Abstract
Background
Although non-Hispanic white women have an increased risk of developing breast cancer, the disease-specific survival is lower for African American and Hispanic women. Little is known about disparities in follow-up after an abnormal mammogram. The goal of this study was to investigate potential disparities in follow-up after an abnormal mammogram.
Methods
A retrospective cohort study of 6722 women with an abnormal mammogram and documented follow-up from January 2000 through December 2002 was performed at an academic medical center in New York City. The outcome was the number of days between the abnormal mammogram and follow-up imaging or biopsy. Cox proportional hazards models were used to assess the effect of race/ethnicity and other potential covariates.
Results
The median number of days to diagnostic follow-up after an abnormal mammogram was greater for African American (20 days) and Hispanic (21 days) women compared with non-Hispanic white (14 days) women (p < 0.001). Racial/ethnic disparities remained significant in a multivariable model controlling for age, Breast Imaging Reporting and Data System (BIRADS) category, insurance status, provider practice location, and median household income.
Conclusions
After an abnormal mammogram, African American and Hispanic women had longer times to diagnostic follow-up compared with non-Hispanic white women. Future efforts will focus on identifying the barriers to follow-up so that effective interventions may be implemented.
Introduction
Breast cancer is the most common type of cancer diagnosed and the second leading cause of cancer deaths in women in the United States.1 There are racial and ethnic differences associated with breast cancer. For example, non-Hispanic white women have an increased risk of developing breast cancer,1 and African American and Hispanic women have a lower disease-specific survival.2,3
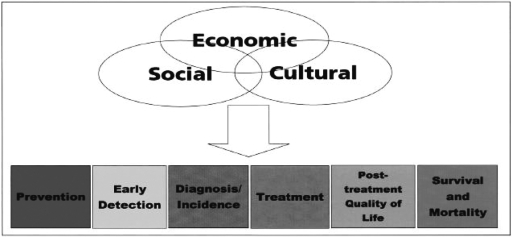
The cancer care continuum disparities model begins with prevention and early detection and continues through the survival period (Fig. 1).4–6 Some of the factors that might contribute to cancer disparities may occur at each end of the continuum or at the stages in between, such as diagnosis and treatment. It has been hypothesized that economic, social, and cultural factors may influence each stage.4,7–9 For example, poverty can decrease survival because it is associated with diminished access to healthcare and a lack of resources.10 Cultural perspectives can impact survival, as they may prevent people from seeking necessary testing or treatment because of reliance on fatalism or folk healing methods.4 The goal of this study was to investigate potential disparities in the stage from detection to diagnosis as depicted in the model (Fig. 1).
FIG. 1.
Factors that may contribute to disparities in the cancer care continuum. (From Ward et al.6)
Several factors may be contributing to these disparities in the cancer care continuum. First, African American and Hispanic women seek medical help at a later stage of breast cancer.11–17 Second, after diagnosis, minority women experience delays in the initiation and completion of treatment.18–20 Third, there are differences in the type of treatment received by African American and Hispanic women.21–23 Fourth, African American women are more likely to receive fewer cycles of the expected treatment compared with white women.24 Lastly, some studies suggest that African American women may present with a more aggressive form of breast cancer.25–27
Prior work has looked at disparities in the stage from detection to diagnosis. Data from the National Breast and Cervical Cancer Early Detection Program showed that among low income and uninsured women in the United States, African American and Hispanic women had longer follow-up times after abnormal mammograms compared with non-Hispanic white women.28 A study based at an academic medical center in California found that minority women had longer follow-up times both to first diagnostic test and to final disposition after abnormal mammograms compared with non-Hispanic white women.29 Similarly, a study based in five cities in Connecticut that examined a cohort of African American and white women found that African American women were more likely than white women to have inadequate follow-up after an abnormal mammogram (>3 months for Breast Imaging Reporting and Data Systems [BIRADS] 0, 4, 5 and >9 months for BIRADS 3).30 Another study of English-speaking non-Hispanic African American women in New York City found that 39% of the women did not have diagnostic resolution within 3 months of an abnormal mammogram.31 One study of 546 women at an urban public university hospital in New Jersey showed no difference in delay by race/ethnicity.32
In comparison to previous studies, our study has a large sample size nearly three times that of previous studies and includes a heterogeneous triethnic population. The specific aim of this study was to investigate potential disparities in follow-up after an abnormal mammogram at an academic medical center in New York, with the hypothesis that African American and Hispanic women would have longer times to diagnostic follow-up after an abnormal mammogram compared with non-Hispanic white women.
