Abstract
Objective
Stress urinary incontinence may serve as a barrier to lifestyle modification among women at high risk for diabetes, but the prevalence of stress urinary incontinence among women with histories of gestational diabetes mellitus (hGDM) is unknown. The purpose of this study was to examine the prevalence of stress incontinence among women with hGDM and to examine its association with their current physical activity.
Methods
We surveyed women with hGDM within the past 5 years who were currently enrolled in a managed care plan (n = 228). In a cross-sectional analysis, self-reported weekly or more frequent stress incontinence was the primary independent variable and measures of physical activity and body mass index (BMI) were the outcomes of interest. We constructed multivariable models that adjusted for participant characteristics associated with the measure of incontinence or outcomes in bivariate analyses.
Results
Of the 228 women with hGDM, 49% reported weekly or more frequent incontinence during pregnancy, and 28% reported that incontinence affected their activities during pregnancy. Fifty percent reported weekly or more frequent incontinence after delivery, with 27% reporting interference of incontinence with activity. Less than a third of women reported optimal physical activity, and 42% were obese. After adjustment for characteristics associated with measures of activity and incontinence, there was minimal association between levels of activity and stress urinary incontinence; similarly, there was no association between BMI and measures of stress incontinence.
Conclusions
Stress urinary incontinence is common among women with hGDM but does not appear to be associated with physical activity levels or BMI.
Introduction
Gestational diabetes mellitus (GDM), or glucose intolerance first identified during pregnancy, is a major risk factor for type 2 diabetes after delivery.1 The Diabetes Prevention Program (DPP), a randomized trial, demonstrated that lifestyle behaviors, such as physical activity, can contribute to weight loss and thus delay the onset of future diabetes.2 Unfortunately, physical activity levels are suboptimal among women with recent histories of GDM.3
The reasons for these low levels of physical activity are incompletely elucidated, but one potential contributor may be stress urinary incontinence. In population-based samples of women of reproductive age, urinary incontinence, particularly stress incontinence, is a significant barrier to physical activity.4,5 Once incontinence is present, decreased physical activity may further exacerbate it, as lack of physical activity and greater weight may also lead to increased stress incontinence.6,7 In addition, although the prevalence of stress incontinence increases with age,8 it is not uncommon in women of reproductive age. The rate of stress incontinence in women aged 30–39 years was approximately 10% in one survey.8 Another survey of women with a mean age of 44 ± 10 years reported that 16% had stress urinary incontinence.4 Finally, in the National Health and Nutrition Examination Survey (NHANES), approximately 14% of women with normal glucose tolerance reported weekly or more often stress incontinence; their average age was 44 years.7
To our knowledge, the prevalence of urinary stress incontinence among women with GDM has not been reported, nor has its association with physical activity among women with histories of GDM been reported. Women with recent histories of GDM may have a high prevalence of urinary incontinence because of their high rates of obesity and macrosomic pregnancies.8–10 On the other hand, women with a recent history of GDM are younger than women with diabetes,3,11 and studies of women of reproductive age generally report lower levels of urinary incontinence than those found in older women.8 Examination of the prevalence of urinary incontinence and the associations between incontinence and physical activity might identify intervention points for diabetes prevention through lifestyle modification in this high-risk population of women.
Therefore, we surveyed women with a history of GDM regarding their urinary incontinence. We also examined its association with their current physical activity. We hypothesized that among women with a history of GDM prevalence of stress urinary incontinence would be high and, moreover, that stress urinary incontinence would be associated with decreased physical activity.
Materials and Methods
Study population and data collection
Study participants were women enrolled in an academic managed care plan and identified as having had a GDM pregnancy within the past 5 years through ICD-9 codes 648.8, 648.83, and 648.84 and with at least one health care service for any reason during the year before the survey. Women were contacted by interviewer using a computer-assisted telephone algorithm. Women were excluded if they stated they had type 1 or type 2 diabetes before their pregnancy, denied having had GDM, were currently pregnant with the index pregnancy (although they were eligible if they were currently pregnant and had already completed another GDM pregnancy), or were unable to give informed consent. Four hundred eight women were initially identified by claims data. A total of 30 women were ineligible, 6 because they had type 1 or type 2 diabetes before their pregnancy, 23 because they denied having GDM, and 1 because she was currently pregnant with her first GDM pregnancy. Four eligible women refused to participate or did not complete an entire survey, and 146 could not be contacted. If women who we were unable to contact had the same rate of eligibility as those contacted and were counted in the denominator, the survey response rate was 65%.12 Surveys were completed by 228 women, with 135 consenting to telephone interviews and 93 opting to complete written surveys. Survey responders were similar to nonresponders in terms of years of age (36 ± 5 vs. 36 ± 5, p = 0.16), months since delivery (32 ± 18 vs. 30 ± 17, p = 0.17), and visits in the year following their GDM delivery (11 ± 11 vs. 10 ± 10, p = 0.34); telephone and written responders were also similar. Ethical approval was obtained from the University of Michigan Institutional Review Board.
