Abstract
Expression of secreted protein acidic and rich in cysteine (SPARC)/osteonectin, a collagen-binding matricellular protein, is frequently associated with tissues with high rates of collagen turnover, such as bone. In the oral cavity, expression of SPARC/osteonectin has been localized to the periodontal ligament (PDL), a collagen-rich tissue with high rates of collagen turnover. The PDL is critical for tooth position within the alveolar bone and for absorbing forces generated by chewing. To characterize the function of SPARC/osteonectin in PDL, SPARC/osteonectin expression in murine PDL was evaluated by immunochemistry at 1, 4, 6, and >18 months. Highest levels of SPARC/osteonectin were detected at 1 and >18 months, with decreased levels associated with adult (4–6 months) PDL. To determine whether the absence of SPARC/osteonectin expression influenced cellular and fibrillar collagen content in PDL, PDL of SPARC-null mice was evaluated using histological stains and compared with that of wild-type (WT). Our results demonstrated decreased numbers of nuclei in PDL of SPARC-null mice at 1 month. In addition, decreased collagen volume fractions were found at 1 and >18 months and decreases in thick collagen fiber volume fraction were detected at 4, 6, and >18 months in SPARC-null PDL. The greatest differences in cell number and in collagen content between SPARC-null and WT PDL coincided with ages at which levels of SPARC/osteonectin expression were highest in WT PDL, at 1 and >18 months. These results support the hypothesis that SPARC/osteonectin is critical in the control of tissue collagen content and indicate that SPARC/osteonectin is necessary for PDL homeostasis. (J Histochem Cytochem 58:871–879, 2010)
Keywords: SPARC, periodontal ligament, collagen, BM-40, osteonectin, extracellular matrix, matricellular, periodontium, aging
Periodontal disease accounts for a significant percent of tooth loss in adults >30 years of age, and 34% of Americans have some degree of periodontal disease (Eke and Genco 2007). Periodontal disease is characterized by chronic inflammation that occurs in response to the presence of a biofilm within the plaque or calculus surrounding the tooth at the gingival sulcus (Schaudinn et al. 2009). Symptoms indicative of periodontal disease include bleeding on probing, increased pocket depth, inflamed and reddened gingiva, and bone loss as determined by radiographs (Savage et al. 2009). Alveolar bone loss can progress to the extent that the roots of the teeth are exposed, leading to increased sensitivity and eventual tooth loss. Current treatments for patients recovering from severe periodontal disease lack efficacy in promoting regeneration of the three essential structures linking tooth to bone: alveolar bone, cementum, and periodontal ligament (PDL).
The principle components of the PDL are blood vessels, fibroblasts, and collagen fibers. The collagen fibers in the PDL are composed primarily of collagen type I. The PDL fibers connect the collagen fibers in the cementum layer of the outer tooth to those in the alveolar bone. Previous studies have found that mesenchymal stem cells associated with the vasculature within the PDL have the potential to differentiate into cell types that populate bone and cementum (McCulloch and Bordin 1991). Hence, resident cells of the PDL are postulated to provide a cellular source for regeneration of the primary tissues injured in periodontal disease. As collagen type I is the principal extracellular matrix (ECM) protein in cementum, bone, and PDL, an appreciation of cellular mechanisms that control collagen assembly and deposition in these tissues is critical to improve treatments that enhance regeneration.
Collagen undergoes postsynthetic processing in the extracellular space that is essential for the formation of collagen fibrils and fibers. Collagen-binding proteins are critical in collagen fibril synthesis, collagen fiber assembly, and collagen deposition into the ECM. One collagen-binding protein with a profound effect on ECM structure and content is secreted protein acidic and rich in cysteine (SPARC)/osteonectin/BM-40.
The gene encoding SPARC/osteonectin is predicted to generate a 33-kDa protein that undergoes further posttranslation glycosylation so that secreted SPARC/osteonectin protein is ∼43 kDa in most tissues. SPARC/osteonectin, a matricellular protein, binds to collagen through the extracellular Ca2+ (EC) domain in the C-terminal portion of the protein (Maurer et al. 1995). In addition to collagen binding, several additional activities have been attributed to SPARC/osteonectin. For example, the EC domain was shown to mediate antiproliferative and counteradhesive activities in cells in culture (Hohenester et al. 1997; Brekken and Sage 2000; Delostrinos et al. 2006; Pavasant and Yongchaitrakul 2008). SPARC/osteonectin is predicted to modulate interactions between the cell and ECM and to influence the efficacy of certain growth factors (Giudici et al. 2008). Expression of SPARC/osteonectin is found to be highest during development in most tissues, and re-expression of SPARC/osteonectin is frequently associated with ECM remodeling events such as wound healing (Lane and Sage 1994; McCurdy et al. 2009). Recently, phenotypic evaluation of the SPARC-null mouse has revealed a function of SPARC/osteonectin in the deposition and accumulation of fibrillar collagen in tissues such as dermis, heart, fat, and long bones (Bradshaw et al. 2003a; Alford and Hankenson 2006; Rentz et al. 2007; McCurdy et al. 2009). Results from studies of primary fibroblasts have implicated SPARC/osteonectin in pericellular processing of procollagen, and a function in collagen turnover at the cell surface has been proposed (Rentz et al. 2007).
