Abstract
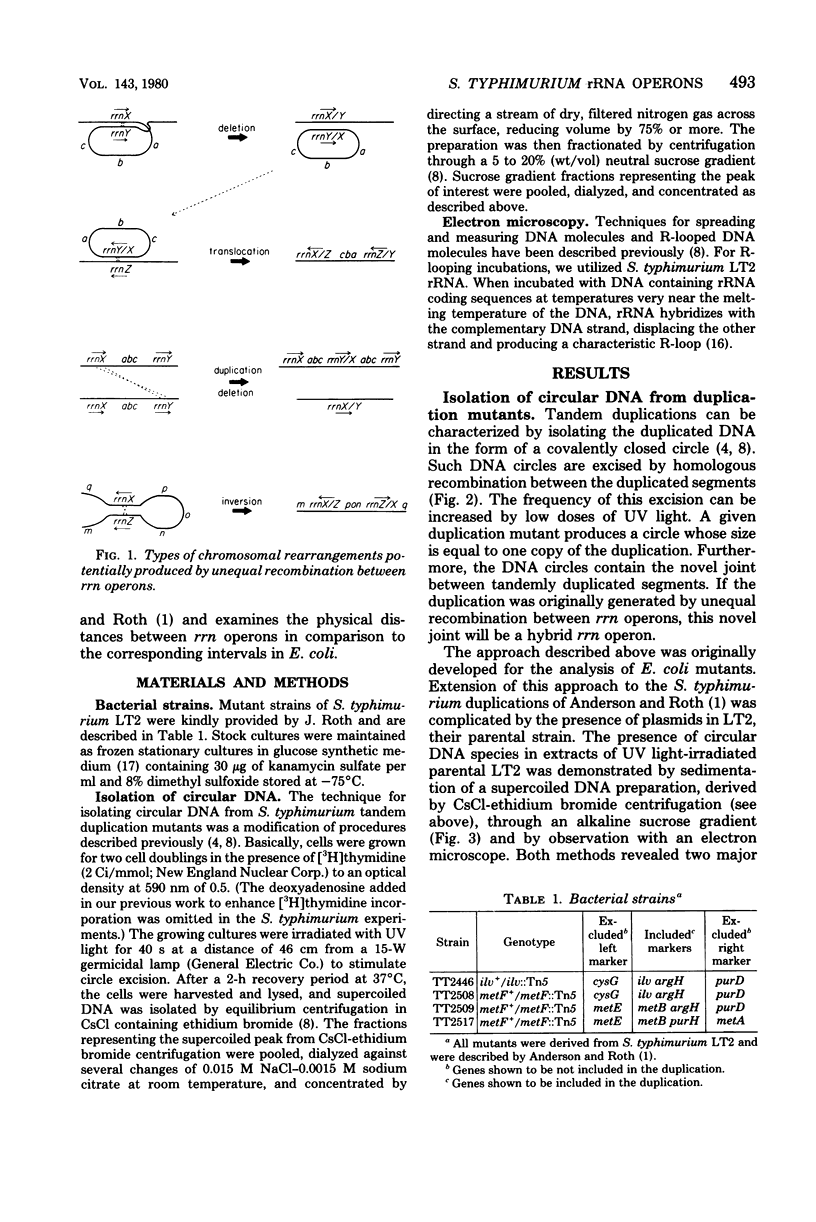
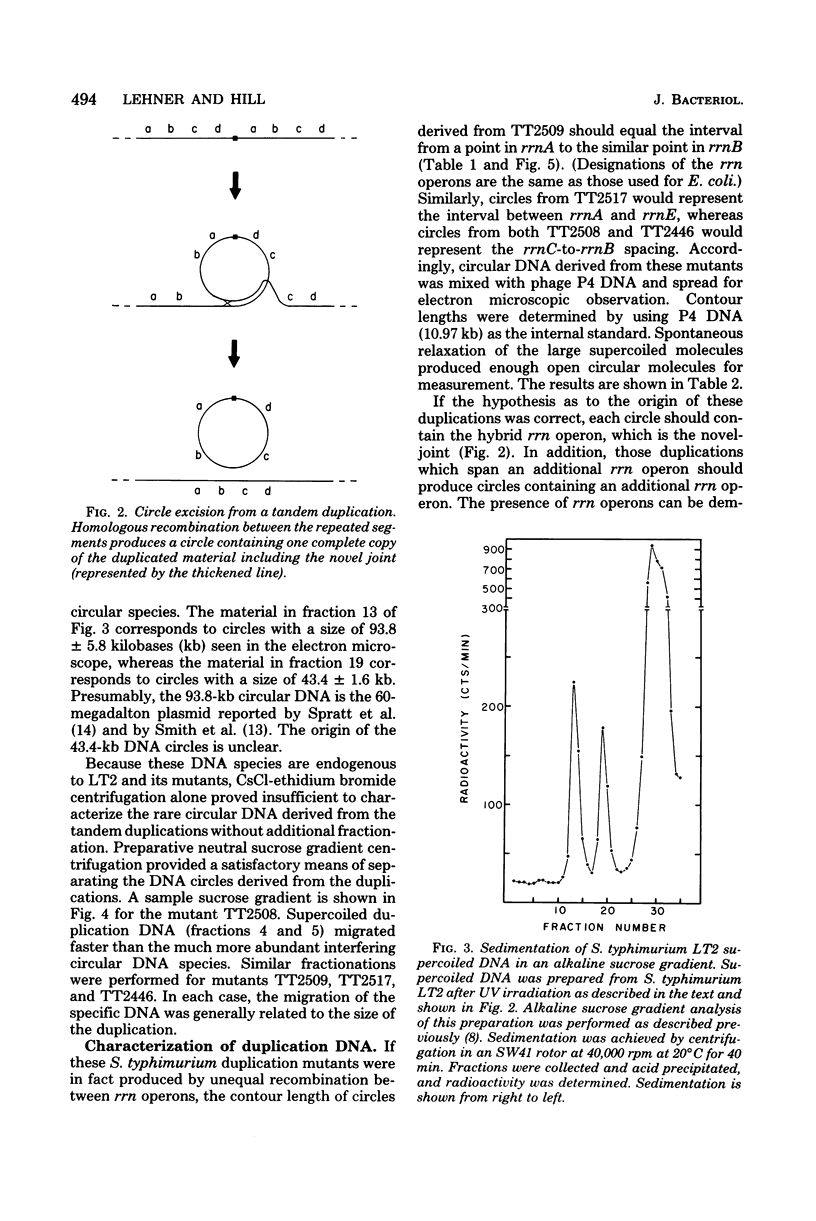
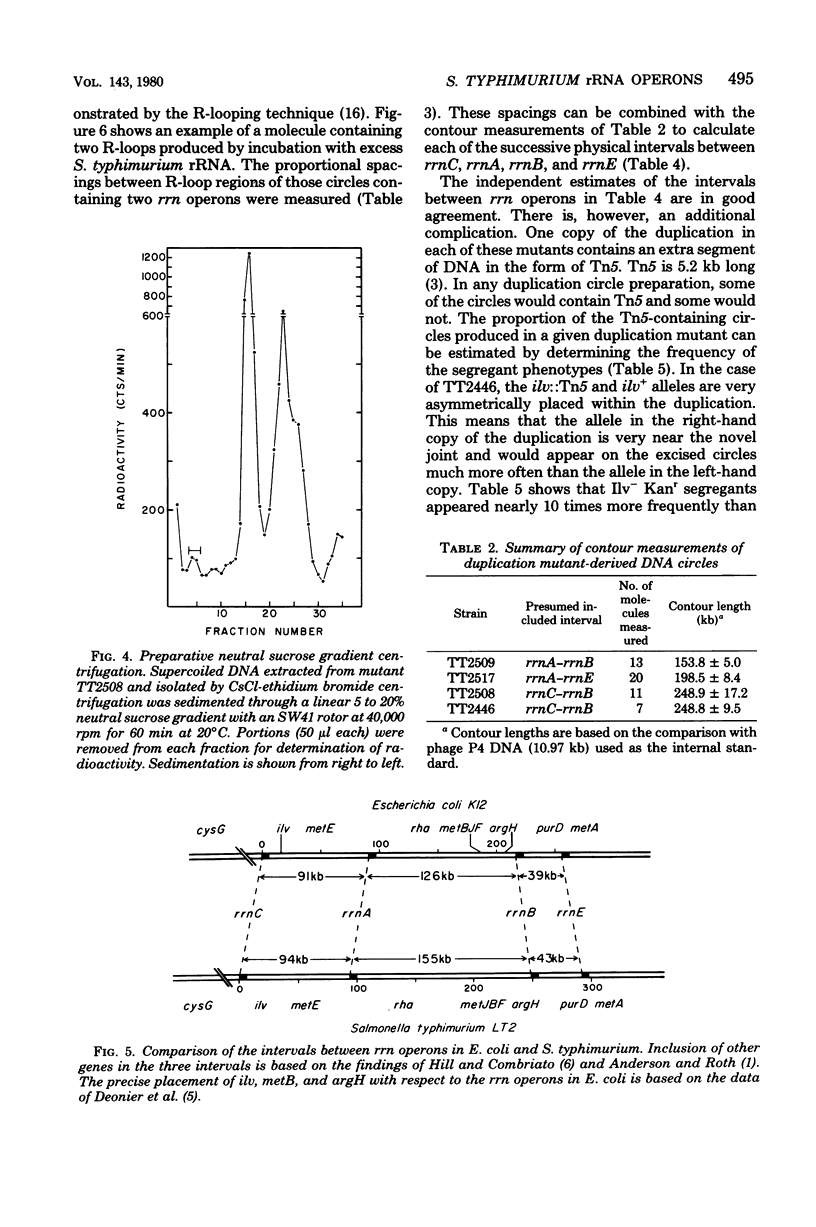
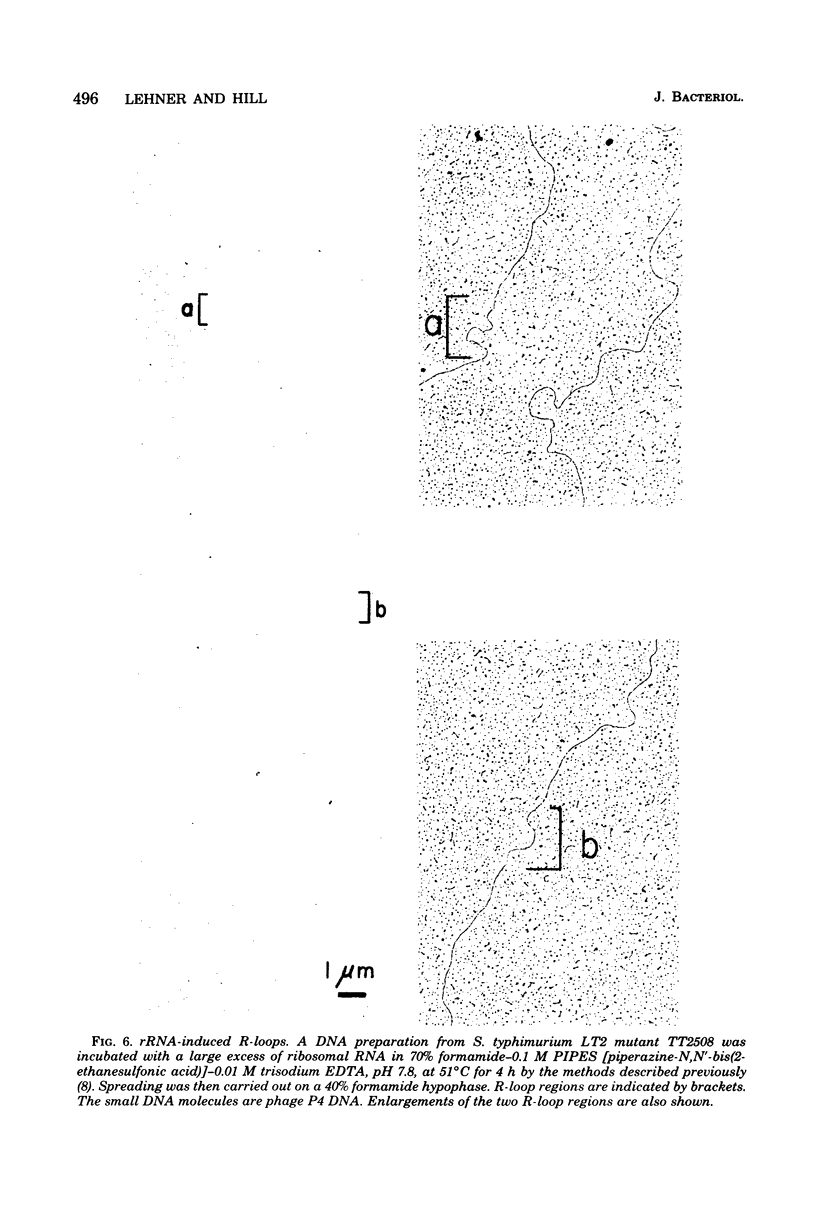
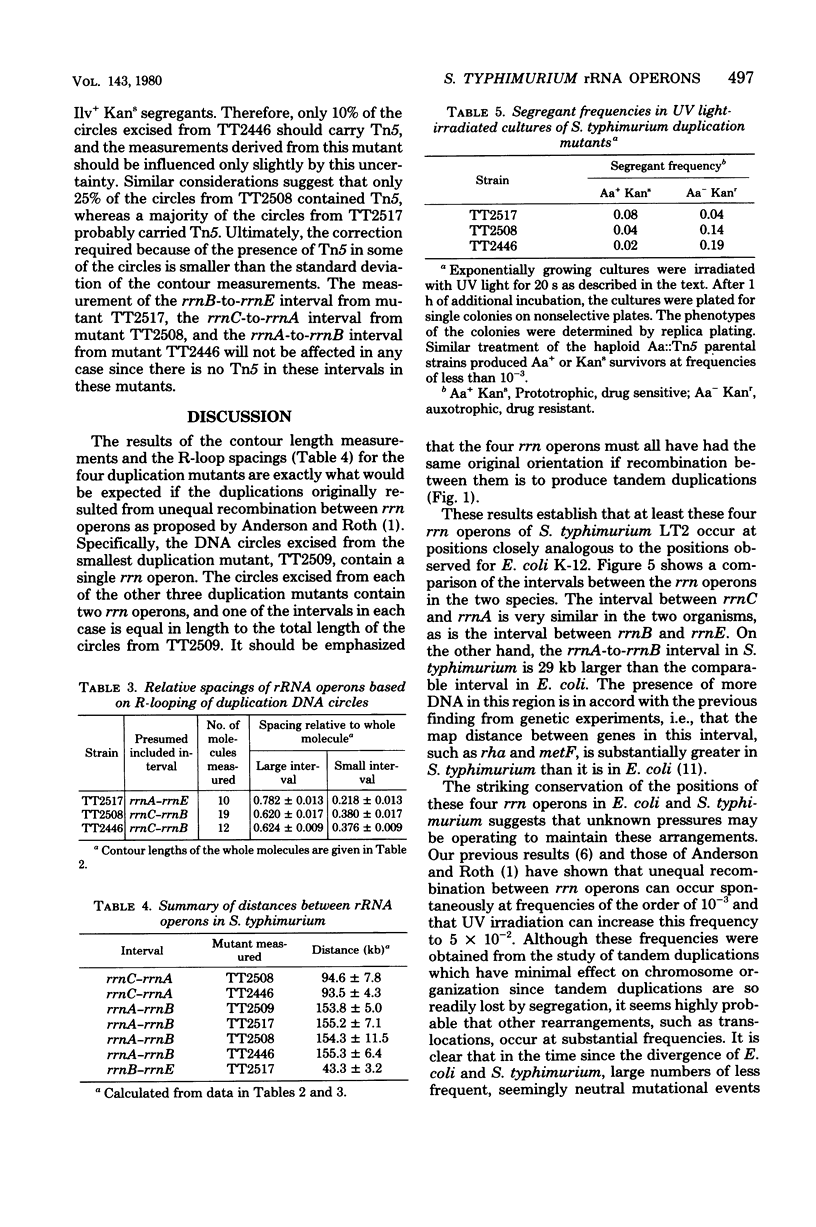
As part of our efforts to understand factors influencing chromosomal organization and rearrangements, we studied a family of Salmonella typhimurium tandum duplication mutants. We found that the duplications were originally generated by unequal recombination between pairs of similarly oriented ribosomal ribonucleic acid operons (rrn). This demonstration involved the physical isolation of the duplicated material as circular deoxyribonucleic acid excised from the duplication. The four rrn operons involved embraced the ilv pur D