Abstract
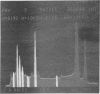
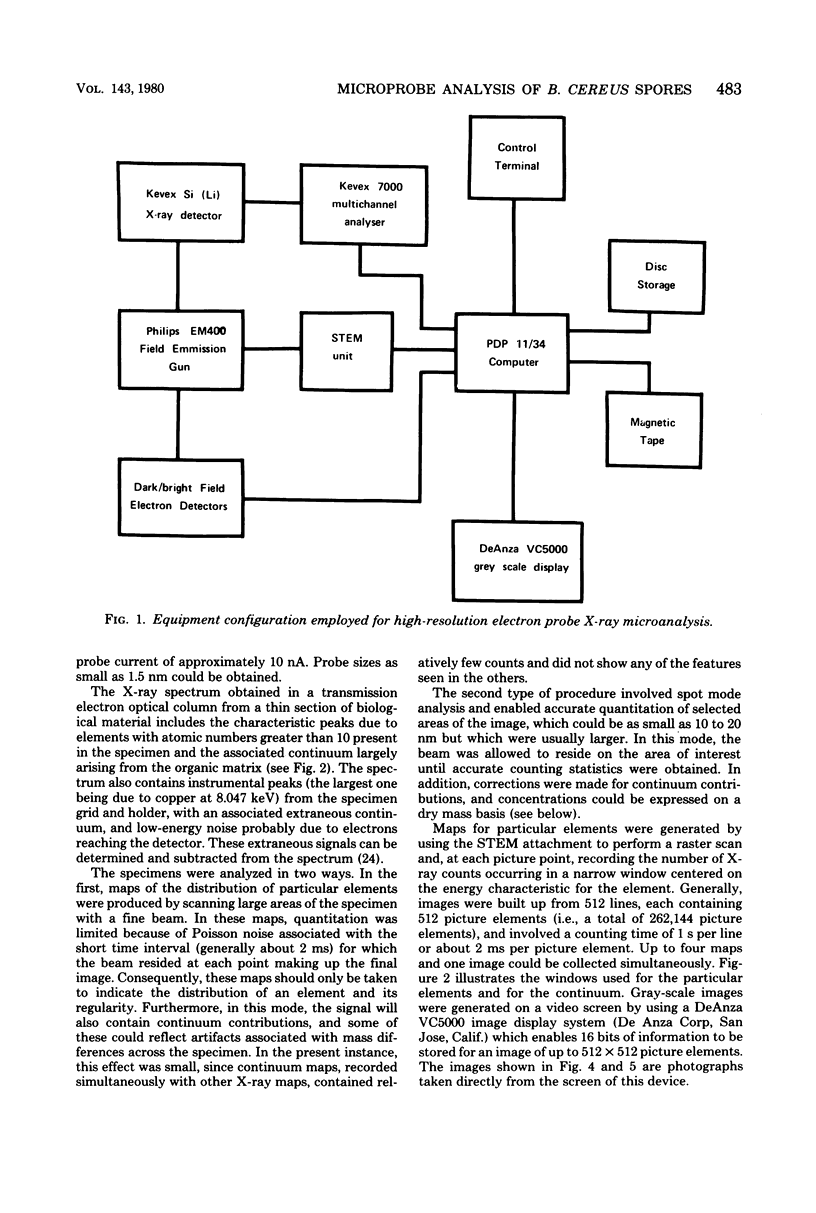
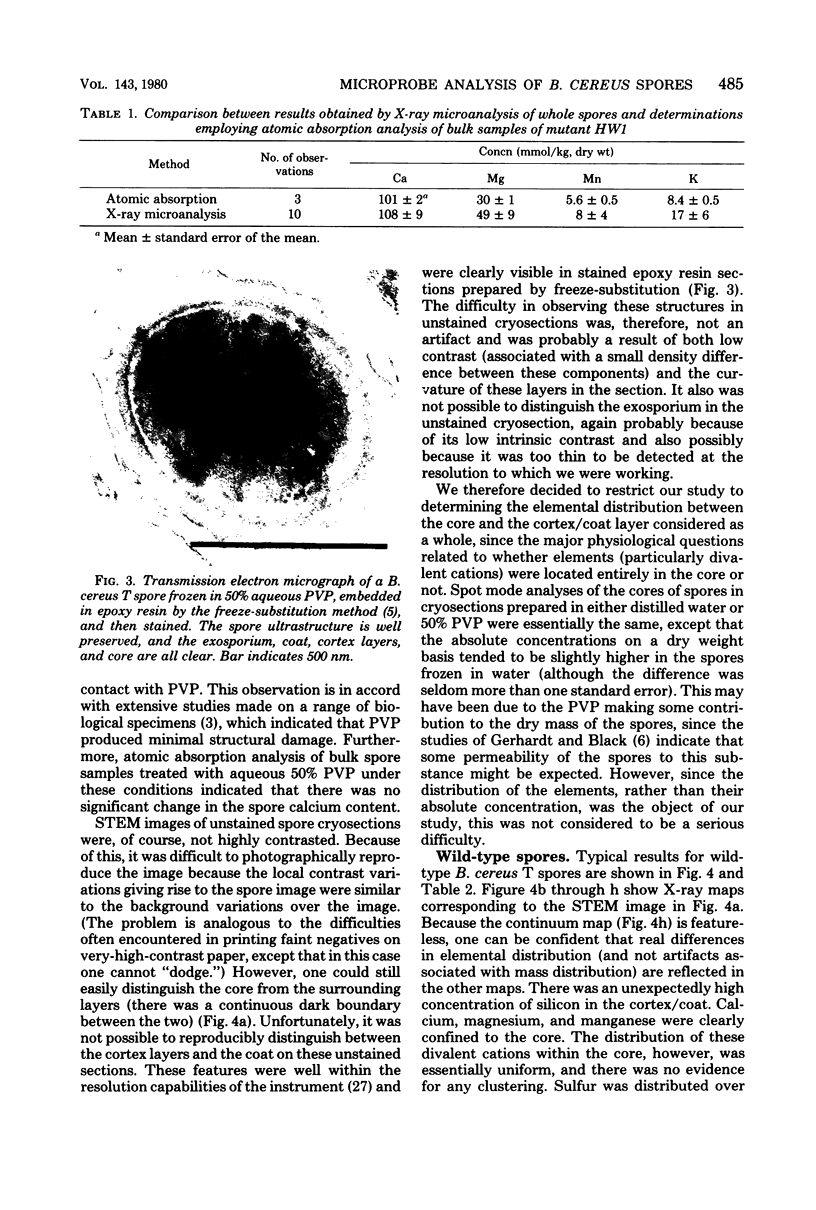
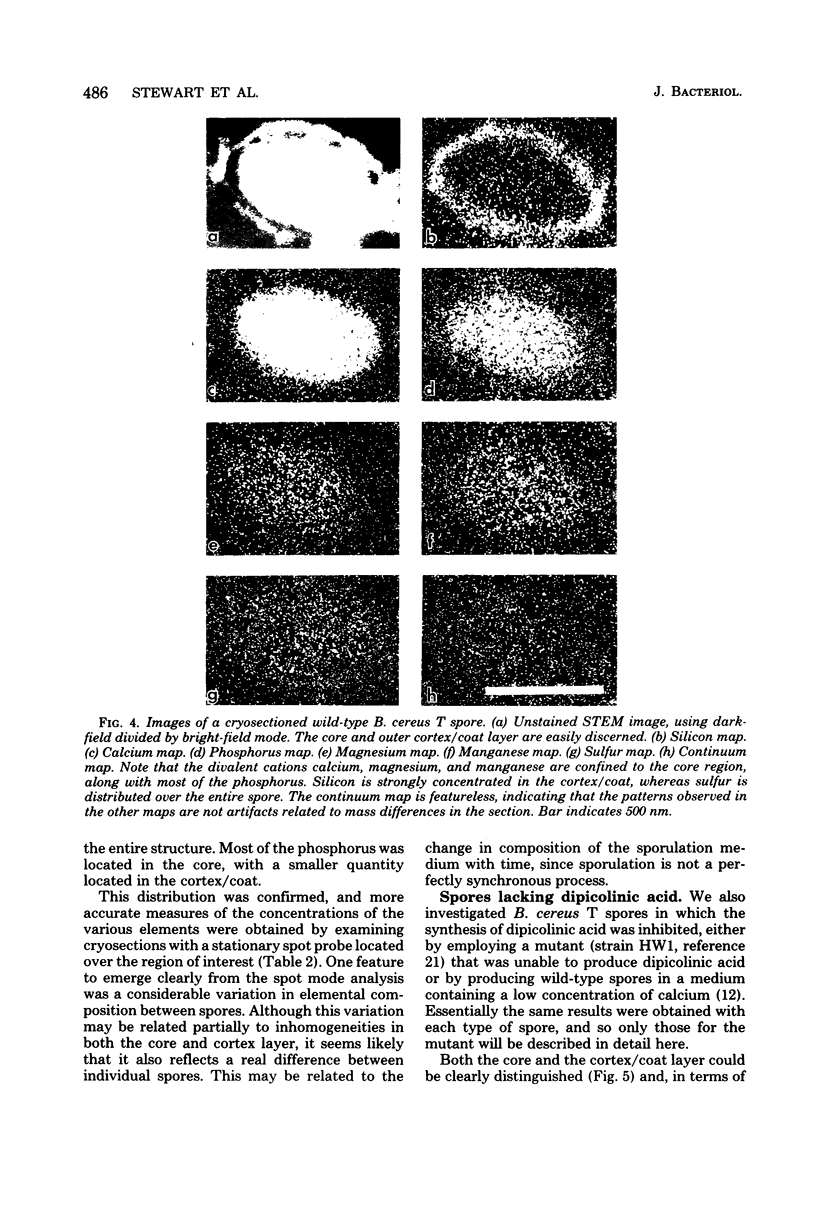
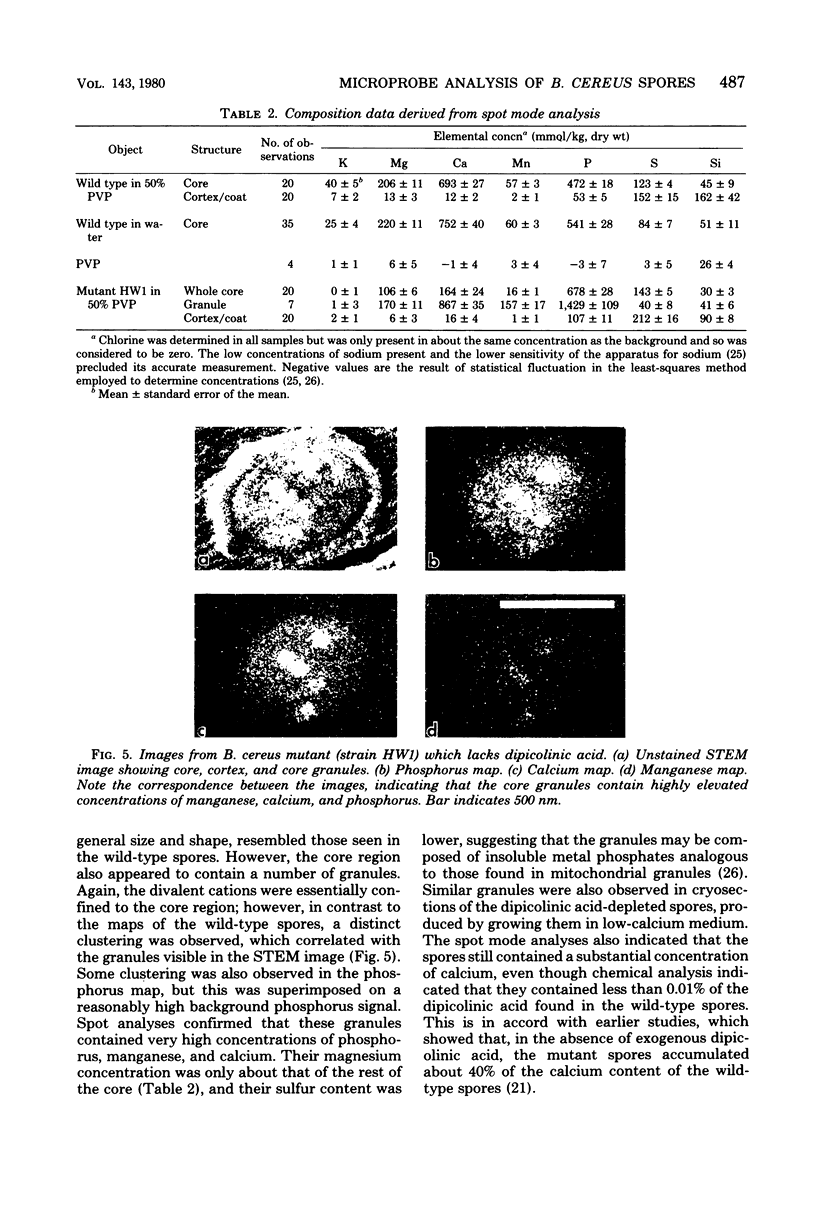
The distribution of a number of key elements in Bacillus cereus T spores was determined by high-resolution scanning electron probe X-ray microanalysis. To circumvent the redistribution of soluble or weakly bound elements, freeze-dried cryosections of spores, which had been rapidly frozen in 50% aqueous polyvinyl pyrrolidone, were employed. The sections were examined by using a modified Philips EM400 electron microscope fitted with a field emission gun, scanning transmission electron microscopy attachment, and a computer-linked energy-dispersive X-ray microanalysis system. X-ray maps for selected elements and the corresponding electron image were produced simultaneously by scanning the cryosections with a fine electron beam in a raster pattern, using the scanning transmission electron microscopy attachment. The results indicated that almost all of the calcium, magnesium, and manganese, together with most of the phosphorus, was located in the core region. An unexpectedly high concentration of silicon was found in the cortex/coat layer. Granules containing high concentrations of calcium, manganese, and phosphorus were demonstrated in spores containing reduced levels of dipicolinic acid. Spot mode analyses, in which a stationary beam was located over the region of interest in the spore cryosection, confirmed