Materials and Methods
The study was conducted at an academic medical center in New York consisting of a large university and an affiliated voluntary not-for-profit hospital. The medical center serves two distinct populations of women. One group, residing in the local community, a federally designated medically underserved area, is predominantly Hispanic (74%) and, to a lesser extent, black (8%). Over 95% of these residents were Caribbean Hispanics, with Dominicans comprising 55% of all Hispanics, followed by Puerto Ricans and Cubans. Spanish was the primary language spoken at home by >90% of the Hispanics in the community.33 Most of these women, who are within the hospital catchment area, receive care in one of the medical center's community-based clinics. Another group of women served by the medical center come from a more diverse geographic area and receive care in one of the medical center's several affiliated private practices. Women from this New York Primary Metropolitan Statistical Area are predominantly white (40%) and, to a lesser extent, Hispanic (25%) or black (23%).
This analysis used a retrospective cohort study design of 6722 women who were found to have an abnormal mammogram at the medical center. All abnormal mammograms were included whether done for screening purposes or based on clinical examination. Women with an abnormal mammogram were identified using the medical center's clinical information system (CIS), which holds data from 1994 to the present for 1.5 million patients.
Inclusion criteria
Abnormal mammograms were defined using the American College of Radiology (ACR) BIRADS. Each mammogram is assigned a BIRADS category (0–5) to indicate the likelihood of a normal, benign, or malignant diagnosis. In this analysis, abnormal mammograms were defined as those mammograms requiring immediate follow-up, either BIRADS category 0 (indeterminate), BIRADS category 4 (suspicious for cancer), or BIRADS category 5 (highly suspicious for cancer).
Using this criterion, 7092 abnormal mammograms were identified from January 2000 through December 2002. There were 370 women (5.2%) without documented follow-up in this specified time period, and they were not included in subsequent analyses. The percent of women without documented follow-up was similar among African American (6%), Hispanic (4%), and non-Hispanic white women (6%) and greater for women of other racial/ethnic groups (11%).
Variables
The dependent variable was number of days to follow-up. This was defined as the number of days between the abnormal mammogram and additional imaging, such as ultrasound or repeat mammogram with spot compression or biopsy. The dates of the studies were obtained from the CIS data, which incorporate information from different sources, including radiology and pathology.
The main independent variable of interest was the race/ethnicity of the patients. This was recorded in the CIS database as either African American, Hispanic, white, Asian, and other. Because of small numbers, Asian and other were combined into one group called Asian/other.
Based on the conceptual model and variables available to us in our dataset, covariates that were hypothesized as potential confounders included age at the time of the mammogram, BIRADS category, location of residence (inside hospital catchment or outside hospital catchment area), insurance status, income, and provider practice location (clinic/nonclinic). The hospital catchment area was defined using ZIP codes. The variable was dichotomized as living inside the medical center's catchment area (residing in one of seven locally adjacent ZIP codes) or outside this local catchment area. Provider practice location was also dichotomized based on clinic codes. Women with at least two visits in the year prior to their mammogram at one of the several internal medicine, family practice, gynecology, or geriatrics clinics were considered as receiving their care in a clinic, that is, clinic patients. All others were considered nonclinic patients.
The insurance status of the patients was included in analyses as one of the following: insured by Medicaid, insured by private insurance and/or Medicare, or self-pay. The CIS database does not record individual-level income data. Therefore, data on median household income was obtained from the 2000 United States Census using the patient's ZIP code of residence. The income, divided into quartiles, was included in multivariate analyses using the following categories: 0–$27,000, $27,000–$30,000, $30,000–$41,000 and ≥$41,000.
Statistical analyses
Continuous variables are reported as mean ± 1 standard deviation (SD) for normally distributed variables and as median and interquartile range (IQR) for nonnormally distributed variables. Categorical variables are reported as percentage frequency. Differences in mean age among groups were assessed by analysis of variance (ANOVA). A chi-square test was used to analyze group differences in the frequency of categorical variables. Number of days to diagnostic follow-up was right skewed; thus, analysis of group differences was performed using the Kruskal-Wallis test. Multivariable analyses were performed using Cox proportional hazards models to determine the risk of having delayed follow-up. In addition to race and ethnicity, the covariates included age, BIRADS category, insurance status, provider practice location, and median household income.
