Abstract
The pulmonary alveolar epithelium is composed of two morphologically distinct cell types, type I (TI) and type II (TII) cells. Alveolar TII cells synthesize, secrete, and recycle surfactant components; contain ion transporters; and secrete immune effector molecules. In response to alveolar injury, TII cells have the capacity to act as progenitor cells, proliferating and transdifferentiating into TI cells. Although various proteins are associated with TII cells, a plasma membrane marker specific to human TII cells that would be useful for identification in tissue and for isolating this cell type has not been described previously. We devised a strategy to produce a monoclonal antibody (MAb) specific to the apical surface of human TII cells and developed an MAb that appears to be specific for human TII cells. The antibody recognizes a 280- to 300-kDa protein, HTII-280, which has the biochemical characteristics of an integral membrane protein. HTII-280 is detected by week 11 of gestation and is developmentally regulated. HTII-280 is useful for isolating human TII cells with purities and viabilities >95%. HTII-280 is likely to be a useful morphological and biochemical marker of human TII cells that may help to advance our understanding of various lung pathological conditions, including the origin and development of various lung tumors. (J Histochem Cytochem 58:891–901, 2010)
Keywords: HTII-280, type II cells, human pulmonary epithelium, membrane protein, biomarker
The human pulmonary alveolar epithelium, composed of type I (TI) and type II (TII) cells, has an internal surface area of ∼100 m2 (Stone et al. 1992). Alveolar TI cells, which cover 95% of this surface area, have a calculated diameter of ∼100 μm; this cell type has the capacity to participate in ion and water transport (Dobbs et al. 1998; Johnson et al. 2006). Gas exchange occurs across the air–blood barrier, which comprises thin cytoplasmic extensions of TI cells and capillary endothelium. Alveolar TII cells, which comprise the remainder of the alveolar epithelium, have a diameter of ∼10 μm; this cell type synthesizes, secretes, and recycles surfactant components (Wright and Dobbs 1991; Andreeva et al. 2007); contains ion transporters; and secretes immune effector molecules (Wright 2005). After injury to the epithelium, TII cells have the capacity to act as progenitor cells, proliferating and transdifferentiating into TI cells (Evans et al. 1975).
Identification of cell surface markers specific for human alveolar TI cells has proved helpful for identification of this cell type in tissue (Dobbs et al. 1999) and for the evaluation of alveolar epithelial disease progression (Newman et al. 1999,2000). Although various proteins have been associated with TII cells, such as surfactant-associated proteins [of which surfactant protein (SP) C is specific for TII cells (Kalina et al. 1992)], ATP-binding cassette sub-family A member 3 (Inagaki et al. 2004), CD44v6 [expressed on the basolateral membrane of TII cells (Fehrenbach et al. 1999)], pepsinogen C (Foster et al. 2004), lysosome-associated membrane glycoprotein 3 (Salaun et al. 2003), and E-cadherin (Kasper et al. 1995), these are expressed intracellularly or are not TII cell specific. Although a TII cell–specific surface antigen has been described for the rat (Gonzalez and Dobbs 1997; Boylan et al. 2001) and has been useful for evaluation of lung injury (Clegg et al. 2005) and for isolating rodent TII cells (Gonzalez et al. 2005,2009), to our knowledge, an apical plasma membrane marker specific to human TII cells has not been described in detail to date.
We previously described differential binding to apical cell membranes of rat TI and TII cells by the lectins Maclura pomifera (TII cells) and Ricinis communis I (TI cells) (Dobbs et al. 1985). Subsequently, we identified integral membrane proteins in the rat lung specific to TI or TII cells (Dobbs et al. 1988; Gonzalez and Dobbs 1997). By immunizing mice with partially purified alveolar epithelial cells and screening candidate monoclonal antibodies (MAbs) against lung cells and tissue, we were able to identify MAbs specific to proteins expressed on the apical membranes of either rat TI or TII cells (Dobbs et al. 1988; Gonzalez and Dobbs 1997). A similar approach was used to produce an MAb specific to human TI cells; this antibody was used to identify and partially characterize an integral membrane protein unique to human TI cells (Dobbs et al. 1999). This antibody has been utilized to identify human TI cells and assess alveolar cell damage (Newman et al. 1999,2000).
In this study, we have used analogous techniques to develop an MAb that identifies a 280- to 300-kDa protein (HTII-280) specific to the lung and, within the lung, specific to the apical surface membrane of TII cells. Although we currently do not know the function of HTII-280, the antibody is useful for isolation of human TII cells with purities and viabilities >95%. Furthermore, the antibody is likely to be useful in a more precise analysis of lung development and pathological conditions of the lung.
Materials and Methods
Isolation of TII Cells and Screening of Hybridomas
TII cells were partially purified from adult human lung tissue by the following method. We used human lung tissue removed during lobectomy or pneumonectomy. The Human Tissue Use Committee at the University of California, San Francisco (UCSF) approved the protocol. A distal airway was cannulated and lavaged eight times with Ca- and Mg-free PBS containing 0.2 mM EDTA at 37C and then instilled with RPMI–Hepes (Cell Culture Facility, UCSF) containing elastase (60 U/ml; Worthington, Freehold, NJ). The enzyme-instilled lungs were incubated at 37C for a total of 50 min. Additional elastase solution was continuously instilled to keep the lung segment inflated. Lungs were minced to 1-mm3 fragments, shaken in a reciprocating water bath for 5 min, and then filtered sequentially through cotton gauze (one ply) and subsequently through 150-, 70-, and 10-μm nylon mesh (Tetko; Elmsford, NY).