Collagen in the PDL has one of the highest turnover rates in the body (Beertsen et al. 1997). Hence, proteins that influence collagen deposition and turnover, such as SPARC, are predicted to play a critical role in the maintenance of this essential ligament. Evidence that SPARC/osteonectin contributes to human PDL disease is supported by significant increases in SPARC/osteonectin expression detected in the gingival crevicular fluid of patients with periodontal disease (Kinney et al. 2007). In addition, increased levels of SPARC/osteonectin have been identified as a marker correlated with lower levels of bone loss in patients with periodontal disease (Ng et al. 2007). To study the role of SPARC/osteonectin in fibrillar collagen deposition and maintenance of the PDL, we have characterized the PDL of SPARC-null mice and compared it to that of wild-type (WT) mice.
Materials and Methods
Animal Use and Care
Homozygous C57BL/6 SPARC-null mice used in this study have global abrogation of SPARC/osteonectin expression as described previously (Norose et al. 1998). WT C57BL/6 mice were used as control. Mice were housed in the Ralph H. Johnson Veterans Administration (VA) animal facility and were supplied with a standard diet. All animal procedures used in this study were approved by the VA Institutional Animal Care and Use Committee.
Histology
Mandibles and maxillas were removed from 1-, 4-, 6-, and >18-month age-matched SPARC-null and WT mice. At least four mice per genotype and age point were analyzed. Specimens were fixed in either 10% formalin (histology) or methyl Carnoy's fixative (SPARC/osteonectin immunochemistry). Following fixation, mandibles and maxillas were tumbled at 4C in 0.5 M EDTA for 2 weeks for decalcification. They were then dehydrated, embedded in paraffin, and sectioned (5–7 μm). Sections were mounted on glass slides and rehydrated through xylene followed by graded ethanol rinses. Slides were stained in hematoxylin for 15 min and rinsed for 15 min under running water followed by a 30-sec incubation in 95% ethanol. The slides were then stained for 1 min in eosin, dehydrated, and cover-slipped (H&E; Sigma, St. Louis, MO). Specimens were analyzed using an Olympus Bx50WI scope (Center Valley, PA) equipped with Infinity 2 Capture software (Electron Microscope Services; Hatfield, PA). Slides were viewed using 4×, 20×, and 60× objectives, and representative fields were captured at various magnifications; scale bars are present in each image.
SPARC/Osteonectin Immunofluorescence
Samples fixed in methyl Carnoy's fixative were used for SPARC/osteonectin immunofluorescence. Sections were deparaffinized and rehydrated in xylene followed by incubation in a series of graded ethanol washes. Once sections were rehydrated, the slides were rinsed with PBS containing 2% Tween-20 (PBST) for 10 min and blocked in 3% normal donkey serum in PBST. Slides were then incubated with SPARC/osteonectin primary antibody (R&D Systems; Minneapolis, MN) for 1 hr, followed by incubation with fluorescein-conjugated anti-goat secondary antibody. ProLong antifade mounting medium containing DAPI (Molecular Probes; Eugene, OR) was used to mount cover slips. Sections were viewed on a Leica microscope (Wetzlar, Germany) equipped for epifluorescence.
Histological Analysis of Collagen Morphology
Sections fixed in 10% formalin were used for picrosirius red (PSR) staining. The slides were stained in iron hematoxylin for 10 min followed by a 10-min rinse in running water. Next, the slides were placed in PSR (Sigma; St Louis, MO) for 1 hr followed by three 5-min rinses in 1% acetic acid. The slides were then dehydrated and mounted with cover slips. PSR stain is specific for collagen, and when viewed under polarized light, the collagen fibers are illuminated against a black background because of the intrinsic birefringence of the collagen fibers. Thicker collagen fibers appear bright red or red/orange, whereas thinner fibers appear green/yellow in color (Junqueira et al. 1979).