Analyses were performed using SAS 8.2 (SAS Institute, Cary, NC). A two-sided p value < 0.05 was considered significant for all analyses.
Results
Baseline characteristics
There were 6722 women included in the final analysis. Of those, 5394 were BIRADS 0, 1116 were BIRADS 4, and 212 were BIRADS 5. Baseline characteristics of the women are shown in Table 1. There were 2143 (32%) non-Hispanic white women, 915 (14%) African American women, 3291 (49%) Hispanic women, and 373 (6%) women from Asian or other racial/ethnic groups. There were significant differences in characteristics between the groups. Compared with non-Hispanic white and African American women, Hispanics were slightly younger (56 years vs. 52 years, respectively, p < 0.001). Compared with non-Hispanic white women, both African American and Hispanic women were more likely to reside in lower income neighborhoods (p < 0.001) and to be covered by Medicaid (p < 0.001). Further, half of all Hispanic women and nearly a third of African American women served by the medical center received their care from the clinics vs. 5% of non-Hispanic white women (p < 0.001). Slightly over one third of non-Hispanic white women had a mammogram result of BIRADS 4 or 5 (suspicious or highly suspicious) vs. <20% for African American and Hispanic women (p < 0.001).
Table 1.
Characteristics of 6722 Women with Abnormal Mammograms, 2000–2002 (by Race/Ethnicity)
| |
Race/ethnicity |
||||
|---|---|---|---|---|---|
| White | African American | Hispanic | Asian/other | p value | |
| No. of participants (%) | 2143 (32) | 915 (14) | 3291 (49) | 373 (6) | |
| Mean age at initial abnormal mammogram, years ± SD | 56 ± 14 | 56 ± 13 | 52 ± 12 | 50 ± 12 | <0.001a |
| BIRADs, n (%) | |||||
| 0 | 1376 (64) | 762 (83) | 2957 (90) | 299 (80) | <0.001b |
| 4 | 665 (31) | 114 (13) | 274 (8) | 63 (17) | <0.001b |
| 5 | 102 (5) | 39 (4) | 60 (2) | 11 (3) | <0.001b |
| Medicaid insurance, n (%) | 86 (4) | 238 (26) | 1613 (49) | 93 (25) | <0.001b |
| Residence within local community, n (%) | 621 (29) | 384 (42) | 2435 (74) | 160 (43) | <0.001b |
| ZIP code population median income < $27,000, n (%) | 193 (9) | 604 (66) | 1448 (44) | 134 (36) | <0.001b |
| Location of care in clinic system, n (%) | 107 (5) | 128 (31) | 1646 (50) | 90 (24) | <0.001b |
Based on ANOVA across groups.
Based on chi-square across groups.
Time to diagnostic follow-up by race and ethnicity
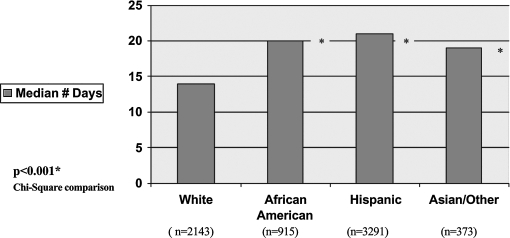
The median number of days to diagnostic follow-up after an abnormal mammogram was significantly greater for both African American and Hispanic women, each compared with non-Hispanic white women. Median times were 21 days for Hispanic women (IQR 15–31) and 20 days for African American women (IQR 13–31) vs. 14 days (IQR 3–22) for non-Hispanic white women (p < 0.001) (Table 2 and Fig. 2). Approximately 50 % of each racial/ethnic group with a mammogram that was BIRADS 4 or 5 had same-day additional imaging. However, among women who did not have same-day imaging we found that the median number of days of follow-up was 26 (IQR 10–44) for African American women, 23 (IQR 9–47) for Hispanic women, and 14 (IQR 7–27) for non-Hispanic white women (p < 0.05) (Table 2).
Table 2.