the results obtained with the scanning mode and also provided a more accurate quantitation of the elemental concentrations on a dry weight bases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando Y. [Properties of ionic forms of spores of a Clostridium perfringens]. Nihon Saikingaku Zasshi. 1976;31(6):713–717. [PubMed] [Google Scholar]
- Echlin P., Skaer H. B., Gardiner B. O., Franks F., Asquith M. H. Polymeric cryoprotectants in the preservation of biological ultrastructure. II. Physiological effects. J Microsc. 1977 Aug;110(3):239–255. doi: 10.1111/j.1365-2818.1977.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Franks F., Asquith M. H., Hammond C. C., Skaer H. B., Echlin P. Polymer cryoprotectants in the preservation of biological ultrastructure. I. Low temperature states of aqueous solutions of hydrophilic polymers. J Microsc. 1977 Aug;110(3):223–228. doi: 10.1111/j.1365-2818.1977.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Heuser J. E., Reese T. S., Somlyo A. P., Somlyo A. V. T-tubule swelling in hypertonic solutions: a freeze substitution study. J Physiol. 1978 Oct;283:133–140. doi: 10.1113/jphysiol.1978.sp012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Murrell W. G. Use of ultraviolet radiation to locate dipicolinic acid in Bacillus cereus spores. J Bacteriol. 1974 Apr;118(1):202–208. doi: 10.1128/jb.118.1.202-208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., Dring G. J. Heat resistance of bacterial endospores and concept of an expanded osmoregulatory cortex. Nature. 1975 Dec 4;258(5534):402–405. doi: 10.1038/258402a0. [DOI] [PubMed] [Google Scholar]