Cell Quantification
Images of H&E stains were analyzed using the Select and Count tools in Adobe Photoshop CS3 extended, version 10.0.1. An area of the PDL was selected that spanned the tooth to the bone (avoiding regions containing epithelial rests of Malassez or blood vessels) and the number of nuclei in each field was determined. Nuclei on the edge of the selection were counted when at least 50% of the nucleus was within the selection. Calculations were then normalized to a set unit of area to standardize each area measured. To measure the nuclei within each half of the PDL (either tooth or bone), the same method was used; however, the width of the PDL was measured first and a midline was established to generate a tooth side and a bone side. Quantification of nuclei in each half was standardized to a half unit of area to represent half the PDL. At least four fields per mouse from at least four different animals per age and genotype contributed to the quantification. Mean, standard deviation, and standard error of the mean (SEM) were calculated for each age. Values greater than two standard deviations from the mean were removed as outliers. Age-matched animals were analyzed using Student's t-test; p values <0.05 were considered significant. One-way ANOVA followed by the Bonferroni t-test was used to determine significance between strains at different ages.
Collagen Volume Fraction Analysis
Images taken of PSR stains were analyzed using Visiopharm Integrator System, version 3.2.9.0 (Visiopharm; Hoersholm, Denmark). Parameters were set in this program to measure the total area of the PDL and the area of total collagen, thick collagen fibers, and thin collagen fibers. Results are presented as volume fractions representing the area of collagen divided by the total area of PDL represented in each section. At least five fields per mouse and four animals per age and genotype contributed to the quantification. Mean, standard deviation, and SEM were calculated for each age. Values greater than two standard deviations from the mean were removed as outliers. Age-matched animals were analyzed using Student's t-test; p values <0.05 were considered significant. One-way ANOVA followed by the Bonferroni t-test was used to determine significance between strains at different ages; p values <0.05 were considered significant.
Results
Age-dependent Changes in SPARC/Osteonectin Expression in PDL
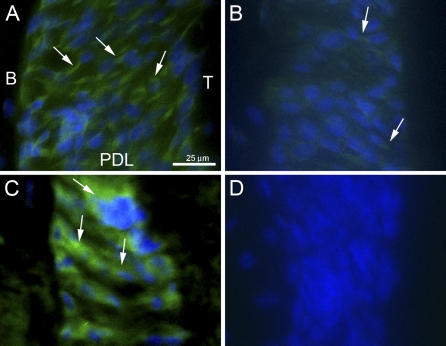
To monitor relative levels of SPARC/osteonectin expression in PDL of increasing age, WT sections were probed for SPARC/osteonectin immunoreactivity at 1, 4, 6, and >18 months of age. Shown in Figure 1 are representative images taken from sections of PDL from mice at 1 month (Figure 1A), 4 months (Figure 1B), and >18 months (Figure 1C) of age. Control SPARC-null sections from mice >18 months of age are shown in Figure 1D. Robust SPARC/osteonectin expression was detected in 1-month sections and appeared to localize to PDL fibroblasts (Figure 1A, arrows). By 4 months, SPARC/osteonectin expression underwent a considerable decrease in intensity (Figure 1B, arrows). SPARC/osteonectin expression was not detected in 6-month PDL (not shown). In sections from aged mice, >18 months, SPARC/osteonectin expression was once again apparent and exhibited robust intensity (Figure 1C, arrows). SPARC/osteonectin staining in younger tissues was restricted to cellular structures, whereas SPARC/osteonectin staining in older sections appeared more diffuse and perhaps localized to extracellular structures (compare Figure 1A with Figure 1C).
Figure 1.
SPARC/osteonectin expression varies with age in murine periodontal ligament (PDL). Secreted protein acidic and rich in cysteine (SPARC)/osteonectin immunoreactivity (green, arrows) was localized in sections of PDL from mice at 1 month (A), 4 months (B), and >18 months (C) of age. (D) SPARC-null control PDL (>18 months). Nuclei are stained blue. Each panel is oriented with bone to the left and tooth to the right. B, bone; T, tooth. Bar in A = 25 μm and applies to all panels.
Morphological Differences in PDL of SPARC-null Mice
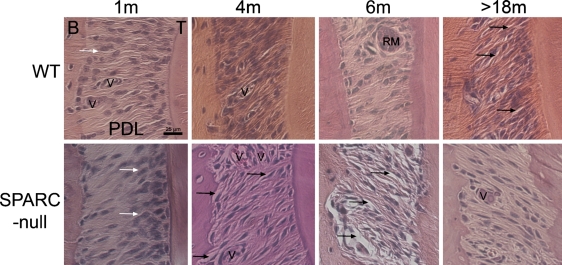
H&E-stained sections were evaluated for overall tissue quality. As shown in Figure 2, WT PDL collagen fibers, the primary component of PDL, spanned the distance from cementum (tooth) to alveolar bone. To a large extent, the fibroblasts within the PDL of younger (1–6 months) WT mice were surrounded by fibers, and the tissue integrity was robust, with infrequent gaps evident in PDL sections. In contrast, SPARC-null mice at 4 and 6 months exhibited collagen fibers that were not tightly surrounded by resident fibroblasts or other ECM components and gave rise to gaps in stained sections consistent with compromised tissue integrity (Figure 2, black arrows). Although tissue processing likely contributed to the gaps in the sections, the increased appearance of interstitial space in the SPARC-null PDL suggested the tissue was of poorer quality before tissue preparation.