segment of the chromosome and occurred at positions closely analogous to those previously observed for Escherichia coli. The interval between rrnC and rrnA of S. typhimurium was similar in size to that of E. coli (43 versus 39 kilobases), as was the interval between rrnB and rrnE (94 versus 91 kilobases). The rrnA-to-rrnB interval of S. typhimurium, however, was 155 kilobases, substantially greater than the 126 kilobases observed for E. coli.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Gene duplication in bacteria: alteration of gene dosage by sister-chromosome exchanges. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1083–1087. doi: 10.1101/sqb.1979.043.01.120. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capage M., Hill C. W. Preferential unequal recombination in the glyS region of the Escherichia coli chromosome. J Mol Biol. 1979 Jan 5;127(1):73–87. doi: 10.1016/0022-2836(79)90460-1. [DOI] [PubMed] [Google Scholar]
- Deonier R. C., Otsubo E., Lee H. J., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. VII. Mapping the ribosomal RNA genes of plasmid F14. J Mol Biol. 1974 Nov 15;89(4):619–629. doi: 10.1016/0022-2836(74)90039-4. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Combriato G. Genetic duplications induced at very high frequency by ultraviolet irradiation in Escherichia coli. Mol Gen Genet. 1973 Dec 31;127(3):197–214. doi: 10.1007/BF00333760. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Grafstrom R. H., Harnish B. W., Hillman B. S. Tandem duplications resulting from recombination between ribosomal RNA genes in Escherichia coli. J Mol Biol. 1977 Nov 5;116(3):407–428. doi: 10.1016/0022-2836(77)90077-8. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Yanofsky C. Nucleotide sequences of trpA of Salmonella typhimurium and Escherichia coli: an evolutionary comparison. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5244–5248. doi: 10.1073/pnas.76.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Morgan E. A. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Evolution of the bacterial genome. Annu Rev Microbiol. 1978;32:519–560. doi: 10.1146/annurev.mi.32.100178.002511. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Genetic relatedness in the family Enterobacteriaceae. Annu Rev Microbiol. 1976;30:327–349. doi: 10.1146/annurev.mi.30.100176.001551. [DOI] [PubMed] [Google Scholar]
- Smith H. R., Humphreys G. O., Grindley N. D., Grindley J. N., Anderson E. S. Molecular studies of an fi+ plasmid from strains of Salmonella typhimurium. Mol Gen Genet. 1973 Nov 2;126(2):143–151. doi: 10.1007/BF00330989. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. The plasmid of Salmonella typhimurium LT2. Mol Gen Genet. 1973 Mar 19;121(4):347–353. doi: 10.1007/BF00433233. [DOI] [PubMed] [Google Scholar]
- Starlinger P. DNA rearrangements in procaryotes. Annu Rev Genet. 1977;11:103–126. doi: 10.1146/annurev.ge.11.120177.000535. [DOI] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]