Abstract
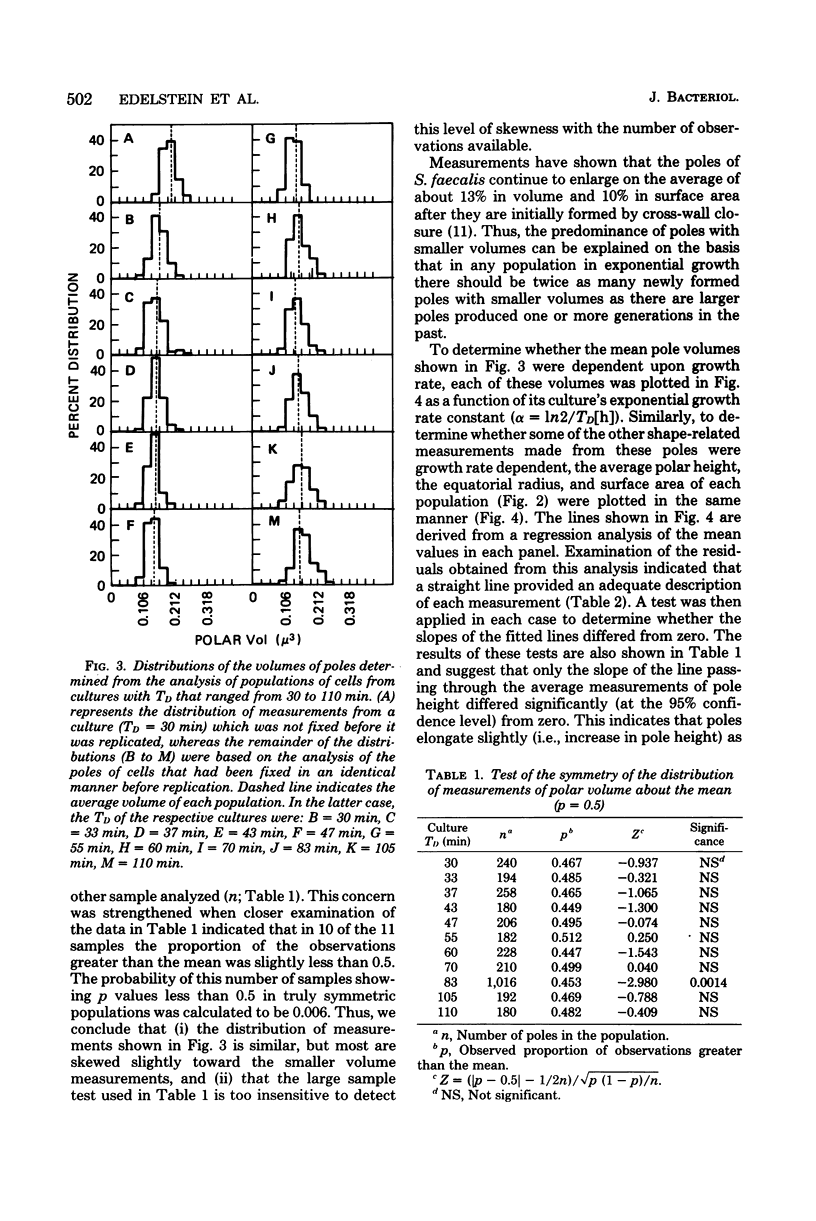
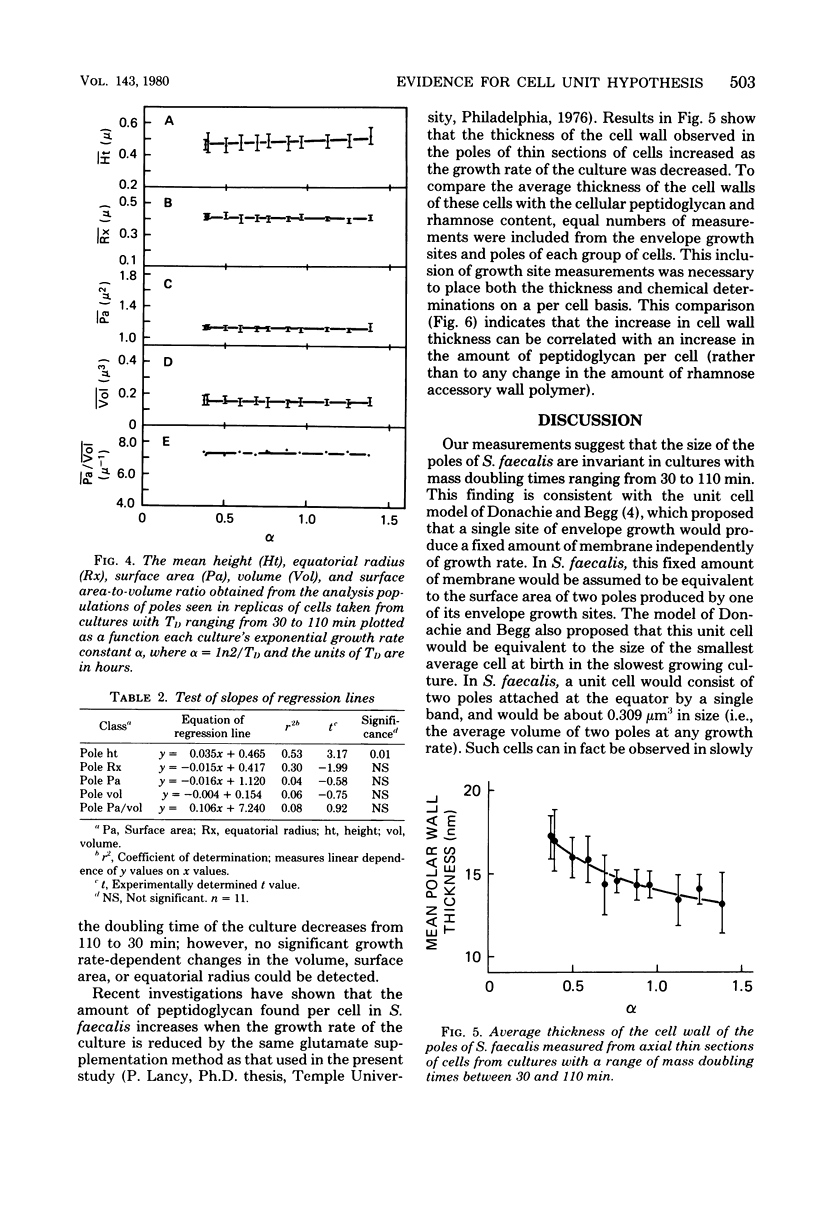
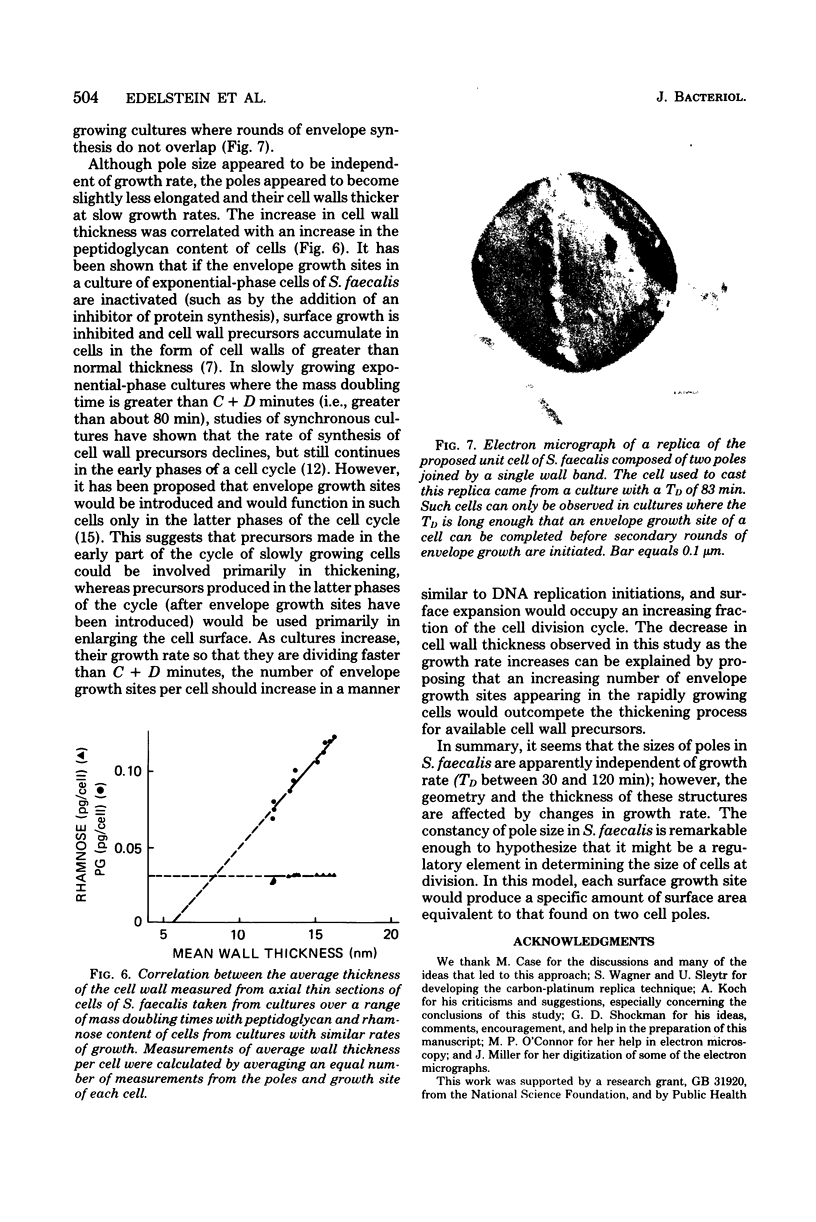
The mass doubling times of exponential-phase cultures of Streptococcus faecalis were varied from 30 to 110 min by omitting glutamine from a defined growth medium and providing different concentrations of glutamate (ranging from 300 to 14 μg/ml). After Formalin fixation, cells were dried by the critical point method, and carbon-platinum replicas were prepared. The surface area and volume of cell poles seen in these replicas were estimated by a computer-assisted, three-dimensional reconstruction technique. It was found that the amount of surface area and volume of poles seen in these replicas were independent of the growth rate of culture from which the samples were taken. These observations were consistent with the unit cell model hypothesis of Donachie and Begg, in which a small number of surface sites would produce a constant amount of new cell surface regardless of the