The resultant cell suspension was centrifuged at 400 × g for 10 min, and the cell pellet was resuspended in Freund's adjuvant to immunize BALB/c mice according to the UCSF MAb production protocol. Booster injections were administered at 3-week intervals. Mouse serum was screened by immunofluorescence for antibodies to human TII cells before splenectomy. Spleens were removed on day 123, and splenocytes were isolated and fused to NS-1 myeloma cells by conventional methods (Dobbs et al. 1988). To rapidly screen propagating clones, we established a method of testing multiple hybridoma media supernatants for the presence of antibody reactive solely to TII cells. Using a 48-well manifold, we centrifuged a crude enzymatic cell digest of lung cells onto a glass slide. Slides were precoated with a solution of 200 μg fibronectin/ml PBS (pH 7.4) to increase adherence of the cells and were secured to the 48-well plexiglass manifold. Cells were diluted to a final concentration of 0.4 million cells/ml; ∼0.1 ml was placed in each well, and the manifold was centrifuged in a Thermo IEC DPR 6000 centrifuge (International Equipment; Needham Heights, MA) at 300 rpm at room temperature for 10 min. The cytocentrifuged cells were then placed in freshly made 4% paraformaldehyde in PBS and stored at 4C for up to a month before use. Hybridoma clones were screened by placing 50 μl of clone supernatant on each dot of cytocentrifuged cells and washed, and FITC–anti-mouse IgG (ICN Immunobiologicals; Costa Mesa, CA) was added to each disc of deposited cells to identify reactive clone supernatants. Alternatively, supernatants were screened on human lung tissue sections placed on 12-mm round glass cover slips (Fisher Scientific Health Care; Pittsburgh, PA), which were inserted in 24-well tissue culture plates for easy handling. During the screening process, we identified TII cells using phase contrast microscopy by the presence of multiple lucent cytoplasmic inclusions verifiable as lamellar bodies by modified Papanicolaou staining (Dobbs et al. 1986). Positive supernatants were further tested on 2-μm-thick cryosections of human lung to establish specificity to alveolar epithelial TII cells. Clones were discarded if supernatants exhibited cross-reactivity with TI cells (Dobbs et al. 1999), airway cells, macrophages, neutrophils, or other types of lung cells in addition to TII cells. We performed four fusions and screened ∼1000 clones per fusion, resulting in a single stable hybridoma cell line that produced an antibody reactive only to human TII cells. The hybridoma clone was “stabilized” by recloning three times. We named this antibody anti-HTII-280. We determined that the antibody was an IgM isotype by using Ouchterlony double-diffusion analysis (ICN Biomedicals; Irvine, CA).
Isolation of TII Cells by Flow Cytometry
Alveolar epithelial cells were isolated as described earlier, cell aggregates were removed by successive filtration through 100-, 40-, and 20-μm nylon mesh filters (Tetko), the cell suspension was centrifuged at 400 × g for 10 min, and the cell pellet was resuspended in RPMI 1640 containing 10% FBS. Human IgG (50 μg/ml; Sigma) and mouse IgG (50 μg/ml; Sigma) were added and incubated at 4C for 10 min. Anti-HTII-280 tissue culture supernatant was added (1:20 dilution), incubated on ice for an additional 5 min, and centrifuged at 300 × g for 12 min. Alexa 488 anti-mouse IgM (Invitrogen; Carlsbad, CA) and anti-HTI-56-Alexa 610-RPE (previously directly labeled anti-HTI-56 using Zenon technology; Invitrogen) were added and incubated on ice for 10 min. Cells were centrifuged at 300 × g for 12 min and sorted by fluorescent-activated cell sorting (FACS) as previously described for rat alveolar epithelial cells (Gonzalez et al. 2009).
Immunohistochemistry
Human lung tissue used for IHC was fixed by instillation of freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4), cryoprotected by 30% sucrose at 4C for 24 hr, immersed in Tissue-Tek OCT compound (Miles Laboratories; Elkhart, IN), and frozen in liquid nitrogen. Cryosections were picked up on Superfrost Plus Slides (Fisher Scientific; Fair Lawn, NJ). Single-antibody reactions of anti-HTII-280 (1:10 dilution of hybridoma supernatant with PBS) and goat anti-mouse IgM secondary antibody conjugated to FITC were performed on 4-μm sections as described previously (Dobbs et al. 1999) and the sections were counterstained with 0.05% pontamine sky blue (Sigma; St Louis, MO) containing 10% DMSO in PBS for 2 min. Pontamine sky blue stains elastin fibers and reduces nonspecific background fluorescence (Baker and McDonald 1992). Cover slips were mounted with ProLong (Molecular Probes, Invitrogen). Immunofluorescence and phase images were photographed with Fujichrome Velvia 35-mm film using a Leitz Orthoplan microscope (Leica Microsystems; Bannockburn, IL) and then scanned at high resolution.