Figure 2.
Tissue organization of SPARC-null PDL differs from that of wild-type (WT) PDL. Sections from WT PDL (top row) and SPARC-null PDL (lower row) at indicated ages were stained with H&E. White arrows indicate disproportionate numbers of fibroblasts in SPARC-null PDL at 1 month. Black arrows indicate regions of PDL with sites of tissue disorganization that present as gaps between collagen fibers and surrounding fibroblasts. Images are representative of at least three mice per age point. Each panel is oriented with bone to the left and tooth to the right. V, blood vessel; RM, epithelial rests of Malassez. Bar = 25 μm and applies to all panels.
However, in aged SPARC-null mice (>18 months), collagen fibers were more frequently observed in a linear orientation, tightly surrounding the fibroblasts, and tissue integrity appeared improved over that of 6-month-old SPARC-null animals (Figure 2, black arrows). In contrast, aged WT PDL exhibited an increased incidence of gaps and appeared less robust in comparison to younger mice as would be expected in aged tissue (Figure 2, black arrows).
Alterations in Fibroblast Number in SPARC-null Mice
H&E-stained sections of PDL from WT and SPARC-null mice at increasing ages were also evaluated for differences in cellularity. As shown in Figure 2, SPARC-null PDL at 1 month appeared less populous in cell number in comparison to WT (white arrows). In addition, SPARC-null PDL displayed a tendency toward an increase in nuclei associated with the tooth side portion of the PDL vs that of WT. Substantial differences in cell number were no longer apparent in mice at 4 and 6 months of age, but the trend toward fewer fibroblasts in SPARC-null PDL continued. By 18 months of age, SPARC-null PDL exhibited cell numbers that approximated those of aged WT PDL.
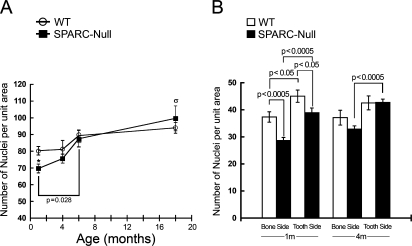
Quantification of cell number in H&E-stained sections is shown in Figure 3A. At 1 month, SPARC-null mice had significantly fewer numbers of total nuclei within the PDL in comparison with WT mice. In general, WT fibroblast numbers did not differ significantly from 1 to >18 months of age. In SPARC-null mice, however, a significant increase in total fibroblast number occurred from 1 to 6 months (Figure 3A). Aged SPARC-null PDL had significantly greater numbers of fibroblasts than younger SPARC-null PDL.
Figure 3.
Fewer nuclei populate SPARC-null PDL at 1 month of age. (A) SPARC-null PDL demonstrated significant increases in numbers of nuclei from 1 to 6 months, whereas significant differences in numbers of nuclei in WT PDL across ages were not detected. (B) Although nuclei in the PDL are more closely associated with the tooth side than the bone side in both WT and SPARC-null mice at 1 month, the differences in distribution remained significant at 4 months in SPARC-null PDL only. Asterisk in A indicates p<0.05 between WT and SPARC-null PDL at 1 month by Student's t-test; σ indicates p<0.05 for SPARC-null PDL >18 months vs SPARC-null PDL at 1 and 4 months by one-way ANOVA (see Materials and Methods). p values represented in B were generated by Student's t-test. Error bars = standard error of the mean (SEM).
To quantify differences in cell distribution within the PDL, a midline was established in each section taken from WT and SPARC-null mice and fibroblasts that populated each half were counted and reported separately (Figures 2 and 3B; see Materials and Methods). At 1 month, both WT and SPARC-null PDL demonstrated a higher number of cells associated with the tooth side than the bone side (Figure 3B). Although SPARC-null PDL contained fewer cells at this age, the percentage of total cells associated with the tooth side did not differ substantially between SPARC-null and WT PDL (56.8% of total SPARC-null cells vs 54% of WT cells were found associated with the tooth side). At 4 months, however, SPARC-null PDL retained a statistically significant difference in cell distribution with more nuclei associated with the tooth half, whereas cell number in tooth vs bone sides was not significant in WT PDL at this age (Figure 3B). In PDL at 6 and >18 months, there were no differences in distribution of nuclei in either SPARC-null or WT PDL.