Main outcome measures
Self-reported physical activity was first assessed using questions from the National Health Interview Survey13 that asked women how often they walked for exercise, the average number of minutes they spent walking each time, and how much their heart and breathing rates increased (i.e., no increase, small, medium, or large) while walking. We calculated the total number of hours per week that women spent walking. We examined the association between walking intensity and scale scores, stratified by duration (no walking, walking with no increase in heart rate, small increase, medium increase, or large increase in heart rate). We also assessed degree of exertion during leisure time activity using a single-item question validated in the Diabetes Intervention Reaching and Educating Communities Together study14,15; women were asked which of the following four activity levels best described their present leisure time activity: none, only light physical activity in most weeks, vigorous activity for ≥20 minutes 1–2 times per week, and vigorous activity for ≥20 minutes ≥3 times per week.
Prepregnancy body mass index (BMI) and current BMI were obtained from self-report. Height is generally estimated within an average of 0.5 inches; men have a greater tendency than women to overestimate height. In population-based surveys that examined the correlation between measured anthropometrics vs. self-reported anthropometrics, the correlation between measured height and self-reported height was 0.92 in women. Similarly, weight is generally underestimated; women aged 20–29 years have a greater tendency to underestimate weight than do other groups. In population-based surveys, the correlation between measured weight and self-reported weight exceeded 0.90.16
Stress urinary incontinence measures
Stress urinary incontinence was determined using questions from NHANES and DPP.17,18 In NHANES, the frequency of stress incontinence was assessed by the question: “During the past 12 months, have you leaked or lost control of even a small amount of urine with an activity like coughing, lifting, or physical activity?” We modified this question by replacing “during the past 12 months” with “during your [GDM] pregnancy” and also “after your [GDM] pregnancy.” Frequency of incontinence was ascertained as everyday, a few times a week, a few times a month, or a few times a year. We categorized frequency as weekly or more frequent incontinence. In the NHANES, participants were also asked about the effect of urine leakage on day-to-day activity in the past 12 months; we replaced “12 months” with “during your [GDM] pregnancy” and “after your [GDM] pregnancy.” The responses to these questions were categorized as “not at all” or “only a little” vs. “somewhat,” “very much,” or “greatly.”
Statistical analyses
We used analysis of variance (ANOVA) to examine the unadjusted association between urinary incontinence measures and participant characteristics (Table 1) and measures of self-reported physical activity and BMI. We constructed multivariable regression models that controlled for patient covariates. These models adjusted for participant characteristics associated with the primary dependent and independent variables in the bivariate analyses described. Candidate participant characteristics included demographic variables (age, race, education, income), cardiovascular risk factors (family history of diabetes and current diabetes, history of dyslipidemia, history of hypertension outside of pregnancy, cigarette smoking), and pregnancy characteristics (breastfeeding, insulin use during pregnancy, and type of prenatal care provider) (Table 1). Because women could have multiple provider types during pregnancy, prenatal provider contact was characterized as six indicator variables: contact with an obstetrician/gynecologist (yes/no), family practitioner (yes/no), endocrinologist (yes/no), midwife (yes/no), dietitian (yes/no), and other provider type (yes/no). Covariates were not collinear.
Table 1.