Double-label immunohistochemical reactions were done sequentially on 2-μm cryosections. All sections were stained with anti-HTII-280 (1:10 dilution) followed by staining with secondary antibody goat anti-mouse IgM conjugated to Alexa 488 (1:3000 dilution; Invitrogen). These slides were then double-stained with one of these antibodies: anti-HTI-56 (1:10 dilution) followed by secondary goat anti-mouse IgG, or antibodies to SP-A (1:500 dilution), SP-B (1:500 dilution), and SP-C (1:300 dilution), all followed by secondary goat anti-rabbit IgG conjugated to Alexa 594 (1:3000 dilution). Double-antibody reactions were recorded with a Leica DC500 camera (Wetzler, Germany); each channel was captured separately and merged in Photoshop CS4. All fluorescence images were paired with phase images to identify TII cells that contained lucent intracytoplasmic lamellar bodies.
Cryoultramicrotomy
For immunoelectron microscopy, ultrathin cryosections were cut at −100C from tissue blocks fixed in 4% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4), cryoprotected by overnight submersion in a 20% solution of polyvinylpyrrolidone in 2.3 M sucrose (Tokuyasu 1989), and frozen in Freon 22 and liquid nitrogen. The sections were picked up on droplets of sucrose, transferred to grids, and collected on agarose–gelatin plates. Immunohistochemical reactions were conducted as described previously, except that Triton X-100 was omitted (Dobbs et al. 1999). Grids were floated face down on drops of each solution on dental wax tablets. Cryosections on grids were first reacted with primary antibody to HTII-280, rinsed, and then reacted with secondary goat anti-mouse IgM conjugated to either 5- or 10-nm colloidal gold (Electron Microscopy Sciences; Fort Washington, PA). After secondary antibody incubation, the grids were rinsed in distilled water, stained with 2% uranyl acetate in 0.15 M oxalate (pH 7.0), and embedded in a thin layer of 0.2% aqueous uranyl acetate and 2% methylcellulose. Sections were examined and photographed using a Zeiss 10 transmission electron microscope. Film negatives were scanned at high resolution.
Tissue Microarray
Tissue microarray technology (Kononen et al. 1998; Simon and Sauter 2003) was used to examine the ontogeny of HTII-280. Core-needle biopsies (diameter ∼2.0 mm) were removed from conventional paraffin blocks of 11-, 12-, 14-, 15-, 18-, 23-, 25-, 26-, 27-, 32-, 36-week-old human fetal lung tissue and 1-month-old postnatal lung. Death was attributed to non-pulmonary causes. Core samples were then reassembled in a single empty paraffin block at predefined positions. A section of the resulting tissue array was assayed for HTII-280 by indirect immunofluorescence. Preparation of the array allowed simultaneous staining of all samples under identical conditions on a single slide, enhancing comparative analysis of the relative staining intensities.
Western Blotting
For organ specificity blots, we used tissue obtained from adult autopsy material as approved by the Human Tissue Use Committee at UCSF. For ontogeny blots, we obtained lung tissue samples (11–24 weeks gestation) from Advanced Bioscience Resources (Alameda, CA) and the Birth Defects Laboratory at the University of Washington (Seattle, WA); 32-week prenatal lung and postnatal lung was obtained from postmortem tissue under protocols approved by The Children's Hospital of Philadelphia. Organ samples were minced into 1-mm3 fragments and homogenized in 50 mM Tris base (pH 7.8) using a polytron homogenizer (Brinkman; Lucerne, Switzerland). The tissue homogenate was centrifuged at 10,000 × g for 5 min, and the protein content of the supernatant was determined using a bicinchoninic acid (BCA) colorimetric reaction (BCA method; Pierce, Rockford, IL). Samples were equilibrated for protein content and diluted 1:1 with 2× sample buffer [(4% SDS, 2 M urea, 20% glycerol in 5 mM Tris base (pH 8.0)]. Aliquots containing 50 μg protein were electrophoresed on 4–15% gradient gels (or 7% polyacrylamide with 4% stacking gels) containing SDS at 40 mA for 1 hr at 20C. Proteins were transferred onto nitrocellulose paper at 250 mA for 2 hr. HTII-280 was detected with MAb supernatant according to the following protocol. Endogenous peroxidase activity was quenched by treatment with 15% hydrogen peroxide for 10 min, and nonspecific binding was blocked by a 1-hr incubation in a solution of 1% nonfat dry milk, 0.4% gelatin, 0.1% BSA, 0.9% NaCl, and 20 mM Tris base (pH 7.2). Primary antibody to HTII-280 (undiluted hybridoma supernatant) was incubated with the blot for 20 min. The blot was washed 10 times with 20 mM TBS (pH 7.4) containing 0.05% Tween (TBS-T) and incubated with a solution of peroxidase-labeled rabbit anti-mouse IgM secondary antibody (Sigma) in TBS-T (1:2000 dilution). After 20 min of incubation, unbound secondary antibody was removed by 10 washes in TBS-T. Bound secondary antibody was detected by exposure to luminol (ECL Light Detection System; Amersham, GE Healthcare, Piscataway, NJ) followed by autoradiography.