- Grecz N., Smith R. F., Hoffmann C. C. Sorption of water by spores, heat-killed spores, and vegetative cells. Can J Microbiol. 1970 Jul;16(7):573–579. doi: 10.1139/m70-096. [DOI] [PubMed] [Google Scholar]
- KEYNAN A., MURRELL W. G., HALVORSON H. O. Germination properties of spores with low dipicolinic acid content. J Bacteriol. 1962 Feb;83:395–399. doi: 10.1128/jb.83.2.395-399.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNAYSI G. FURTHER OBSERVATIONS ON THE SPODOGRAM OF BACILLUS CEREUS ENDOSPORE. J Bacteriol. 1965 Aug;90:453–455. doi: 10.1128/jb.90.2.453-455.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C., Snell N. S., Burr H. K. Water Permeability of Bacterial Spores and the Concept of a Contractile Cortex. Science. 1960 Aug 26;132(3426):544–545. doi: 10.1126/science.132.3426.544. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Murrell W. J. Symposium on bacterial spores: IX. Biophysical analysis of the spore. J Appl Bacteriol. 1970 Mar;33(1):103–129. doi: 10.1111/j.1365-2672.1970.tb05237.x. [DOI] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Location of calcium within Bacillus spores by electron probe x-ray microanalysis. J Bacteriol. 1972 Oct;112(1):559–568. doi: 10.1128/jb.112.1.559-568.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H. Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol. 1979 May;81(2):316–335. doi: 10.1083/jcb.81.