Participant Characteristics and Stress Urinary Incontinence during and after a Pregnancy Affected by Gestational Diabetes Mellitusa
| Characteristic | Total | ≥ Weekly incontinence during pregnancy n = 112 (49%) | Incontinence affected activities during pregnancy n = 64 (28%) | ≥ Weekly incontinence after delivery n = 115 (50%) | Incontinence affected activities after delivery n = 62 (27%) |
|---|---|---|---|---|---|
| Age, years | |||||
| <34 | 34% | 38% | 28% | 28% | 31% |
| 34–38 | 35% | 30% | 39% | 35% | 42% |
| >38 | 31% | 32% | 33% | 37% | 27% |
| Race | |||||
| Non-Hispanic white | 71% | 71% | 78% | 74% | 81% |
| Asian/Pacific Islander | 13% | 12% | 13% | 14% | 10% |
| African American | 7% | 3% | 5% | 3% | 5% |
| Other | 9% | 14% | 5% | 10% | 5% |
| Education | |||||
| High school graduate or less | 8% | 6% | 13% | 7% | 17% |
| Some college | 28% | 28% | 33% | 26% | 29% |
| College graduate | 64% | 66% | 54% | 67% | 55% |
| Annual household income | |||||
| <$15,000 | 4% | 3% | 5% | 4% | 5% |
| $15,000–<$40,000 | 12% | 14% | 18% | 9% | 13% |
| $40,000–<$75,000 | 33% | 35% | 34% | 32% | 38% |
| ≥$75,000 | 51% | 49% | 44% | 56% | 44% |
| Current cigarette use | 11% | 7% | 17% | 10% | 16% |
| Duration of breastfeeding with formula | |||||
| 0–3 months | 54% | 57% | 58% | 50% | 52% |
| 3 months-<1 year | 32% | 30% | 33% | 36% | 33% |
| ≥1 year | 14% | 13% | 9% | 15% | 15% |
| Number of months since delivery | |||||
| <15 | 32% | 30% | 27% | 25% | 25% |
| 15–33 | 32% | 40% | 41% | 34% | 41% |
| >33 | 35% | 30% | 32% | 41% | 34% |
| Prenatal provider typeb | |||||
| Obstetrician | 91% | 93% | 89% | 91% | 87% |
| Family practitioner | 15% | 14% | 17% | 17% | 21% |
| Endocrinologist | 42% | 45% | 45% | 42% | 44% |
| Midwife | 6% | 6% | 9% | 6% | 6% |
| Dietician | 60% | 63% | 66% | 63% | 65% |
| Other | 5% | 4% | 5% | 6% | 6% |
| Insulin during pregnancy | 44% | 46% | 45% | 43% | 44% |
Bold indicates association at p < 0.05.
Women could see more than one type of provider, so percents do not sum to 100.
Separate models were constructed to examine the effect of women's (1) self-reported frequency of urine leakage during pregnancy, (2) interference of incontinence with activities during pregnancy, (3) self-reported frequency of urinary leakage after pregnancy, and (4) interference of incontinence with activities after pregnancy on each measure of physical activity. Similar models were constructed with prepregnancy BMI and current BMI as the dependent variables, with BMI categorized as <25 kg/m2, 25–29.9 kg/m2, and ≥30 kg/m2. Finally, to determine if the relationship between BMI and incontinence was confounded by physical activity, we constructed models where the dependent variable was BMI and the independent variable was a measure of incontinence, and we included a measure of physical activity as an adjuster. Analyses were conducted with SAS Version 9.0 software (SAS Institute, Cary, NC).
Results
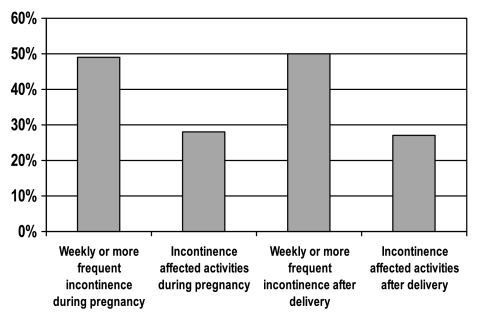
Approximately half of women reported having urinary incontinence at least once a week, and approximately a quarter reported that incontinence interfered with activity during and after pregnancy (Fig. 1). Although 28% of women reported that incontinence affected their activities during their GDM pregnancy, no specific patient characteristics were significantly associated with urinary incontinence and prenatal interference with activity (Table 1). Women were an average of 36 ± 5 years of age. Urinary incontinence affected activities after delivery more frequently among women who were less well educated. Performance of physical activity, whether measured by hours per week walking, perceived walking intensity, or leisure time vigorous activity, was suboptimal, with only 31% of women reporting the recommended levels of vigorous physical activity. Regarding obesity, the population was markedly disadvantaged, with 42% reporting a prepregnancy BMI ≥ 30 kg/m2 before pregnancy and 46% reporting a current BMI ≥ 30 kg/m2 after delivery.