Age-dependent Alterations in Collagen Fiber Morphology and Collagen Content in WT and SPARC-null PDL
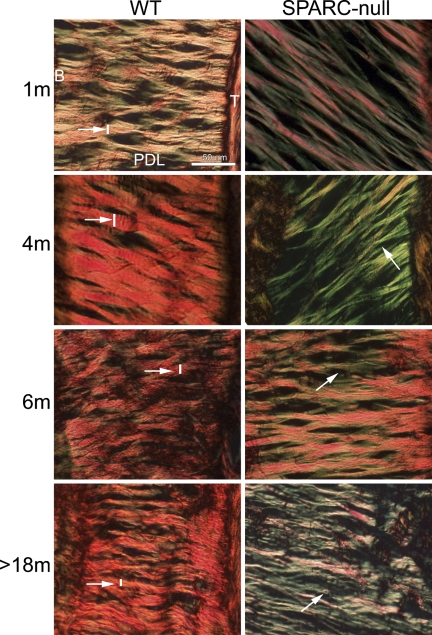
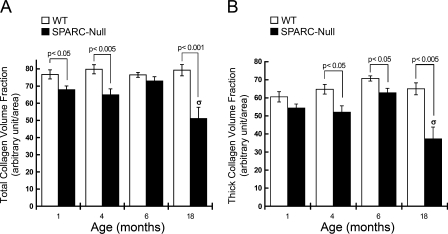
A decrease in collagen content has been found in several tissues in SPARC-null mice (Bradshaw and Sage 2001). PSR-stained sections from PDL were evaluated to determine whether collagen content and fiber morphology were affected in SPARC-null PDL. At 1 month, PSR-stained sections of SPARC-null PDL viewed under polarized light exhibited an overall reduction in the number of collagen fibers (Figure 4, 1 month). In addition, fewer collagen fibers that stained red/orange (indicative of large, crosslinked fibers) and a greater number of fibers that stained yellow/green were observed in SPARC-null vs WT sections at 1 month (Figure 4). Quantification of collagen volume fractions demonstrated a significant decrease in collagen content in SPARC-null PDL at 1 month (Figure 5A).
Figure 4.
Collagen fiber morphology is altered in SPARC-null PDL. Picrosirius red–stained sections photographed under polarized light highlight differences in collagen fiber size and morphology. WT sections (first column) and SPARC-null sections (second column) are shown at indicated ages. The intensity and color of collagen fibers viewed in these conditions reflect collagen fiber size and maturity; thicker fibers stain red/orange, whereas thinner fibers stain yellow/green. Note thicker fibers present in the WT PDL (white arrow and line) vs fibers in SPARC-null PDL (white arrow and line). Each panel is oriented with bone to the left and tooth to the right. Bar = 50 μm and applies to all panels.
Figure 5.
Decreased collagen volume fraction and altered collagen fiber morphology in PDL of SPARC-null mice. (A) Collagen volume fraction in SPARC-null (black bars) and WT (white bars) PDL. (B) Fractional area of thick collagen fibers was calculated as described in Materials and Methods. WT (white bars) and SPARC-null (black bars) PDL measurements from mice aged 1, 4, 6, and >18 months are shown. σ indicates p<0.05 as determined by ANOVA for SPARC-null PDL from mice aged >18 months vs all other points from WT and SPARC-null PDL at all other ages. p values shown were generated by Student's t-test at each age. Error bars = SEM.
At 4 months, SPARC-null PDL continued to exhibit reductions in collagen content (Figures 4 and 5A). A reduction in collagen fibers that stained red/orange was also detected in SPARC-null PDL at 4 months, which indicated a reduction in large collagen fibers (Figure 4, white arrows and lines, and Figure 5B). The differences in color produced through PSR staining of collagen fibers can be used to detect differences in width of collagen fibers (Junqueira et al. 1979). PSR-stained images of SPARC-null PDL more closely resembled those of WT PDL at 6 months than at the other ages studied (Figure 4). However, quantification of thick collagen fiber volume revealed significantly fewer large collagen fibers in SPARC-null PDL at this age as well (Figure 5B).
A reduction in the integrity of the PDL has been reported in studies of aged animals and aged human tissues (Schneir et al. 1976). Here, aged WT mice (>18 months) maintained total collagen levels similar to those quantified at 6 months, but had an overall disorganized appearance (Figures 2 and 4). In contrast, collagen content and collagen fibers of aged SPARC-null PDL were significantly decreased in comparison to those of younger ages, as determined by ANOVA. Similarly, collagen volume fraction and the volume fraction of thick fibers were significantly decreased in aged SPARC-null PDL in comparison to aged WT PDL and in comparison to younger SPARC-null PDL (Figure 5B). The SPARC-null PDL at >18 months appeared more structured and organized than the aged WT PDL, despite overall decreases in collagen content.