Follow-Up after Abnormal Mammogram by Race/Ethnicity
| |
Race/ethnicity |
||||
|---|---|---|---|---|---|
| White | African American | Hispanic | Asian/other | p valuea | |
| No. of days, median (IQR)b | 14 (3–22) | 20c (13–30) | 21c (15–31) | 19c (11–30) | <0.001 |
| BIRADs 4 or 5d | |||||
| Number of days, median (IQR) | 14 (7–27) | 26c (10–44) | 23c (9–47) | 10c (5–23) | <0.05 |
| Follow-up time ≤ 30 days, % | 86 | 75c | 74c | 78c | <0.001 |
| Follow-up time ≤ 60 days, % | 94 | 92e | 91c | 91c | <0.01 |
Based on ANOVA or chi-square analysis across groups.
IQR, interquartile range.
p < 0.05 by chi-square analysis for each racial/ethnic groups vs. referent group of non-Hispanic white women.
Excluding those with same day follow-up.
NS by chi-square analysis for African American vs. non-Hispanic white women.
FIG. 2.
Bivariate analysis of time to diagnostic follow-up after abnormal mammogram by race/ethnicity.
Within the follow-up interval of 30 days, 86% of non-Hispanic white women had follow-up compared with 75% of African American and 74% of Hispanic women (p < 0.001 for each racial/ethnic group vs. non-Hispanic white women) (Table 2). By 60 days, the differences were markedly attenuated, and >90% of the women in all three groups had received appropriate follow-up. However, some minority women still remained slightly less likely to have had the follow-up imaging (p < 0.01 for Hispanic vs. non-Hispanic white women) (Table 2).
Multivariable analysis
All the hypothesized covariates were found to be significantly related to the outcome. For example, women who received care within the medical center's clinic system had a median number of days of follow-up of 22 (IQR 16–32) compared with 17 (IQR 9–27) for women who received care outside the clinic system (p < 0.001). Also, women living within the hospital catchment area had a median number of days of follow-up of 21 (IQR 14–30), compared with 16 days (IQR 7–26) for women from outside the catchment area (p < 0.001). Thus, multivariable analyses were used to adjust for these potential confounders.
In the multivariable model, the strongest predictor of delayed follow-up risk was BIRADS status, with a result of BIRADS 4 or 5 (suspicious or highly suspicious) being associated with shorter time to follow-up (BIRADS 4: HR 0.64, 95% CI 0.58-0.69, p < 0.001; BIRADS 5: HR 0.48, 95% CI 0.40-0.56, p < 0.001) (Table 3). Other significant predictors for delayed follow-up included living in the hospital catchment area (HR 1.09, 95% CI 1.02-1.18, p < 0.05) and having Medicaid insurance (HR 1.09, 95% CI 1.02-1.17, p < 0.05) (Table 3). Receiving care within the hospital clinic system was not independently predictive of increased risk (HR1.05, 95% CI 0.99-1.12, NS) (Table 3). After these covariates were adjusted for, African American women (HR 1.20, 95% CI 1.09-1.33, p < 0.001) and Hispanic women (HR 1.23, 95% CI 1.13-1.33, p < 0.001) were still at greater risk of having a longer time to diagnostic follow-up compared with white women (Table 3).
Table 3.
Multivariable Analysis of Delay in Follow-Up after Abnormal Mammogram
| Hazard ratio | 95% CI | p value | |
|---|---|---|---|
| Race/ethnicity | |||
| White | 1.0 | ||
| African American | 1.20 | 1.09-1.33 | <0.001 |
| Hispanic | 1.23 | 1.13-1.33 | <0.001 |
| Asian/other | 1.14 | 1.00-1.30 | <0.05 |
| Hospital catchment | |||
| Outside catchment | 1.0 | ||
| Within catchment | 1.09 | 1.02-1.18 | <0.05 |
| Provider practice location | |||
| Outside clinic system | 1.0 | ||
| Within clinic system | 1.05 | 0.99-1.12 | NS |
| Insurance status | |||
| Private and/or Medicare | 1.0 | ||
| Medicaid | 1.09 | 1.02-1.17 | <0.05 |
| BIRADS | |||
| 0 | 1.0 | ||
| 4 | 0.64 | 0.58-0.69 | <0.001 |
| 5 | 0.48 | 0.40-0.56 | <0.001 |
Discussion
Disparities in the stage of diagnosis, treatment, and outcomes of breast cancer for racial and ethnic minority women have been extensively described.1,3,12,28,29 In this ethnically diverse cohort of 6722 women with an abnormal mammogram, longer times to diagnostic follow-up were found for African American and Hispanic women compared with non-Hispanic white women. These racial/ethnic differences in risk of having a longer time to diagnostic follow-up remained after adjusting for age, BIRADS status, insurance, income, and provider practice location.