FIG. 1.
Percent of women with a history of GDM reporting measures of incontinence.
Despite the high prevalence of urinary incontinence and suboptimal levels of physical activity, incontinence was not associated with any of the measures of physical activity or current BMI (Table 2). The lack of association persisted in multivariate models (Table 3). Incontinence was not associated with any measure of physical activity after adjustment for participant characteristics associated with the measure of activity or the measure of incontinence, nor was incontinence associated with category of BMI before or after adjustment for activity (data not shown). Moreover, the point estimates did not change in a consistent pattern between urinary incontinence with increasing levels of physical activity.
Table 2.
Physical Activity and Stress Urinary Incontinence during and after a Pregnancy Affected by Gestational Diabetes Mellitusa
| Total | ≥ Weekly incontinence during pregnancy | Incontinence affected activity during pregnancy | ≥ Weekly incontinence after delivery | Incontinence affected activity after delivery | |
|---|---|---|---|---|---|
| Hour/week walking | |||||
| 0–1 | 21% | 22% | 20% | 19% | 21% |
| 2 | 21% | 20% | 20% | 19% | 17% |
| 3 | 21% | 26% | 27% | 28% | 26% |
| ≥4 | 37% | 32% | 34% | 35% | 36% |
| Perceived walking intensity | |||||
| No walking at all | 4% | 6% | 6% | 6% | 2% |
| No increase in heart rate | 8% | 5% | 6% | 5% | 8% |
| Small increase in heart rate | 42% | 42% | 37% | 47% | 35% |
| Medium increase in heart rate | 37% | 40% | 42% | 35% | 47% |
| Large increase in heart rate | 9% | 7% | 9% | 6% | 8% |
| Leisure time vigorous activity | |||||
| No activity | 4% | 5% | 3% | 4% | 5% |
| Only light physical activity | 42% | 45% | 40% | 46% | 44% |
| Vigorous activity for 20 minutes ≤2 times per week | 22% | 23% | 32% | 24% | 29% |
| Vigorous activity for 20 minutes 3 times per week | 31% | 28% | 25% | 27% | 23% |
| Prepregnancy BMI (kg/m2) | |||||
| <25 | 33% | 35% | 27% | ||
| 25–29.9 | 24% | 17% | 22% | ||
| ≥30 | 42% | 48% | 51% | ||
| Current BMI (kg/m2) | |||||
| <25 | 30% | 30% | 23% | ||
| 25–29.9 | 24% | 22% | 19% | ||
| ≥30 | 46% | 49% | 58% | ||
Bold indicates association at p < 0.05.
Table 3.
Association between Urinary Incontinence Measures during Pregnancy and after Delivery and Physical Activity, Adjusted for Patient Characteristics
| Measure of physical activity | ≥ Weekly incontinence during pregnancy | Incontinence affected activity during pregnancy | ≥ Weekly incontinence after delivery | Incontinence affected activity after delivery |
|---|---|---|---|---|
| Number of hours spent walking over 2 weeks, reference = 0–1 hours | ||||
| 2 | 0.77 (0.31–1.89)a | 1.00 (0.38–2.66)d | 1.03 (0.43–2.49)g | 0.71 (0.25–2.04)j |
| 3 | 1.13 (0.46–2.79)a | 1.52 (0.59–3.86)d | 2.35 (0.96–5.79)g | 1.40 (0.53–3.73)j |
| ≥4 | 0.65 (0.29–1.45)a | 0.96 (0.40–2.28)d | 1.04 (0.48–2.28)g | 0.84 (0.34–2.09)j |
| Perceived walking intensity, reference = no walking | ||||
| Walking, no increase in heart rate | 0.16 (0.02–1.39)b | 0.47 (0.07–3.21)e | 0.19 (0.03–1.41)h | 2.51 (0.22–29.28)k |
| Walking with small increase in heart rate | 0.29 (0.04–1.87)b | 0.55 (0.12–2.60)e | 0.48 (0.08–2.86)h | 2.16 (0.23–19.81)k |
| Walking with medium increase in heart rate | 0.31 (0.05–2.02)b | 0.71 (0.15–3.34)e | 0.27 (0.05–1.64)h | 3.10 (0.34–28.06)k |
| Walking with large increase in heart rate | 0.17 (0.02–1.36)b | 0.53 (0.09–3.21)e | 0.16 (0.02–1.18)h | 1.65 (0.14–19.30)k |
| Leisure time vigorous activity, reference = no activity | ||||
| Only light physical activity | 0.88 (0.20–3.88)c | 1.50 (0.27–8.24)f | 2.07 (0.47–9.06)i | 0.95 (0.18–4.99)l |
| Vigorous activity for 20 minutes 1–2 times per week | 0.80 (0.18–3.62)c | 2.71 (0.48–15.12)f | 1.54 (0.34–6.99)i | 1.44 (0.27–7.71)l |
| Vigorous activity for 20 minutes 3 times per week | 0.66 (0.15–2.85)c | 0.94 (0.17–5.19)f | 0.93 (0.22–4.01)i | 0.45 (0.08–2.42)l |
Adjusted for race (non-Hispanic white, Asian/Pacific Islander, African American, other), current smoking.