Discussion
Collagen homeostasis is pivotal to maintain healthy PDL. Results presented here demonstrate that SPARC/osteonectin is a critical component in the homeostatic regulation of PDL in adult mice and contributes to collagen content and age-associated reductions in tissue integrity in PDL of older animals. High levels of expression of SPARC/osteonectin were found in young PDL, with decreased expression detected in mid-age PDL and a robust expression that returned in aged PDL (Figure 1). The expression pattern suggested that SPARC/osteonectin was most influential in developing and aging PDL. The SPARC-null PDL phenotype was distinguishable throughout development, but the most notable changes in cell number at 1 month and collagen volume fraction at >18 months coincided with the greatest levels of SPARC/osteonectin expression found in WT PDL.
At 1 month, SPARC-null PDL had fewer nuclei and less total collagen volume fraction than WT PDL. The finding that numbers of fibroblast nuclei were decreased in SPARC-null PDL at 1 month was unexpected. Previous studies performed primarily in cultured endothelial cells have indicated an antiproliferative activity associated with SPARC/osteonectin (Brekken and Sage 2000). The absence of SPARC/osteonectin might then be anticipated to increase cell numbers in SPARC-null mice, in contrast to the findings reported here at 1 month. One explanation is perhaps a deficiency in fibroblast migration. SPARC/osteonectin has been shown to elicit counteradhesive activity in cultured cells and has been implicated previously in fibroblast migration (Wu et al. 2006; Pavasant and Yongchaitrakul 2008). Fibroblasts that populate PDL originate from the dental follicle during development; the follicle later differentiates to form tooth. As the root develops, fibroblasts migrate coronally and toward the bone (Beertsen and Hoeben 1987; McCulloch and Bordin 1991; Weinreb et al. 1997). Possibly, the lack of SPARC influenced cell migration during development. Results reported here showed an increased number of nuclei associated with the tooth side of the PDL at 1 month in both WT and SPARC-null mice, perhaps indicative of cell origin at the tooth surface before migration into the PDL. Notably, at 4 months in SPARC-null PDL, a statistically significant difference in cells associated with the tooth was found, whereas the distribution of nuclei in WT was no longer significant. These data might indicate a lag in cell migration of SPARC-null fibroblasts from the tooth side to populate the PDL.
Coincident with decreased SPARC/osteonectin expression at 4 months and further decreases at 6 months in WT mice, phenotypic differences in cell number were less substantial at these ages in SPARC-null tissue. The increase in fibroblast number from 1 to 6 months in SPARC-null PDL suggested that fibroblasts from SPARC-null PDL underwent greater rates of proliferation than those from WT PDL over this period—a finding that is consistent with previously reported antiproliferative activity associated with SPARC/osteonectin. Increases in cell proliferation perhaps occurred to compensate for decreased collagen content in SPARC-null PDL at early times.
Prior studies have implicated SPARC/osteonectin in collagen fiber assembly and deposition (Bradshaw 2009). The collagen content in the PDL at 1 and >18 months was consistent with a function of SPARC/osteonectin in collagen incorporation into the ECM as decreases in collagen volume fractions were observed. Possibly, diminished collagen content and reductions in fiber thickness in adult SPARC-null PDL were carried over from development abnormalities resulting from a lack of SPARC/osteonectin expression during crucial periods in tissue formation. In addition, at 4, 6, and >18 months, fewer thick fibers were present, suggesting SPARC/osteonectin was involved in the growth of collagen fibers.
High rates of collagen deposition in development frequently slow upon maturation of tissues when the period of rapid growth ends. Although the decrease in total collagen volume fraction in SPARC-null PDL at 6 months was not statistically significant, there was a trend toward less total collagen at this age. In addition, a significant decrease in thick collagen fiber volume fraction was observed (Figures 4 and 5). Collagen fiber growth has been shown to occur through the addition of collagen monomers to existing fibers and then by fusion to form larger fibers (Birk et al. 1995). Conditions that favor the formation of thicker collagen fibers are predicted to reflect lower collagen turnover in the ECM. Similar differences in collagen fiber size were detected in SPARC-null dermis in which smaller collagen fibrils and fibers were shown to populate SPARC-null tissue in comparison to its WT counterpart (Bradshaw et al. 2003b).