In this study, most of the follow-up occurred within the first 30 days after an abnormal mammogram. Within this time period, however, the percentage of African American and Hispanic women with follow-up was significantly less than the percentage of non-Hispanic white women. There is no consensus in the literature as to what is a reasonable follow-up interval after an abnormal mammogram. Some investigators have found that follow-up intervals of up to 3 months may not impact overall survival,18,34 whereas others have shown that women who waited more than 30 days for evaluation after breast cancer detection were more likely to experience breast cancer recurrence or death.35 This study was unable to address the long-term clinical significance of the delay in follow-up after an abnormal mammogram; however, our findings document that minority women received follow-up approximately 1 week later than nonminority women. If similar disparities are present at other points along the cancer care continuum, they may have a cumulative clinically significant impact overall on mortality.
Similar to the findings in this study, a smaller study in California found a delay in follow-up of 7 days for minority women compared with white women. This study included 76 minority women and adjusted for income but not for location of care, location of residence, or insurance status.29 These investigators suggested that the delay may be due to a combination of factors related to the healthcare system, the patients, and the providers. Another study, which did not include Hispanic women, found a longer diagnostic interval for African American than for non-Hispanic white women.35 This was found after adjusting for income and insurance status but not for location of care or location of residence.
Other investigators have documented similar racial differences in follow-up after abnormal mammograms within strata of socioeconomic status. Data from 1991–1995 in the National Breast and Cervical Cancer Early Detection Program found that the time from initial abnormal mammogram to diagnosis was 7 days longer for African American women and 9 days longer for Hispanic women, each compared with non-Hispanic white women.28 This study included only uninsured or low-income women.
The source of these documented differences in follow-up after abnormal mammograms is most likely multifactorial. The National Cancer Institute's Presidents Cancer Panel Report for 2000–2001 presented four categories of barriers: (1) healthcare system barriers, (2) financial barriers, (3) physical barriers, and (4) physician and patient level information and education barriers.4 One of the common healthcare system barriers is difficulty in contacting the patients because of frequent mobility, with inaccurate phone numbers and addresses. The patient level financial barriers include inadequate resources for phones, transportation, childcare, and elder care. In addition, patients also face barriers because of language differences, leading to difficulty in communication. Finally, patient-related fears and concerns may contribute to delays in appropriate follow-up evaluations and treatments. 20,21,32,36–42
In order to successfully implement interventions that overcome such barriers, the emerging consensus is that such initiatives need to be culturally tailored to the specific group of individuals in need.43–47 Interventions should also involve the local community and should be related to each aspect of the described barriers that may be contributing to the delays in follow-up. For example, community health workers could be used to assist in educating patients and helping them to navigate the complex healthcare system. Studies that have looked at the efficacy of these types of interventions, in both cancer and other diseases, have shown them to be useful.48–52
Strengths and limitations
The strengths of this study include a large triethnic cohort of over 6000 women and the ability to capitalize on data from the medical center's CIS. In addition, the medical center serves a racially diverse community. Our study in particular focuses on Caribbean Hispanics, the fastest growing component of the U.S. Hispanic population.53 Furthermore, >10% of the population seen at the medical center is uninsured, and there are various screening programs for the uninsured. However, several caveats apply. First, the use of hospital administrative data for the classification of race and ethnicity may lead to misclassification. Studies using Medicare data in which race was recorded using methodology similar to that used in this study have shown race to have a sensitivity and positive predictive value exceeding 94%.54 It may, however, underestimate the proportion of Hispanics. Thus, the racial/ethnic classification was validated using data collected as part of a distinct study having self-reported racial/ethnic data in 1007 patients followed in one of our clinical sites.55,56 Using the self-reported data as the gold standard, the sensitivity of the CIS database was 67% for Hispanics and 72% for African Americans, and the specificity was 94% for both Hispanics and African Americans. This misclassification would have resulted in an underrepresentation of the proportion of minority women included in the analysis and biased the results toward the null. Another limitation is that it was not possible to control for additional potential confounders, such as the number of previous mammograms, prior history of mammography screening, family or personal history of breast cancer, language barriers, or psychosocial predictors. Our study was not able to obtain follow-up information on the 5% of women without documented follow-up in order to determine if they received care at another institution or did not follow up at all. This percent of women without documented follow-up did not vary significantly by racial/ethnic group. Our study was also not able to address the causes of delay, and this is an area that will require additional investigation.