For the Western ontogeny blot, lung extracts (12–24 weeks gestation, 50 μg protein; 32 weeks to 3 months gestation, 5 μg protein) were run on a 10% NuPAGE Bis–Tris gel with MES SDS Running Buffer under reducing conditions (Invitrogen). Proteins were transferred to Duralose membrane (Stratagene; La Jolla, CA) and blocked with 40 mM Tris (pH 7.5) containing 5% nonfat dry milk and 0.1% Tween. For band detection, HTII-280 antibody was added (1:1000 dilution) overnight at 4C, followed by addition of horseradish peroxidase–tagged goat anti-mouse secondary antibody (1:10,000 dilution) for 1 hr, and the band was detected with the SuperSignal West Femto ECL kit (Pierce) and autoradiography.
Liquid-phase Isoelectric Focusing
Human lung tissue (25 g wet weight) was frozen in liquid nitrogen, pulverized, and extracted at 20C for 1 hr with 100 ml of a solution containing 9.5 M urea, 5% octylphenoxy ethanol (NP-40), 2 mM EDTA, 0.5 mM PMSF, 5 mM iodoacetamide, and 2 mg DNase (all reagents from Sigma). The lung homogenate was centrifuged at 100,000 × g for 2 hr. To 45 ml of this supernatant, we added 5 ml glycerol and ampholines (2.8 ml, pH 2.5–4.0, and 1.4 ml, pH 4.0–6.0; Bio-Rad, Richmond, CA). The homogenate was loaded into a preparative isoelectric focusing chamber (Rotofor Cell; Bio-Rad), and 12 W constant power was applied until the voltage stabilized (Fullmer 1984; Deutscher 1990). After voltage stabilization, fractions were collected and assayed for protein content, pH, and immunoreactivity to anti-HTII-280 MAb. Protein was measured by the BCA method. The fractions containing immunoreactive protein were pooled and then refocused.
Results
HTII-280 Is Specific to Human Lung TII Cells
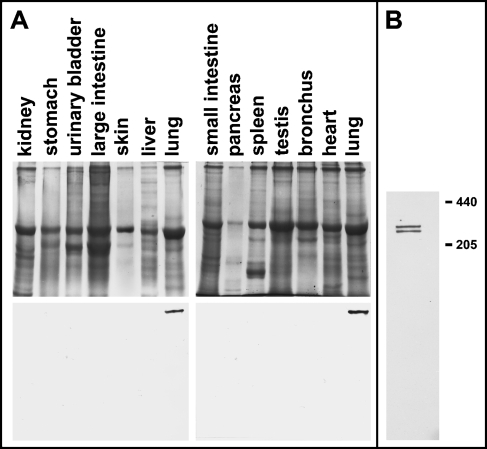
Figure 1 shows an image of a silver-stained SDS-PAGE gel of various adult human organ homogenates and a Western blot of an identical gel probed with anti-HTII-280. Only the lane containing lung homogenate is immunoreactive with our antibody. Using immunofluorescence to examine cryosections of the same organs shown in the Western blot for the presence of HTII-280, we confirmed by IHC that only lung tissue contains detectable antigen (data not shown). Therefore, within the sensitivities of Western blotting and IHC, HTII-280 is specific to the lung, among the tissues tested.
Figure 1.
HTII-280 is specific to the lung by Western blotting. (A) Silver-stained SDS-PAGE (4–15% gradient gels) of various organ homogenates (top) and an accompanying Western blot for HTII-280 (bottom). Ten μg protein from various organs (labeled above the appropriate lanes) was loaded into each lane. Only the lane containing homogenate of lung tissue exhibited immunoreactivity. (B) When lung homogenate is resolved on a 7% gel by SDS-PAGE under reducing conditions, HTII-280 migrates as a dimer with an apparent molecular mass of 280–300 kDa.
Within the lung, immunoreactivity is localized only to alveolar TII cells (Figures 2A, 2B, 2D, and 2F). Airways, lymphatic vessels, and blood vessels are negative for HTII-280 (Figures 2D and 2F). Lung mesothelium was also negative (data not shown). Within the alveoli, TI cells, interstitial cells, vasculature, and macrophages are negative; only TII cells are positive. Figure 2B shows anti-HTII-280 localizing to a group of hyperplastic alveolar TII cells in a section of injured adult human lung. Figures 3A and 3B show anti-HTII-280 localizing to adult alveolar TII cells; adjacent TI cells stained with an antibody against a TI cell–specific protein, HTI-56, are negative. In the matching phase contrast image (Figure 3C), small lucent lamellar bodies further identify the positive cells as TII cells. HTII-280-positive adult human TII cells co-express SP-A, SP-B, and SP-C (Figures 3H–3L). In Figure 3H, TII cell apical surfaces are positive for HTII-280 (green), and the cytoplasm of the same cells express SP-C (red). A similar pattern is present for SP-B (Figure 3J). Figure 3K shows positive staining for SP-A in both a TII cell and a macrophage; resident alveolar macrophages are known to ingest SP-A (Walker et al. 1986).
Figure 2.
IHC of HTII-280 demonstrating localization to type II (TII) cells within the lung (green: indirect IHC for HTII-280; red: elastin fibers stain red because the tissue was counterstained with pontamine sky blue). (A) Green: HTII-280-positive TII cell; red: elastin staining with pontamine. Inset: a phase-contrast image of the TII cell, showing lucent lamellar bodies. (B,C) Paired immunofluorescence and phase-contrast images of injured lung tissue, showing HTII-280-positive hyperplastic TII cells (arrows). Neither blood vessels (BV; D,E) nor airways (F,G) exhibit staining for HTII-280.
Figure 3.