Adjusted for race (non-Hispanic white, Asian/Pacific Islander, African American, other), current smoking, prepregnancy BMI, obstetrician/gynecologist provider.
Adjusted for race (non-Hispanic white, Asian/Pacific Islander, African American, other), current smoking, time since delivery (months), family practice provider.
No covariates included, as no participant characteristic were significantly associated with incontinence in bivariate analyses.
Adjusted for pregnancy BMI, contact with an obstetrician/gynecologist.
Adjusted for race (non-Hispanic white, Asian/Pacific Islander, African American, other), current smoking, time since delivery (months), family practice provider.
Adjusted for time since delivery (months).
Adjusted for time since delivery (months), current BMI, contact with an obstetrician/gynecologist.
Adjusted for race (non-Hispanic white, Asian/Pacific Islander, African American, other), current smoking, time since delivery (months), family practice provider.
Adjusted for education (less than high school, high school, some college or more), current BMI.
Adjusted for education (less than high school, high school, some college or more), current BMI, contact with an obstetrician/gynecologist.
Adjusted for race (non-Hispanic white, Asian/Pacific Islander, African American, other), education (less than high school, high school, some college or more), current BMI, current smoking, time since delivery (months), family practice provider.
Discussion
Women with a history of GDM are at increased risk for glucose intolerance after delivery, and lifestyle behaviors are crucial to preserving glucose tolerance. Multiple potential barriers to physical activity exist in this population, however. Among women with a recent history of GDM, we found a high prevalence of urinary stress incontinence, inconvenience associated with that incontinence, and suboptimal levels of physical activity. Half of these women reported greater than weekly incontinence, and approximately a quarter reported incontinence that interfered with activity. These difficulties were present during the GDM pregnancy as well. Although such reported barriers could potentially explain the low levels of physical activity observed, we did not find an association between incontinence and physical activity levels among women with a recent history of GDM.
The high rates of incontinence with activity in our sample contrast with the rates in other studies, which were generally much lower and ranged between about 10% and 16% in women of similar or slightly older age.4,7,8 The high prevalence of stress urinary incontinence during and after pregnancy in our population may have been partially attributable to the high rates of obesity we observed in our sample, 42% during pregnancy and 46% after delivery. Because BMI was obtained by self-report, it is possible that these are actually underestimates of obesity, with women underreporting their weight during and after pregnancy. The exact mechanism remains unclear; excess body weight may increase abdominal pressure during physical activity, which in turn increases bladder pressure and urethral mobility, thus leading to incontinence.19 It is also possible that the glucose intolerance experienced by women with GDM during pregnancy, and particularly for women who have persistent glucose intolerance after delivery, may have contributed to the high rates of urinary incontinence. Rates of microvascular disease among women with recent GDM have not been reported, and most women are thought to have normal glucose levels after delivery.20 However, examinations of older women with microvascular disease due to diabetes have found increased rates of incontinence, possibly through disturbances of the nerve supply to the urethral sphincter and bladder, causing sphincter damage and involuntary bladder contractions, resulting in incontinence.7 Such changes appeared to be present even among women with prediabetes, suggesting that microvascular disease may precede the diagnosis of frank diabetes.