In conclusion, this study found a 6-day delay in follow-up after an abnormal mammogram for African American women and a 7-day delay for Hispanic women compared to non-Hispanic white women. This provides additional evidence of racial and ethnic disparities that exist among women in the cancer care continuum. This information may now be used in subsequent analyses that will identify the barriers that lead to these disparities. Necessary interventions should be implemented in order to address the racial and ethnic disparities that are currently seen in breast cancer diagnosis and treatment.
Footnotes
This work was supported in part by grants from the Avon Foundation, the Norman and Rosita Winston Foundation (R.P., E.V.G.) and an NIH/NCMHD EXPORT award (O.C.).
Disclosure Statement
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Surveillance, Epidemiology, End Results (SEER) [Nov;2002 ]. www.cancer.org www.cancer.org
- 2.Bradley CJ. Given CW. Roberts C. Disparities in cancer diagnosis and survival. Cancer. 2001;91:178–188. doi: 10.1002/1097-0142(20010101)91:1<178::aid-cncr23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Li CI. Malone KE. Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Freeman HP. Reuben SH. Presidents Cancer Panel Report of the chairman, 2000–2001. Bethesda, MD: National Cancer Program National Cancer Institute; 2001. Voices of a broken system. Real problems, real people. [Google Scholar]
- 5.Zapka JG. Taplin SH. Solberg LI. Manos MM. A framework for improving the quality of cancer care: The case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12:4–13. [PubMed] [Google Scholar]
- 6.Ward E. Jemal A. Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 7.Li FP. Digianni LM. Reducing the unequal burden of cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:230s–231s. [PubMed] [Google Scholar]
- 8.Kaplan CP. Eisenberg M. Erickson PI. Crane LA. Duffey S. Barriers to breast abnormality follow-up: Minority, low-income patients' and their providers' view. Ethnicity Dis. 2005;15:720–726. [PubMed] [Google Scholar]
- 9.Weinmann S. Taplin SH. Gilbert J, et al. Characteristics of women refusing follow-up for tests or symptoms suggestive of breast cancer. Natl Cancer Inst. 2005;35:33–38. doi: 10.1093/jncimonographs/lgi035. [DOI] [PubMed] [Google Scholar]
- 10.Freeman HP. Commentary on the meaning of race in science and society. Cancer Epidemiol Biomarkers Prev. 2003;12(Suppl):232s–236s. [PubMed] [Google Scholar]
- 11.Grau AM. Ata A. Foster L, et al. Effect of race on long-term survival of breast cancer patients: Transinstitutional analysis from an inner city hospital and university medical center. Am Surg. 2005;71:164–70. [PubMed] [Google Scholar]
- 12.Chen F. Trapido EJ. Davis K. Differences in stage at presentation of breast and gynecologic cancers among whites, blacks, and Hispanics. Cancer. 1994;73:2838–2842. doi: 10.1002/1097-0142(19940601)73:11<2838::aid-cncr2820731129>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Davidson PL. Bastani R. Nakazono TT. Carreon DC. Role of community risk factors and resources on breast carcinoma stage at diagnosis. Cancer. 2005;103:922–930. doi: 10.1002/cncr.20852. [DOI] [PubMed] [Google Scholar]
- 14.Lannin DR. Mathews HR. Mitchell J. Swanson MS. Swanson FH. Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentaion of breast cancer. JAMA. 1998;279:1801–1807. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- 15.Velanovich V. Marianne UY. Bawle U, et al. Racial differences in the presentation and surgical management of breast cancer. Surgery. 1999;125:372–379. [PubMed] [Google Scholar]