IHC of normal and injured lung tissues. (A–L) Green: HTII-280, specific for TII cells; (A–G) red: HTI-56, specific for type I (TI) cells. (A,B) Green: HTII-280 localizes to alveolar TII cells; red: TI cells exhibit staining for HTI-56. In the matching phase contrast image (C), small lucent lamellar bodies identify TII cells. A macrophage (mac) is negative for HTII-280. (D,E) In 24-week prenatal lung, HTII-280 (green) is localized mostly to the tips of the branching potential airspaces. (F,G) Higher magnification immunofluorescence and phase-contrast views showing an area (arrows) of co-localization of HTI-56 and HTII-280. (H–L) Co-localization of surfactant proteins (SPs) A, B, and C (red) and HTII-280 (green); accompanying phase-contrast views or insets are shown. (H) TII cells (arrows) are positive for both HTII-280 (green) and SP-C (red). (J) TII cells (arrows) positive for both HTII-280 (green) and SP-B (red); the inset shows the accompanying phase-contrast views of the areas indicated by the arrows. (K) TII cell (arrow) positive for SP-A (red) and HTII-280 (green) and a mac containing SP-A. (M–P) In injured lung tissue, areas of co-localization of HTI-56 and HTII-280 can be seen. (M) Merged red and green channels. TII cells are green and TI cells are red; asterisks indicate areas of co-localization (yellow). (N) Red (HTI-56) channel; (O) green (HTII-280) channel; (P) accompanying phase-contrast image.
HTII-280 Is Localized to the Apical Plasma Membrane of TII Cells
Immunofluorescence at the light microscopic level suggests that HTII-280 is predominantly localized to the apical plasma membrane (Figure 2A). At the electron microscope level using immunogold to localize HTII-280, gold particles are found mostly on the apical surface of TII cells. The cytoplasm and the basolateral membrane are negative (Figure 4).
Figure 4.
Immunoelectron microscopy demonstrating predominant localization of HTII-280 to the apical plasma membrane of TII cells. Electron microscopic localization of HTII-280 with 10-nm immunogold (A,B) and 5-nm immunogold (C). The TII cells can be identified by the presence of lamellar bodies (LB, arrows). The apical plasma membrane is immunoreactive; rare immunogold particles can be seen in the cytoplasm. (B,C) Arrows show junctions between TI and TII cells.
We checked our MAb for species crossreactivity; this antibody does not crossreact with lung tissue from chimpanzee, dog, rabbit, guinea pig, rat, or mouse (data not shown).
Ontogeny of HTII-280
We examined the ontogeny of HTII-280 by both Western blotting and indirect immunofluorescence. Figure 5A is a Western blot of various human fetal lung tissue samples at prenatal weeks 12 through 32 and postnatal days 3 and 90. By Western blotting, HTII-280 is first detected at week 17. By immunofluorescence of a separate set of fetal lung samples, there is rare staining for HTII-280 by week 11; its expression became more widespread with increasing gestational age. HTII-280 appeared to increase with gestational age but not linearly, with markedly higher concentrations in late gestation and postnatal samples (less protein loaded). Variation between quantities of HTII-280 detected in early gestation may be due to biological variability of our samples or to the injured state and relatively prolonged time between lung removal and tissue preservation of various specimens. Figures 3D–3G are images of prenatal lung at week 24. During this stage of lung development (cannalicular), HTII-280 is detected mostly at the tips of potential branching airspaces. Groups of HTII-280-positive cells are interspersed with HTI-56-positive cells in the undifferentiated epithelium (Figures 3D and 3F). Some of these undifferentiated epithelial cells show co-localization of both HTII-280 and HTI-56 [a TI cell–specific antigen (Dobbs et al. 1999)] (Figure 3F). We did not detect cells positive for both HTII-280 and HTI-56 in normal adult tissue; however, in areas of injury, some alveolar epithelial cells co-express both antigens (Figure 3M).
Figure 5.
Western blot and indirect immunofluorescence of fetal lung tissue at various gestational ages. In the upper panel, tissue extracts (12–24 weeks gestation, 50 μg protein; 32 weeks to 3 months gestation, 5 μg protein) were run on a 10% NuPAGE Bis–Tris gel. HTII-280 was detectable at week 17, with variable intensity up to week 24. The fetal week 32 and postnatal (PN) 3 day and 3 month time points show increased amount of HTII-280 (the lanes were loaded with one-tenth of the protein of the lanes at earlier gestational ages). The lower panel of images shows indirect immunofluorescence for HTII-280, using goat anti-mouse IgM Alexa 594 (red) as a secondary antibody on a fetal lung tissue array. All images were stained identically and captured at the same magnification.
Isolation of Human TII Cells With High Degrees of Purity and Viability Using Anti-HTII-280 and FACS
Before FACS, the mixed cell preparation contained a mixture of different types of cells and cell aggregates. After FACS, we obtained an Alexa 488-positive population consisting of single TII cells. The isolated TII cells were >98% pure TII cells, and viability was >95% (Figure 6).
Figure 6.
Human TII cells isolated by fluorescent-activated cell sorting using anti-HTII-280 goat anti-mouse IgM Alexa 488. (A) Alexa 488 staining (green) of sorted TII cells. (B) Matching phase contrast image of sorted cells.