Other characteristics of our sample are less likely to have contributed to the high observed rates of incontinence. In the report by Melville et al.,8 higher levels of education and income were associated with lower levels of incontinence in univariate analyses. Our sample was better educated and more affluent than that population, and, therefore, these characteristics would have actually lowered the observed rates of incontinence in our sample. Although non-Hispanic white race is associated with greater rates of stress incontinence and our population was predominantly white, we actually had a greater proportion of nonwhite participants than in other population-based studies, which also would have lowered the observed rates of incontinence. Finally, the mean age or our participants was 36 ± 5 years, approximately 8 years less than the glucose-tolerant population surveyed in NHANES,7 but the rates of stress incontinence were nearly double those observed in that survey despite the use of similar measures of incontinence.
Despite the suboptimal rates of activity and the high rates of incontinence, we did not observe an association between the two. Although explanations are speculative, women may have not had optimal levels of physical activity because of the presence of multiple other barriers. Previous studies suggest that the condition of pregnancy itself may not decrease activity,21 but the presence of young children may eventually have a detrimental effect on physical activity.22 Therefore, incontinence may not have been a significant barrier in and of itself. Another possible explanation is that women acclimate to incontinence, and the impact of incontinence on activity is subsequently minimal. In a Finnish cohort of incontinent women, approximately one quarter were still highly active, and activity levels did not change after treatment.23 To our knowledge, no studies examined the success of interventions aimed at stress incontinence that subsequently improve glucose intolerance. In contrast, the evidence is more suggestive that greater activity levels may serve to decrease levels of incontinence. In a Swedish study, women who engaged in low-impact physical activity reported improved urinary continence.24 In the DPP, women randomized to the lifestyle intervention group reported a reduction in rates of stress incontinence, mostly due to changes in weight. Of note, rates of incontinence were seen to be as high as 38%.18 Women with a history of GDM tend to be poor and racial/ethnic minorities and also have low education levels and incomes, as opposed to our largely non-Hispanic white, well-educated, and affluent sample. It is possible that less advantaged women are more affected by stress urinary incontinence than our sample, perhaps because they have low self-efficacy for physical activity already, and stress incontinence is more difficult to surmount. It is also possible that other populations of women have activity levels that are even less affected by stress urinary incontinence than our sample because incontinence is a minor barrier compared to other existing barriers. Our findings cannot be extrapolated to other samples of women.
Our report has several other limitations. The average time from pregnancy was approximately 2 years (SD 17 months), so that women in our sample may have delivered in the past 6 months to up to 5 years ago. Although we did not find that the relationship between recall of incontinence and time from delivery was consistent, the accuracy of the incontinence measure may have decreased with time. Specifically, women may have failed to recall incontinence after delivery because of the extended period; thus, the rates of reported incontinence, while already high, may have been artificially lowered. It is also possible that peripartum incontinence rates were much higher than those even only a few months later, which would have artificially elevated incontinence rates. In addition, recall bias may have affected our results in several ways. If women who were less active were more likely to recall incontinence interfering with activity than women who were more active, this would have exaggerated the relationship between incontinence and lack of physical activity. As we did not observe an association, despite the fact that a quarter of women reported incontinence interfering with activity, this bias may not have had a large impact on our results. However, if women who were less active were less likely to recall incontinence than women who were more active, this would have biased the results to the null and, therefore, contributed to our negative results. It is also possible that the activity triggered the incontinence. We assessed the prevalence of incontinence among women with a history of GDM using survey measures assessing weekly incontinence, but we were not able to assess a more precise frequency and quantity of urine loss. The measures of physical activity and incontinence we used may not have been sensitive enough to capture an association.19 In a study of postmenopausal women with diabetes, diabetes was associated primarily with severe incontinence,25 and in other studies of diabetic women, diabetes was associated with daily incontinence,26 suggesting that perhaps a larger sample size or measures more sensitive to greater frequency or severity of incontinence may have captured an association.