- 16.Lanz PM. Mujahid M. Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96:2173–2178. doi: 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sassi F. Luft H. Guadagnoli E. Reducing racial/ethnic disparities in female breast cancer: Screening rates and stage at diagnosis. Am J Public Health. 2006;96:2165–2172. doi: 10.2105/AJPH.2005.071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards MA. Westcombe AM. Love SB. Littlejohns P. Ramirez AJ. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 19.Dennis CR. Gardner B. Lim B. Analysis or survival and recurrence vs. patient and doctor delay in treatment of breast cancer. Cancer. 1975;35:714–720. doi: 10.1002/1097-0142(197503)35:3<714::aid-cncr2820350326>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Gwyn K. Bondy M. Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 21.Tropman SE. Ricketts TC. Paskett E. Hatzell TA. Cooper MR. Aldrich T. Rural breast cancer treatment: Evidence from the Reaching Communities for Cancer Care (REACH) project. Breast Cancer Res Treat. 1999;56:59–66. doi: 10.1023/a:1006279117650. [DOI] [PubMed] [Google Scholar]
- 22.Chu KC. Lamar CA. Freeman HP. Racial disparities in breast carcinoma survival rates: Separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003;97:2853–2860. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 23.Harlan LC. Abrams J. Warren JL. Clegg L. Stevens J. Ballard-Barbash R. Adjuvant therapy for breast cancer: Practice patterns of community physicians. J Clin Oncol. 2002;20:1809–1817. doi: 10.1200/JCO.2002.07.052. [DOI] [PubMed] [Google Scholar]
- 24.Hershman D. McBride R. Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23:6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 25.Joslyn S. West M. Racial differences in breast carcinoma survival. Cancer. 2000;88:114–123. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Elledge RA. Clark GM. Chamness GC. Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 27.Jones BA. Kasl SV. Howe CL, et al. African-American/white differences in breast carcinoma: p53 alterations and other tumor characteristics. Cancer. 2004;101:1293–1301. doi: 10.1002/cncr.20500. [DOI] [PubMed] [Google Scholar]
- 28.Caplan LS. May DS. Richardson LC. Time to diagnosis and treatment of breast cancer: Results from the National Breast and Cervical Cancer Early Detection Program, 1991–1995. Am J Public Health. 2000;90:130–134. doi: 10.2105/ajph.90.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang SW. Kerlikauske K. Napoles-Springer A. Posner S. Sickles EA. Perez-Stable E. Racial differences in timeliness of follow-up after abnormal screening mammography. Cancer. 1996;78:1395–1402. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1395::AID-CNCR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Jones BA. Dailey A. Calvocoessi L, et al. Inadequate follow-up of abnormal screening mammograms: Findings from the Race Differences in Screening Mammography Process Study (United States) Cancer Causes Control. 2005;16:809–821. doi: 10.1007/s10552-005-2905-7. [DOI] [PubMed] [Google Scholar]
- 31.Kerner J. Yedidia M. Padgett D, et al. Realizing the promise of breast cancer screening: Clinical follow-up after abnormal screening among black women. Prev Med. 2003;37:92–101. doi: 10.1016/s0091-7435(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 32.Ferrante JM. Rovi S. Das K. Kim S. Family physicians expedite diagnosis of breast disease in urban minority women. J Am Board Fam Med. 2007;20:52–59. doi: 10.3122/jabfm.2007.01.060117. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Census Bureau. “American Fast Finder”. www.factfinder.census.gov. [Jun;2007 ]. www.factfinder.census.gov
- 34.Kerlikowske K. Timeliness of follow-up after abnormal screening mammography. Breast Cancer Res Treat. 1996;41:53–64. doi: 10.1007/BF01806002. [DOI] [PubMed] [Google Scholar]
- 35.Elmore JG. Nakano CY. Linden HM. Reisch LM. Ayanian JZ. Larson EB. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care. 2005;43:141–148. doi: 10.1097/00005650-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Lerman C. Trock B. Rimer BK. Boyce A. Jepson C. Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med. 1991;114:657–661. doi: 10.7326/0003-4819-114-8-657. [DOI] [PubMed] [Google Scholar]
- 37.Ashing-Giwa KT. Padilla G. Tejero J, et al. Understanding the breast cancer experience of women: A qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psychooncology. 2004;13:408–428. doi: 10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coyne C. Hohman K. Levinson A. Reaching special populations with breast and cervical cancer public education. J Cancer Educ. 1992;7:293–303. doi: 10.1080/08858199209528186. [DOI] [PubMed] [Google Scholar]