HTII-280 Solubility
HTII-280 has the biochemical characteristics of an integral membrane protein. HTIl-280 is poorly soluble in aqueous media or in buffers containing 2 M urea, 2 M NaCl, or 2% SDS, agents that are known to solubilize peripheral membrane proteins (Fullmer 1984; Deutscher 1990). However, HTII-280 is solubilized with 8 M guanidine hydrochloride, 4% NP-40, or 4% octanoyl-N-methylglucamide in 6 M urea, conditions known to extract integral membrane proteins (data not shown).
Molecular Mass and Isoelectric Focusing of HTII-280
By Western blotting under reducing conditions, HTll-280 migrates as a dimer with an apparent molecular mass of 280–300 kDa (Figure 1). Fewer than 2% of 530 oligomeric proteins surveyed by Gianazza and Giorgio Righetti (1980) had polypeptide with molecular mass greater than 250 kDa.
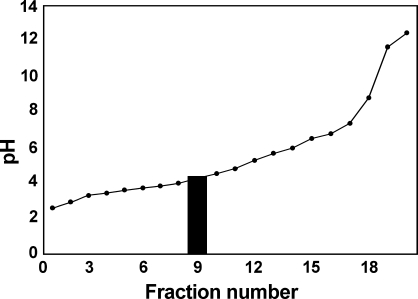
We used liquid isoelectric focusing to establish the isoelectric point (pI) of HTII-280. When we used ampholines in the pH range 3–10, most of the antigenic activity was found in the acidic range close to pH 3.0, in fractions containing ∼3% of the loaded proteins. We, therefore, refocused the homogenate in a column with an expanded acidic range (see Materials and Methods) and found that most of the antigenic activity is in fraction 9 (Figure 7), establishing the pI to be ∼4.4.
Figure 7.
Liquid-phase isoelectric focusing of lung homogenate. Liquid-phase isoelectric focusing was performed as described in Materials and Methods, using ampholines in the pH range of 2.5–6.0. Shaded area indicates the fraction containing immunoreactivity against HTII-280. The pI of HTII-280 is ∼4.4.
Discussion
Cell-specific surface markers have proved invaluable to the study of many organ systems, most notably the hematopoietic system (Tarnok et al. 2009). In the heart, liver, and kidney, the measurement of organ-specific proteins has proved useful to assess the duration and extent of organ injury, to predict outcomes, and to assess the response to various therapeutic regimens. In patients with heart injury, serum levels of cardiac-specific iso-enzymes of creatine kinase and troponin are measured to assess damage; hepatic transaminases, bilirubin, and alkaline phosphatase have been used to assess liver injury; and renal pentraxim-3 and kim-1 have been used to measure renal injury (Fearon et al. 1999; Richards 2009). Presently, there are no widely accepted lung-specific markers routinely used to assess alveolar epithelial disease progression, lung repair, or other pathological processes. The development of useful reagents for measuring disease progression in the lung using biomarkers specific for TI and TII cells is an important development. Additionally, these two reagents can be used to isolate and compare normal and injured TI and TII cells, gaining insight into complex disease processes.
We previously produced MAbs to two marker proteins that are specific to rat alveolar TI and TII cells. The first antibody, anti-RTI-40, identified a 40-kDa apical membrane protein that is found in rat TI cells. The second antibody, anti-RTll-70, labeled a 70-kDa protein found on the apical membrane of alveolar TII cells (Gonzalez and Dobbs 1997). These two antibodies have been utilized to sort very pure populations of rat TI and TII cells using FACS, and expression profiling of these cells using microarray analysis has helped to define the rat alveolar TI and TII cell phenotypes (Gonzalez et al. 2009). Additionally, these antibodies have been useful in assessing alveolar epithelial cell damage in disease models. Anti-RTI-40 was utilized to screen an expression library, identifying RTI-40 as an ortholog of mouse podoplanin [E1a, OTS-8, T1a (Nose et al. 1990; Rishi et al. 1995; Wetterwald et al. 1996; Ramirez et al. 1999)]. Detection of RTI-40 has proved useful in measuring lung damage due to infection (McElroy et al. 1995; Matute-Bello et al. 2008), oxidant-induced injury (McElroy et al. 1997), or bleomycin-induced injury (Koslowski et al. 1998). Similarly, antibody to RTII-70 has been useful in detecting hyperplastic TII cell proliferation after Staphylococcus aureus–induced acute lung damage (Clegg et al. 2005). Using a similar approach to the production of MAbs to rat alveolar epithelial cells, we previously produced an MAb specific to human TI cells that identifies a 56-kDa apical membrane protein (Dobbs et al. 1999). This antibody was subsequently utilized to identify human TI cells and assess the extent of alveolar cell damage (Newman et al. 1999,2000).
We now report the production of an antibody specific to human TII cells and partial characterization of a 280-kDa apical membrane protein found in human alveolar TII cells. Together, the antibodies against TI or TII cells can be used to facilitate FACS of very pure populations of human alveolar TI and TII cells. Furthermore, characterization of expression of these two cell-specific proteins may be useful in the study of lung development, human TI and TII cell functions, and improved characterization of lung tissue in various disease stages. Isolated cell preparations may also have therapeutic potential. Intratracheal instillation of alveolar TII cells has been shown to ameliorate bleomycin-induced lung fibrosis in rats (Serrano-Mollar et al. 2007).