One of the advantages of the urinary incontinence measure adapted in this paper is that it allows comparisons with larger population-based studies, such as the NHANES,6,7 which demonstrated lower rates of stress incontinence (14%) among women with normal glucose tolerance and from which we adopted the incontinence questions. This measure is comparable to other studies using different measures, one of which found that the prevalence of urinary incontinence among women aged 30–39 years was also approximately 14%, with the prevalence increasing with age and the odds increasing with each delivery (OR 1.17, 95% CI 1.11–1.24).8
Our primary objective was to assess the association between incontinence and lifestyle behaviors in women with a history of GDM, but we were not able to assess the impact of GDM on incontinence, as this would have required a comparison group. Although the objective of our study was to examine the prevalence of incontinence among women with GDM and its association with physical activity, we were limited in our ability to assess the effect of other variables that might affect incontinence, such as the mode or number of deliveries, hysterectomy, and comorbidities, including depression. We expected the prevalence of these to be low given the age of our population. We expect that adjustment for these factors would have biased any association to the null. However, the effect of this bias is not known. More detailed information would allow quantification of the risk of urinary incontinence within the population affected by GDM, such as women who delivered by cesarean section or women who were grand multiparas. Larger studies or longitudinal studies may capture an association between urinary incontinence and physical activity. Given the point estimates observed in our cross-sectional study, the magnitude of the association might be smaller than we previously hypothesized. However, such studies would allow more accurate assessment of incontinence by interviewing women at a specific time after their delivery.
Conclusions
Stress urinary incontinence is common among women with a history of GDM but does not appear to be associated with levels of physical activity or with BMI. Studies that examine the onset of incontinence in relation to changes in physical activity are needed to determine definitively the barrier that stress incontinence poses for physical activity in this population of women at high risk for diabetes. Ideally, these reports would also adjust for the other barriers to physical activity that are present in women of reproductive age to determine the relative impact of stress incontinence on activity. In addition, such reports might quantify other contributors to incontinence, particularly the presence of microvascular disease and data on mode and number of deliveries. Such studies might reveal a useful leverage point for future diabetes prevention interventions.
Footnotes
C.K. was supported by K23DK071552 from the National Institute of Health of Diabetes and Digestive and Kidney Diseases (NIDDK). J.D.P. is a VA Research Career Scientist. This work used the Biostatistics and Measurement Cores of the Michigan Diabetes Research and Training Program funded by NIH 5P60 DK20572 from the NIDDK. This study was partially supported by funds from the Translating Research Into Action for Diabetes (TRIAD) study, which was supported by the Centers for Disease Control and Prevention (CDC) U58/CCU523525-03. This study was jointly funded by Program Announcement number 04005 from the CDC (Division of Diabetes Translation) and the NIDDK.
Disclosure Statement
No competing financial interests exist.
References
- 1.Metzger B. Coustan D. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus: The Organizing Committee. Diabetes Care. 1998;21:B161. [PubMed] [Google Scholar]
- 2.Knowler W. Barrett-Connor E. Fowler S, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kieffer E. Sinco B. Kim C. Health behaviors in women with a history of gestational diabetes mellitus in the Behavioral Risk Factor Surveillance System. Diabetes Care. 2006;29:1788. doi: 10.2337/dc06-0199. [DOI] [PubMed] [Google Scholar]
- 4.Nygaard I. Girts T. Fultz N. Kinchen K. Pohl G. Sternfeld B. Is urinary incontinence a barrier to exercise in women? Obstet Gynecol. 2005;106:307. doi: 10.1097/01.AOG.0000168455.39156.0f. [DOI] [PubMed] [Google Scholar]
- 5.Brown W. Miller Y. Too wet to exercise? Leaking urine as a barrier to physical activity in women. J Sci Med Sport. 2001;4:373. doi: 10.1016/s1440-2440(01)80046-3. [DOI] [PubMed] [Google Scholar]