- 39.Jones BA. Reams K. Calvocoressi L. Dailey A. Kasi SV. Liston NM. Adequacy of communicating results from screening mammograms to African American and white women. Am J Public Health. 2007;97:531–538. doi: 10.2105/AJPH.2005.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karliner KS. Kaplan CP. Juarbe T. Pasick R. Perez-Stable EJ. Poor patient comprehension of abnormal mammography results. J Gen Intern Med. 2005;20:432–437. doi: 10.1111/j.1525-1497.2005.40281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan CP. Crane L. Stewart S. Juarez-Reyes M. Factors affecting follow-up among low-income women with breast abnormalities. J Womens Health. 2004;13:195–206. doi: 10.1089/154099904322966182. [DOI] [PubMed] [Google Scholar]
- 42.Arnsberger P. Fox P. Ryder P. Nussey B. Zhang X. Otero-Sabogol R. Timely follow-up among multicultural women with abnormal mammograms. Am J Health Behav. 2006;30:51–61. doi: 10.5555/ajhb.2006.30.1.51. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman-Goetz L. Friedman DB. Disparities in the coverage of cancer informaton in ethnic minority and mainstream mass print media. Ethn Dis. 2005;15:332–340. [PubMed] [Google Scholar]
- 44.Godfrey J. Toward optimal health: Judy Ann Bigby, M.D., discusses the need for cultural competence in the healthcare of women. J Womens Health. 2006;15:480–483. doi: 10.1089/jwh.2006.15.480. [DOI] [PubMed] [Google Scholar]
- 45.Azaiza F. Cohen M. Health beliefs and rates of breast cancer screening among Arab women. J Womens Health. 2006;15:520–529. doi: 10.1089/jwh.2006.15.520. [DOI] [PubMed] [Google Scholar]
- 46.Shirazi M. Champeau D. Talebi A. Predictors of breast cancer screening among immigrant Iranian women in California. J Womens Health. 2006;15:485–506. doi: 10.1089/jwh.2006.15.485. [DOI] [PubMed] [Google Scholar]
- 47.Kreuter MW. Sugg-Skinner C. Holt CL, et al. Cultural tailoring for mammography and fruit and vegetable intake among low-income African-American women in urban public health centers. Prev Med. 2005;41:53–62. doi: 10.1016/j.ypmed.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Corkery E. Palmer C. Foley ME. Schecter CB. Risher L. Roman SH. Effect of a bicultural community health worker on completion of diabetes education in a Hispanic popultion. Diabetes Care. 1997;20:254–257. doi: 10.2337/diacare.20.3.254. [DOI] [PubMed] [Google Scholar]
- 49.Weber BE. Reilly BM. Enhancing mammography use in the inner city. A randomized trial of intensive case management. Arch Intern Med. 1997;157:2345–2349. [PubMed] [Google Scholar]
- 50.Fernandez ME. DeBor M. Candreia MJ. Wagner AK. Stewart KR. Evaluation of ENCOREplus. A community-based breast and cervical cancer screening program. Am J Prev Med. 1999;16(3 Suppl):35–49. doi: 10.1016/s0749-3797(98)00145-7. [DOI] [PubMed] [Google Scholar]
- 51.Ell K. Vourlekis B. Lee PJ. Xie B. Patient navigation and case management following an abnormal mammogram: A randomized clinical trial. Prev Med. 2007;44:26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Battaglia TA. Roloff K. Posner MA. Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population; A patient navigation intervention. Cancer. 2007;109(2 Suppl):359–367. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- 53.Ramirez RR. de la Cruz GP. The Hispanic population in the United States: March 2002. Current population report. Washington, DC: U.S. Census Bureau; 2002. pp. 20–545. [Google Scholar]
- 54.Arday SL. Arday DR. Monroe S. Zhang J. HCFA's racial and ethnic data: Current accuracy and recent improvements. Health Care Financing Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 55.Olfson M. Lewis-Fernandez R. Weissman MM, et al. Psychotic symptoms in an urban general medicine practice. Am J Psychiatry. 2002;159:1412–1419. doi: 10.1176/appi.ajp.159.8.1412. [DOI] [PubMed] [Google Scholar]
- 56.Olfson M. Shea S. Feder A, et al. Prevalence of anxiety, depression, and substance use disorders in an urban general medicine practice. Arch Fam Med. 2000;9:876–883. doi: 10.1001/archfami.9.9.876. [DOI] [PubMed] [Google Scholar]