Anti-HTII-280 was used to detect and partially characterize the antigen. By immunoelectron microscopy using gold-labeled secondary antibody, HTII-280 was localized specifically to the apical plasma membranes of alveolar TII cells. Using non-ionic detergent and chaotropic agents, we found HTII-280 to be insoluble in conditions that solubilize peripheral membrane proteins, but soluble in conditions that solubilize integral membrane proteins. We found HTII-280 to have an apparent molecular mass of ∼280 kDa and a pI of 4.4. Fewer than 2% of 530 oligomeric proteins surveyed by Gianazza and Giorgio Righetti (1980) had polypeptides with molecular mass greater than 250 kDa. Furthermore, fewer than 13% of 500 proteins surveyed have pIs in this range (Gianazza and Giorgio Righetti 1980). These unusual physiochemical characteristics should facilitate further purification and characterization of this protein by the sequential removal of contaminating proteins that do not share these intrinsic properties.
In the developing lung, we first detected HTII-280 at prenatal week 11 in morphologically undifferentiated airspace epithelium. HTII-280 is developmentally regulated, and its expression increases with gestational age. At prenatal week 24, it is expressed occasionally in cells that co-express HTI-56, a TI cell–specific antigen. Clegg et al. (2005) found transitional alveolar epithelial cells expressing both TI and TII cell marker proteins in injured adult rat lungs, but not in healthy control lungs. We have found similar results in human lungs. In normal human lungs, HTI-56 and HTII-280 are not co-expressed in the same cell. However, during human lung development and in injured adult lung, co-expression appears to occur in some cells. HTII-280 should prove useful in studying development and alveolar epithelial cell response to injury. Furthermore, identification and detection of a cell-surface protein specific to human alveolar TII cells, in combination with HTI-56 detection (a TI cell–specific marker), may prove useful in assessing both the extent of lung injury and in the evaluation of various treatment modalities designed to improve the course of lung repair following injury.
Acknowledgments
This work was supported by National Institutes of Health Grants HL-099820 (to LGD), HL-24075 (to LGD), HL-57426 (to LGD), HL-088193 (to PLB and LWG), and HL-086323 (to PLB and LWG).
We thank Ping Wang and Sreedevi Angampalli for expert technical assistance and Portia Krieger for preparation of the tissue microarrays.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA (2007) Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 293:L259–271 [DOI] [PubMed] [Google Scholar]
- Baker DG, McDonald DM (1992) Distribution of catecholamine-containing nerves on blood vessels of the rat trachea. J Comp Neurol 325:38–46 [DOI] [PubMed] [Google Scholar]
- Boylan GM, Pryde JG, Dobbs LG, McElroy MC (2001) Identification of a novel antigen on the apical surface of rat alveolar epithelial type II and Clara cells. Am J Physiol Lung Cell Mol Physiol 280:L1318–1326 [DOI] [PubMed] [Google Scholar]
- Clegg GR, Tyrrell C, McKechnie SR, Beers MF, Harrison D, McElroy MC (2005) Coexpression of RTI40 with alveolar epithelial type II cell proteins in lungs following injury: identification of alveolar intermediate cell types. Am J Physiol Lung Cell Mol Physiol 289:L382–390 [DOI] [PubMed] [Google Scholar]
- Deutscher M (1990) Guide to Protein Purification. Methods in Enzymology, vol. 182. London, Academic Press [PubMed]
- Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS (1998) Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc Natl Acad Sci USA 95:2991–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LG, Gonzalez R, Williams MC (1986) An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134:141–145 [DOI] [PubMed] [Google Scholar]
- Dobbs LG, Gonzalez RF, Allen L, Froh DK (1999) HTI-56, an integral membrane protein specific to human alveolar type I cells. J Histochem Cytochem 47:129–137 [DOI] [PubMed] [Google Scholar]
- Dobbs LG, Williams MC, Brandt AE (1985) Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta 846:155–166 [DOI] [PubMed] [Google Scholar]
- Dobbs LG, Williams MC, Gonzalez R (1988) Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta 970:146–156 [DOI] [PubMed] [Google Scholar]
- Evans MJ, Cabral LJ, Stephens RJ, Freeman G (1975) Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22:142–150 [DOI] [PubMed] [Google Scholar]
- Fearon WF, Lee FH, Froelicher VF (1999) Does elevated cardiac troponin I in patients with unstable angina predict ischemia on stress testing? Am J Cardiol 84:1440–1442 [DOI] [PubMed] [Google Scholar]
- Fehrenbach H, Kasper M, Tschernig T, Pan T, Schuh D, Shannon JM, Muller M, et al. (1999) Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cells in vivo is resolved by differentiation into type I cells and by apoptosis. Eur Respir J 14:534–544 [DOI] [PubMed] [Google Scholar]
- Foster C, Aktar A, Kopf D, Zhang P, Guttentag S (2004) Pepsinogen C: a type 2 cell-specific protease. Am J Physiol Lung Cell Mol Physiol 286:L382–387 [DOI] [PubMed] [Google Scholar]
- Fullmer CS (1984) Identification of cysteine-containing peptides in protein digests by high-performance liquid chromatography. Anal Biochem 142:336–339 [DOI] [PubMed] [Google Scholar]