WT PDL showed predictable signs of increased disorganization in old mice vs younger mice, whereas total collagen levels were maintained. In contrast, SPARC-null PDL had less total collagen volume than WT PDL, but had more tissue organization characterized by fewer intercellular gaps. In the period from 6 to >18 months, coincident with increased levels of SPARC expression, WT PDL maintained similar levels of collagen volume fraction and collagen fiber thickness. However, in SPARC-null mice over the same period, there was significantly lower collagen volume fraction at >18 months and also significantly less thick collagen volume fraction as compared with younger SPARC-null PDL. These results suggested that differences between collagen morphology and content in WT from 6 to >18 months was due to increased expression of SPARC, and in the absence of SPARC, collagen content was not maintained in aged PDL.
In many aged tissues, collagen undergoes slower rates of turnover than in younger counterparts (Schneir et al. 1976). Additional modifications of collagen in the ECM that are enhanced in aged tissues, such as crosslinks generated from advanced glycation end products, are postulated to increase collagen insolubility and resistance to degradation (Baynes 2001). Earlier reports in rat have shown a reduction in collagen turnover in aged PDL, which contributed to increased collagen content (Toto et al. 1975). Slower turnover of ECM is also thought to contribute to increased severity of periodontal disease in the aged human population. In support of a role of SPARC/osteonectin in older patients, increases in SPARC/osteonectin expression in fibroblasts extracted from aged individuals have been reported (Shiba et al. 2000). The evidence presented here suggested that an increase in SPARC/osteonectin expression in aged tissue contributed to diminished collagen turnover in the PDL and that the absence of SPARC/osteonectin resulted in continued remodeling of collagen fibers in old PDL in a manner similar to that of younger tissues.
The mechanism by which SPARC/osteonectin may contribute to the regulation of collagen turnover in PDL is indicated by studies carried out in vitro using primary murine dermal fibroblasts (Rentz et al. 2007). In these studies, procollagen produced by SPARC-null cells exhibited increased association with cell layers vs WT cells. The removal of procollagen propeptides was enhanced in SPARC-null cells, but was associated with inefficient incorporation of collagen into insoluble ECM. The hypothesis put forth was that SPARC/osteonectin bound to newly secreted procollagen functioned to regulate procollagen processing and diminish collagen interaction with certain collagen cell surface receptors (Rentz et al. 2007). In the absence of SPARC/osteonectin, nascent collagen tethered to receptors favored phagocytosis and/or increased degradation by pericellular collagenase activity. In the event that a similar scenario holds true in PDL, the absence of SPARC/osteonectin may give rise to higher collagen turnover at fibroblast cell surfaces at the expense of efficient incorporation of collagen to insoluble ECM and result in the phenotype reported here: lower collagen content and smaller collagen fibers.
One important aspect of SPARC/osteonectin in the context of the PDL is the potential for SPARC/osteonectin to enhance collagen deposition and regeneration of diseased tissue. Periodontal disease is difficult to treat because of the associated bone loss that occurs when the PDL is degraded. SPARC/osteonectin production in bones is substantial and is also expressed in cementum, the outer layer of the tooth connecting to the PDL (Zeichner-David 2006; Delany and Hankenson 2009). In bone, and now in the PDL, the absence of SPARC/osteonectin was reported to cause decreases in total collagen and compromised organization of these tissues. The production of SPARC/osteonectin in these three collagen-rich structures, cementum, PDL, and alveolar bone, affected by periodontal disease make SPARC/osteonectin a strong candidate for improving the regeneration of periodontal attachments that are lost as a consequence of disease. Future studies are planned to further elucidate the mechanisms of SPARC/osteonectin in maintaining the PDL and to contribute to the design of novel strategies to treat diseased tissues.
Acknowledgments
This work was supported by National Institutes of Health Grants T32 DE-017551 (to JMT), 2P20RR017696 (to ADB), and HL-094517 (to ADB) and by a Veterans Administration Merit Award (to ADB).