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Stewart M. Electron probe analysis of muscle and X-ray mapping of biological specimens with a field emission gun. Scan Electron Microsc. 1979;(2):711–722. [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Elemental distribution in striated muscle and the effects of hypertonicity. Electron probe analysis of cryo sections. J Cell Biol. 1977 Sep;74(3):828–857. doi: 10.1083/jcb.74.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS R. S. ULTRASTRUCTURAL LOCALIZATION OF MINERAL MATTER IN BACTERIAL SPORES BY MICRONINCINERATION. J Cell Biol. 1964 Oct;23:113–133. doi: 10.1083/jcb.23.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D. Liquid chromatographic determination of dipicolinic Acid from bacterial spores. Appl Environ Microbiol. 1979 Dec;38(6):1029–1033. doi: 10.1128/aem.38.6.1029-1033.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D. Molecular structure of the bacterial spore. Adv Microb Physiol. 1978;17:1–45. doi: 10.1016/s0065-2911(08)60056-9. [DOI] [PubMed] [Google Scholar]
- Woodruff W. H., Spiro T. G., Gilvarg C. Raman spectroscopy in vivo: evidence on the structure of dipicolinate in intact spores of Bacillus megaterium. Biochem Biophys Res Commun. 1974 May 7;58(1):197–203. doi: 10.1016/0006-291x(74)90911-5. [DOI] [PubMed] [Google Scholar]
- YOUNG I. E., JAMES P. C. Chemical and morphological studies of bacterial spore formation. IV. The development of spore refractility. J Cell Biol. 1962 Jan;12:115–133. doi: 10.1083/jcb.12.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]