- 6.Danforth K. Shah A. Townsend M, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol. 2007;109:721. doi: 10.1097/01.AOG.0000255973.92450.24. [DOI] [PubMed] [Google Scholar]
- 7.Brown J. Vittinghoff E. Lin F. Nyberg L. Kusek J. Kanaya A. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose. Diabetes Care. 2006;29:1307. doi: 10.2337/dc05-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melville J. Katon W. Delaney K. Newton K. Urinary incontinence in U.S. women: A population-based study. Arch Intern Med. 2005;165:537. doi: 10.1001/archinte.165.5.537. [DOI] [PubMed] [Google Scholar]
- 9.Saydah S. Chandra A. Eberhardt M. Pregnancy experience among women with and without gestational diabetes in the U.S. 1995 National Survey of Family Growth. Diabetes Care. 2005;28:1035. doi: 10.2337/diacare.28.5.1035. [DOI] [PubMed] [Google Scholar]
- 10.Casey B. Schaffer J. Bloom S. Heartwell S. McIntire D. Leveno K. Obstetric antecedents for postpartum pelvic floor dysfunction. Am J Obstet Gynecol. 2005;192:1655. doi: 10.1016/j.ajog.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Saadine J. Cadwell B. Gregg E, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med. 2006;144:465. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 12.Frankel L. The report of the CASRO Task Force on response rates. In: Wiseman F, editor. Improving data quality in a sample survey. Cambridge, MA: Marketing Science Institute; 1983. [Google Scholar]
- 13.Gregg E. Gerzoff R. Caspersen C. Williamson D. Narayan K. Relationship of walking to mortality among U.S. adults with diabetes. Arch Intern Med. 2003;163:1440. doi: 10.1001/archinte.163.12.1440. [DOI] [PubMed] [Google Scholar]
- 14.Böthig S. WHO MONICA Project: objectives and design. Int J Epidemiol. 1989;18:529. [PubMed] [Google Scholar]
- 15.Herman W. Thompson T. Visscher W, et al. Diabetes mellitus and its complications in an African-American community: Project DIRECT. J Natl Med Assoc. 1998;90:147. [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson D. Holtzman D. Bolen J. Stanwyck C. Mack K. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System. Soz Praventivmed. 2001;46(Suppl 1):S3. [PubMed] [Google Scholar]
- 17.Grady D. Brown J. Vittonghoff E. Applegate W. Varner E. Snyder T. Postmenopausal hormones and incontinence: The Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97:116. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 18.Brown J. Wing R. Barrett-Connor E, et al. Lifestyle intervention is associated with lower prevalence of urinary incontinence. Diabetes Care. 2006;29:385. doi: 10.2337/diacare.29.02.06.dc05-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown J. Nyberg L. Kusek J, et al. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases International Symposium on Epidemiologic Issues in Urinary Incontinence in Women. Am J Obstet Gynecol. 2003;188:S77. doi: 10.1067/mob.2003.353. [DOI] [PubMed] [Google Scholar]
- 20.Kjos S. Buchanan T. Greenspoon J. Montoro M. Bernstein I. Mestman J. Gestational diabetes mellitus: The prevalence of glucose intolerance and diabetes mellitus in the first two months postpartum. Am J Obstet Gynecol. 1990;163:93. doi: 10.1016/s0002-9378(11)90676-0. [DOI] [PubMed] [Google Scholar]
- 21.Kim C. Brawarsky P. Jackson R. Fuentes-Afflick E. Haas J. Changes in health status experienced by women with gestational diabetes and pregnancy-induced hypertension. J Womens Health. 2005;14:729. doi: 10.1089/jwh.2005.14.729. [DOI] [PubMed] [Google Scholar]
- 22.Sternfeld B. Ainsworth B. Quesenberry C. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 23.Stach-Lempinen B. Nygard C. Laippala P. Metsanoja R. Kujansuu E. Is physical activity influenced by urinary incontinence? Br J Obstet Gynecol. 2004;111:475. doi: 10.1111/j.1471-0528.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 24.Eliasson K. Nordlander I. Larson B. Hammarstrom M. Mattsson E. Influence of physical activity on urinary leakage in primparous women. Scand J Med Sci Sports. 2005;15:87. doi: 10.1111/j.1600-0838.2004.407.x. [DOI] [PubMed] [Google Scholar]
- 25.Jackson R. Vittinghoff E. Kanaya A, et al. Urinary incontinence in elderly women: Findings from the Health, Aging, and Body Composition Study. Obstet Gynecol. 2004;104:301. doi: 10.1097/01.AOG.0000133482.20685.d1. [DOI] [PubMed] [Google Scholar]
- 26.Brown J. Seeley D. Fong J. Black D. Ensrud K. Grady D. Urinary incontinence in older women: Who is at risk? Study of Osteoporotic Fractures Research Group. Obstet Gynecol. 1996;87:715. doi: 10.1016/0029-7844(96)00013-0. [DOI] [PubMed] [Google Scholar]