mass doubling time of the culture. However, measurements of the thickness of the cell wall taken from thin sections of the same cells showed that the cell wall increased in thickness as a function of the increase in cellular peptidoglycan content which occurs when the growth rate of this organism is slowed down by a decrease in glutamate concentration. Thus, it would seem that although the size of polar shells made by S. faecalis is invariant with growth rate, the amount of wall precursors used to construct these shells is not.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L., Boothby D., Shockman G. D. Effect of inhibition of deoxyribonucleic acid and protein synthesis on the direction of cell wall growth in Streptococcus faecalis. J Bacteriol. 1974 May;118(2):681–692. doi: 10.1128/jb.118.2.681-692.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Reinitiation of cell wall growth after threonine starvation of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):1175–1183. doi: 10.1128/jb.105.3.1175-1183.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Study of cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976 Sep;127(3):1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976 Sep;127(3):1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Relationship between cellular autolytic activity, peptidoglycan synthesis, septation, and the cell cycle in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Jun;134(3):1074–1080. doi: 10.1128/jb.134.3.1074-1080.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Hohman R. J. Electron microscopic study of cell surface rings during cell division and morphogenesis of Arthrobacter crystallopoietes. J Bacteriol. 1977 Jun;130(3):1345–1356. doi: 10.1128/jb.130.3.1345-1356.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., ISZARD L., ROGERS N. B., SHOCKMAN G. D. Cell multiplication studied with an electronic particle counter. J Bacteriol. 1961 Dec;82:857–866. doi: 10.1128/jb.82.6.857-866.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]