- Gianazza E, Giorgio Righetti P (1980) Size and charge distribution of macromolecules in living systems. J Chromatogr A 193:1–8 [Google Scholar]
- Gonzalez R, Dobbs L (1997) Characterization and utility of a monoclonal antibody specific to the apical surface of rat alveolar type II cells. Mol Biol Cell 8:1981 [Google Scholar]
- Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L (2005) Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 288:L179–189 [DOI] [PubMed] [Google Scholar]
- Gonzalez RF, Allen L, Dobbs LG (2009) Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol 297:L1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Yamada K, Zhao L-X, Yamano G, Tanaka Y, Funahashi H, Zhou C-J, et al. (2004) Cloning, tissue distribution and function of ABCA transporters. Int Congr Ser 1262:578–581 [Google Scholar]
- Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, et al. (2006) Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA 103:4964–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalina M, Mason RJ, Shannon JM (1992) Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am J Respir Cell Mol Biol 6:594–600 [DOI] [PubMed] [Google Scholar]
- Kasper M, Behrens J, Schuh D, Müller M (1995) Distribution of E-cadherin and Ep-CAM in the human lung during development and after injury. Histochem Cell Biol 103:281–286 [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, et al. (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847 [DOI] [PubMed] [Google Scholar]
- Koslowski R, Dobbs LG, Wenzel KW, Schuh D, Muller M, Kasper M (1998) Loss of immunoreactivity for RTI40, a type I cell-specific protein in the alveolar epithelium of rat lungs with bleomycin-induced fibrosis. Eur Respir J 12:1397–1403 [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295:L379–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy MC, Pittet JF, Hashimoto S, Allen L, Wiener-Kronish JP, Dobbs LG (1995) A type I cell-specific protein is a biochemical marker of epithelial injury in a rat model of pneumonia. Am J Physiol 268:L181–186 [DOI] [PubMed] [Google Scholar]
- McElroy MC, Wiener-Kronish JP, Miyazaki H, Sawa T, Modelska K, Dobbs LG, Pittet JF (1997) Nitric oxide attenuates lung endothelial injury caused by sublethal hyperoxia in rats. Am J Physiol 272:L631–638 [DOI] [PubMed] [Google Scholar]
- Newman V, Gonzalez R, Matthay M, Dobbs L (1999) HTI-56, an integral apical membrane protein of the human alveolar type I cell, is a biochemical marker of acute lung injury. Chest 116:35S–36S [PubMed] [Google Scholar]
- Newman V, Gonzalez RF, Matthay MA, Dobbs LG (2000) A novel alveolar type I cell-specific biochemical marker of human acute lung injury. Am J Respir Crit Care Med 161:990–995 [DOI] [PubMed] [Google Scholar]
- Nose K, Saito H, Kuroki T (1990) Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ 1:511–518 [PubMed] [Google Scholar]
- Ramirez MI, Cao YX, Williams MC (1999) 1.3 Kilobases of the lung type I cell TI alpha gene promoter mimics endogenous gene expression patterns during development but lacks sequences to enhance expression in perinatal and adult lung. Dev Dyn 215:319–331 [DOI] [PubMed] [Google Scholar]
- Richards L (2009) Biomarkers: rapid urine test for kidney disease. Nat Rev Nephrol 5:548–548 [Google Scholar]
- Rishi AK, Joyce-Brady M, Fisher J, Dobbs LG, Floros J, VanderSpek J, Brody JS, et al. (1995) Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol 167:294–306. [DOI] [PubMed] [Google Scholar]
- Salaun B, de Saint-Vis B, Clair-Moninot V, Pin JJ, Barthélemy-Dubois C, Kissenpfennig A, Peronne C, et al. (2003) Cloning and characterization of the mouse homologue of the human dendritic cell maturation marker CD208/DC-LAMP. Eur J Immunol 33:2619–2629 [DOI] [PubMed] [Google Scholar]
- Serrano-Mollar A, Nacher M, Gay-Jordi G, Closa D, Xaubet A, Bulbena O (2007) Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 176:1261–1268 [DOI] [PubMed] [Google Scholar]
- Simon R, Sauter G (2003) Tissue microarray (TMA) applications: implications for molecular medicine. Expert Rev Mol Med 5:1–12 [DOI] [PubMed] [Google Scholar]
- Stone KC, Mercer RR, Freeman BA, Chang LY, Crapo JD (1992) Distribution of lung cell numbers and volumes between alveolar and nonalveolar tissue. Am Rev Respir Dis 146:454–456 [DOI] [PubMed] [Google Scholar]
- Tarnok A, Ulrich H, Bocsi J (2009) Phenotypes of stem cells from diverse origin. Cytometry A 77:6–10 [DOI] [PubMed] [Google Scholar]
- Tokuyasu KT (1989) Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J 21:163–171 [DOI] [PubMed] [Google Scholar]
- Walker S, Williams M, Benson B (1986) Immunocytochemical localization of the major surfactant apoproteins in type II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem 34:1137–1148 [DOI] [PubMed] [Google Scholar]
- Wetterwald A, Hofstetter W, Cecchini MG, Lanske B, Wagner C, Fleisch H, Atkinson M (1996) Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone 18:125–132 [DOI] [PubMed] [Google Scholar]
- Wright JR (2005) Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68 [DOI] [PubMed] [Google Scholar]
- Wright JR, Dobbs LG (1991) Regulation of pulmonary surfactant secretion and clearance. Annu Rev Physiol 53:395–414 [DOI] [PubMed] [Google Scholar]