We thank Kylie Martin, Yuhua Zhang, Lauren Card, and Robert Zinna for technical support.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Alford AI, Hankenson KD (2006) Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone 38:749–757 [DOI] [PubMed] [Google Scholar]
- Baynes JW (2001) The role of AGEs in aging: causation or correlation. Exp Gerontol 36:1527–1537 [DOI] [PubMed] [Google Scholar]
- Beertsen W, Hoeben KA (1987) Movement of fibroblasts in the periodontal ligament of the mouse incisor is related to eruption. J Dent Res 66:1006–1010 [DOI] [PubMed] [Google Scholar]
- Beertsen W, McCulloch CA, Sodek J (1997) The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000 13:20–40 [DOI] [PubMed] [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI (1995) Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn 202:229–243 [DOI] [PubMed] [Google Scholar]
- Bradshaw AD (2009) The role of SPARC in extracellular matrix assembly. J Cell Commun Signal 3:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Graves DC, Motamed K, Sage EH (2003a) SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci USA 100:6045–6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Sage EH (2003b) SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol 120:949–955 [DOI] [PubMed] [Google Scholar]
- Bradshaw AD, Sage EH (2001) SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekken RA, Sage EH (2000) SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol 19:569–580 [DOI] [PubMed] [Google Scholar]
- Delany AM, Hankenson KD (2009) Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. J Cell Commun Signal 3:227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delostrinos CF, Hudson AE, Feng WC, Kosman J, Bassuk JA (2006) The C-terminal Ca2+-binding domain of SPARC confers anti-spreading activity to human urothelial cells. J Cell Physiol 206:211–220 [DOI] [PubMed] [Google Scholar]
- Eke PI, Genco RJ (2007) CDC Periodontal Disease Surveillance Project: background, objectives, and progress report. J Periodontol 78:1366–1371 [DOI] [PubMed] [Google Scholar]
- Giudici C, Raynal N, Wiedemann H, Cabral WA, Marini JC, Timpl R, Bachinger HP, et al. (2008) Mapping of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. J Biol Chem 283:19551–19560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester E, Maurer P, Timpl R (1997) Crystal structure of a pair of follistatin-like and EF-hand calcium-binding domains in BM-40. EMBO J 16:3778–3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11:447–455 [DOI] [PubMed] [Google Scholar]
- Kinney JS, Ramseier CA, Giannobile WV (2007) Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci 1098:230–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TF, Sage EH (1994) The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J 8:163–173 [PubMed] [Google Scholar]
- Maurer P, Hohenadl C, Hohenester E, Gohring W, Timpl R, Engel J (1995) The C-terminal portion of BM-40 (SPARC/osteonectin) is an autonomously folding and crystallisable domain that binds calcium and collagen IV. J Mol Biol 253:347–357 [DOI] [PubMed] [Google Scholar]
- McCulloch CA, Bordin S (1991) Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res 26:144–154 [DOI] [PubMed] [Google Scholar]
- McCurdy S, Baicu CF, Heymans S, Bradshaw AD (2009) Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC). J Mol Cell Cardiol 48:544–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PY, Donley M, Hausmann E, Hutson AD, Rossomando EF, Scannapieco FA (2007) Candidate salivary biomarkers associated with alveolar bone loss: cross-sectional and in vitro studies. FEMS Immunol Med Microbiol 49:252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC (1998) SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci 39:2674–2680 [PubMed] [Google Scholar]
- Pavasant P, Yongchaitrakul T (2008) Secreted protein acidic, rich in cysteine induces pulp cell migration via alphavbeta3 integrin and extracellular signal-regulated kinase. Oral Dis 14:335–340 [DOI] [PubMed] [Google Scholar]
- Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD (2007) SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem 282:22062–22071 [DOI] [PubMed] [Google Scholar]
- Savage A, Eaton KA, Moles DR, Needleman I (2009) A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol 36:458–467 [DOI] [PubMed] [Google Scholar]
- Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW (2009) Periodontitis: an archetypical biofilm disease. J Am Dent Assoc 140:978–986 [DOI] [PubMed] [Google Scholar]
- Schneir M, Furuto D, Berger K (1976) Collagens of oral soft tissues. I. The influence of age on the synthesis and maturation of collagen in rat palatal mucosa as determined in vitro. J Periodontal Res 11:235–241 [DOI] [PubMed] [Google Scholar]
- Shiba H, Nakanishi K, Sakata M, Fujita T, Uchida Y, Kurihara H (2000) Effects of ageing on proliferative ability, and the expressions of secreted protein, acidic and rich in cysteine (SPARC) and osteoprotegerin (osteoclastogenesis inhibitory factor) in cultures of human periodontal ligament cells. Mech Ageing Dev 117:69–77 [DOI] [PubMed] [Google Scholar]
- Toto PD, Rubinstein AS, Gargiulo AW (1975) Labeling index and cell density of aging rat oral tissues. J Dent Res 54:553–556 [DOI] [PubMed] [Google Scholar]
- Weinreb M, Gal D, Weinreb MM, Pitaru S (1997) Changes in the shape and orientation of periodontal ligament fibroblasts in the continuously erupting rat incisor following removal of the occlusal load. J Dent Res 76:1660–1666 [DOI] [PubMed] [Google Scholar]
- Wu RX, Laser M, Han H, Varadarajulu J, Schuh K, Hallhuber M, Hu K, et al. (2006) Fibroblast migration after myocardial infarction is regulated by transient SPARC expression. J Mol Med 84:241–252 [DOI] [PubMed] [Google Scholar]
- Zeichner-David M (2006) Regeneration of periodontal tissues: cementogenesis revisited. Periodontol 2000 41:196–217 [DOI] [PubMed] [Google Scholar]