterbor JW, Brown D, Weiss H, Frost AR (2002) Hormone receptors and proliferation in breast carcinomas of equivalent histologic grades in pre- and postmenopausal women. Int J Cancer 98:118–127 [DOI] [PubMed] [Google Scholar]
- Tlsty TD (2001) Stromal cells can contribute oncogenic signals. Semin Cancer Biol 11:97–104 [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Treier M (2008) Transcriptional regulators in kidney disease: gatekeepers of renal homeostasis. Trends Genet 24:361–371 [DOI] [PubMed] [Google Scholar]
- Wheatley DN, Wang AM, Strugnell GE (1996) Expression of primary cilia in mammalian cells. Cell Biol Int 20:73–81 [DOI] [PubMed] [Google Scholar]
- Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH Jr, Dlugosz AA, et al. (2009) Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 15:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, et al. (2007) The genomic landscapes of human breast and colorectal cancers. Science 318:1108–1113 [DOI] [PubMed] [Google Scholar]
- Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE Jr, Schafer JA, Balkovetz DF (2002) Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282:F541–552 [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R (2007) Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67:10123–10128 [DOI] [PubMed] [Google Scholar]